Biology Group . 2023 August 22;4(8):1236-1241. doi: 10.37871/jbres1790.
Five New Species of Helicotylenchus of Citrus Trees in North of Khuzestan Province of Iran
Zahra Rashno-Bone Abbasi, Somaye Alvani, Esmat Mahdikhani-Moghadam* and Sare Baghaei
- Helicotylenchus
- Citrus
- Iran
- Nematode
- Khuzestan
Abstract
In order to study spiral nematodes of citrus trees in Khuzestan province of Iran, 115 soil and root samples were collected during summer and autumn of 2016 and 2017. Nematodes were extracted and transferred into glycerin. The permanent slides were prepared. The nematodes were identified under light microscopy and drawing tube based on morphological and morphometrical characters. In this study, 14 species of genus Helicotylenchus were identified. Among them H. morassi, H. urobelus, H. angularis, H. thornei and H. pisi are new for Iranian nematofauna.
Introduction
Plant parasitic nematodes belonging to the family Hoplolaimidae [1] have global distribution in agricultural soils. Spiral nematodes of the genus Helicotylenchus [2] comprise of the most common and consistent species of hoplolaimid population attacking agricultural crops under diverse climatic and edaphic conditions. They are ectoparasites of plant roots. They insert their stylets into root epidermis to feed. Some species live half-buried in the root tissue, and others penetrate the root and live inside. They lay eggs on, around, or inside the roots, and within two or three days, the juveniles emerge to feed [3]. This genus is associated with a wide variety of host plant taxa [4]. Most species are not very damaging to the plant. Four species out of over 200 are destructive plant pests, which can suppress plant growth: H. dihystera, H. multicinctus [5], H. pseudorobustus [6] and H. digonicus [7]. A few others are potential pests [4]. Plants infested with aggressive species may become stunted and yellowed, but usually there is no sign of infestation in the herbage. An exception is H. multicinctus [5], which can cause enough root necrosis to seriously weaken the plant. This species may be the most economically important, occurring in crops such as bananas of the Cavendish group. Other species have caused occasional damage to maize and Kentucky bluegrass [3].
This study aimed on determining the plant-parasitic nematodes of the family Haplolaimidae of citrus trees in North of Khuzestan province. During nematode surveys conducted in 2016-2017, we detected some species of Haplolaimidae associated with root and soil samples. This list includes H. abunaamai [8], H. angularis [9], H. californicus [10], H. conicephalus [8], H. crenacauda [10], H. egyptiensis [11], H. falcatus [12], H. insignis [13], H. microlobus [7], H. morassi [14], H. pisi [15], H. pseudorobustus [6], H. thornei [16] and H. urobelus [17]. (Species shown in black were recorded for the first time in Iran).
Materials and Methods
Surveys (115 soil and root samples) were conducted during summer and autumn of 2016-2017 to determine the plant-parasitic nematodes of the family Haplolaimidae associated with citrus from the tropical region of Khuzestan in Iran. To obtain a cleaner suspension of nematodes, the tray method was used [18]. Nematodes of interest were handpicked, heat-killed by adding boiling 4% formalin solution, transferred to anhydrous glycerin according to De Grisse AT [19], mounted in permanent slides, and examined using a light microscope. Drawings were made using a drawing tube attached to the microscope. Genera and species were identified based on morphological and morphometrical characters.
Results and Discussion
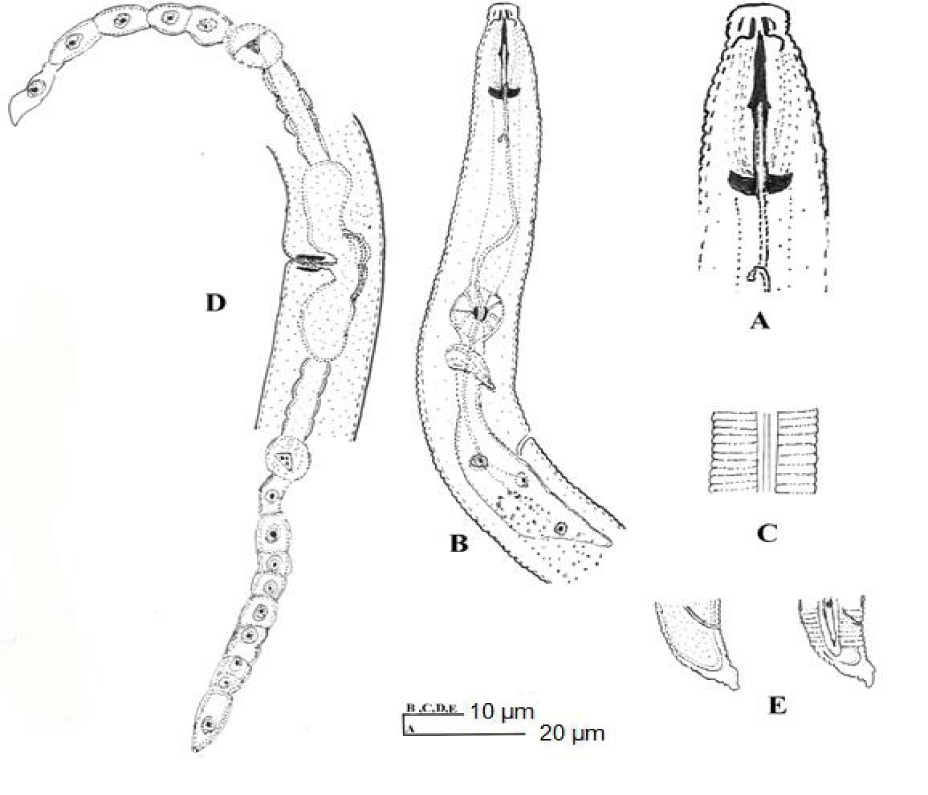
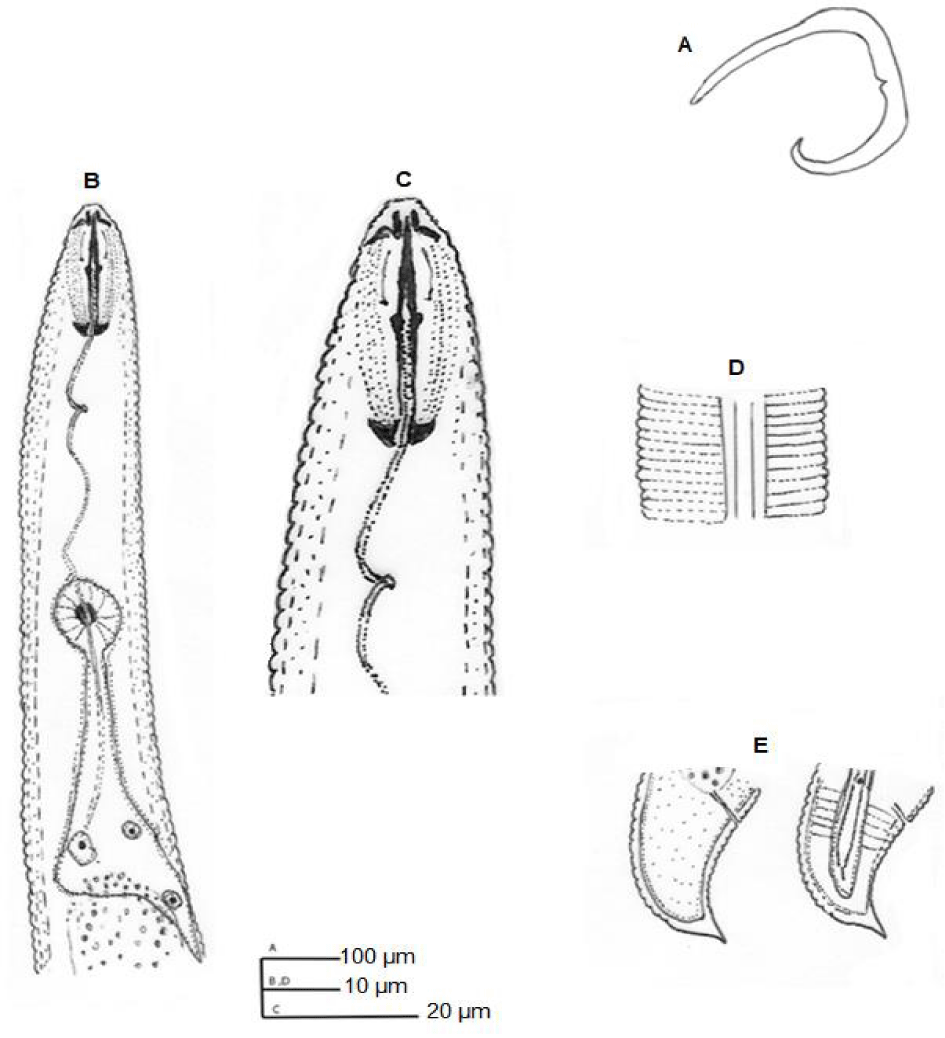
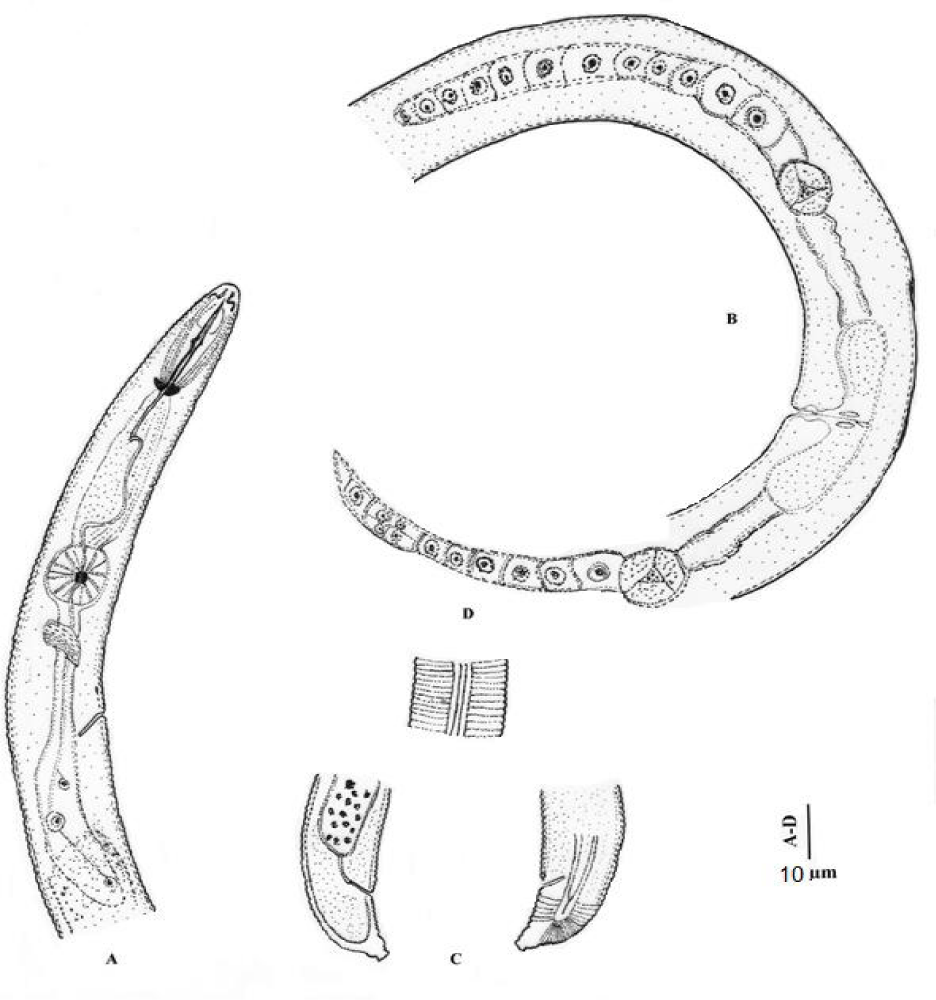
H. angularis (Figures 1,2) (Table 1) [9]
3.1.1. Female: Body spirally curved when relaxed by heat. Annules 1.5-1.8 μm wide at midbody. Lateral fields with four smooth incisures, one-fifth to one-fourth body width. Lip region angular, distinctly offset, wider than adjacent body, with 2-4 annules, framework heavily sclerotized. Stylet well developed, conus 43-49% of spear length, basal knobs anteriorly flattened to concave. Orifice of dorsal oesophageal gland 10-11 μm from stylet knobs. Excretory pore 85-110 μm from anterior end. Vulva transverse, depressed. Ovaries paired, outstretched. Spermatheca empty. Tail dorsally convex-conoid to a well-developed terminal peglike projection, with 7-12 annules ventrally. Phasmids 3-6 annules anterior to anal level.
| Table 1: Helicotylenchus species. | |||||
| Characters | Helicotylenchus species | ||||
| H. angularis | H. morassi | H. pisi | H. thornei | H. urobelus | |
| L | 600-630 | 550-620 | 740-850 | 600-750 | 650-700 |
| a | 28.5-29 | 27.2-32.5 | 18-23.8 | 22-28 | 30.9-41 |
| b | 5-5.2 | 4.3-5 | 5.5-6.7 | 4.2-5.3 | 5-5.2 |
| c | 40-42 | 27-39 | 33-41 | 24-27.7 | 35-40 |
| c' | 1.3-1.5 | 1.6-1.7 | 1.3-2 | 1.2-1.9 | 1.3-1.4 |
| V | 60-62 | 59-63 | 60-65 | 60-63 | 56-58.4 |
| Stylet | 22-24 | 18-20 | 25-30 | 24-25 | 24-26 |
| O | 45-49 | 42.1-50 | 48-56 | 45-53 | 40-46 |
Male: Not found.
Remarks: This species differs from all nominal species of the genus in having an offset, angular lip region.
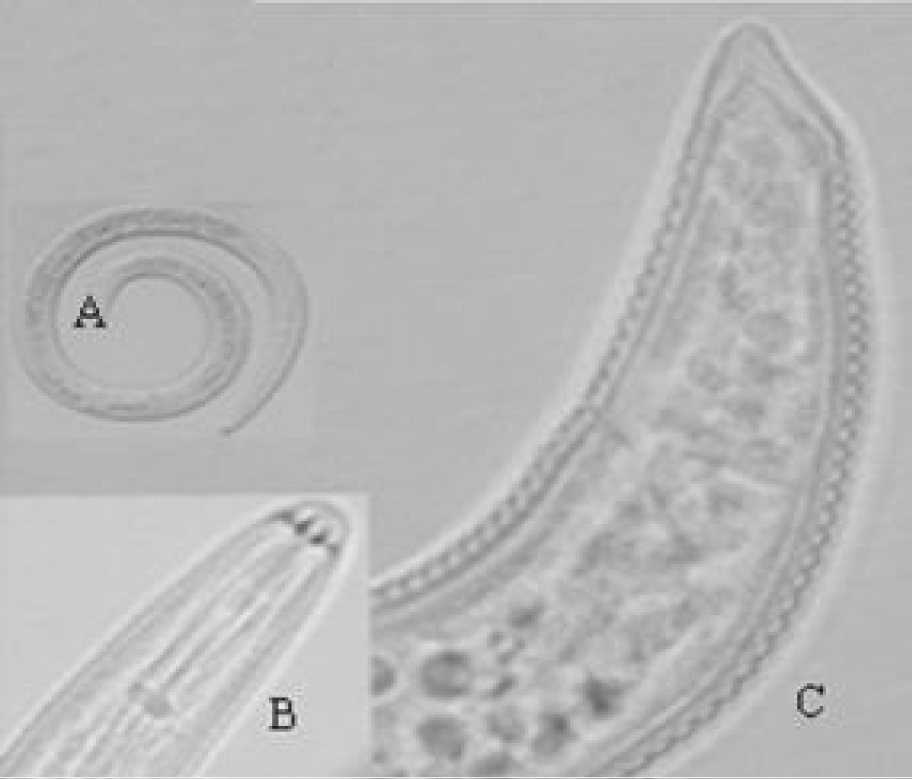
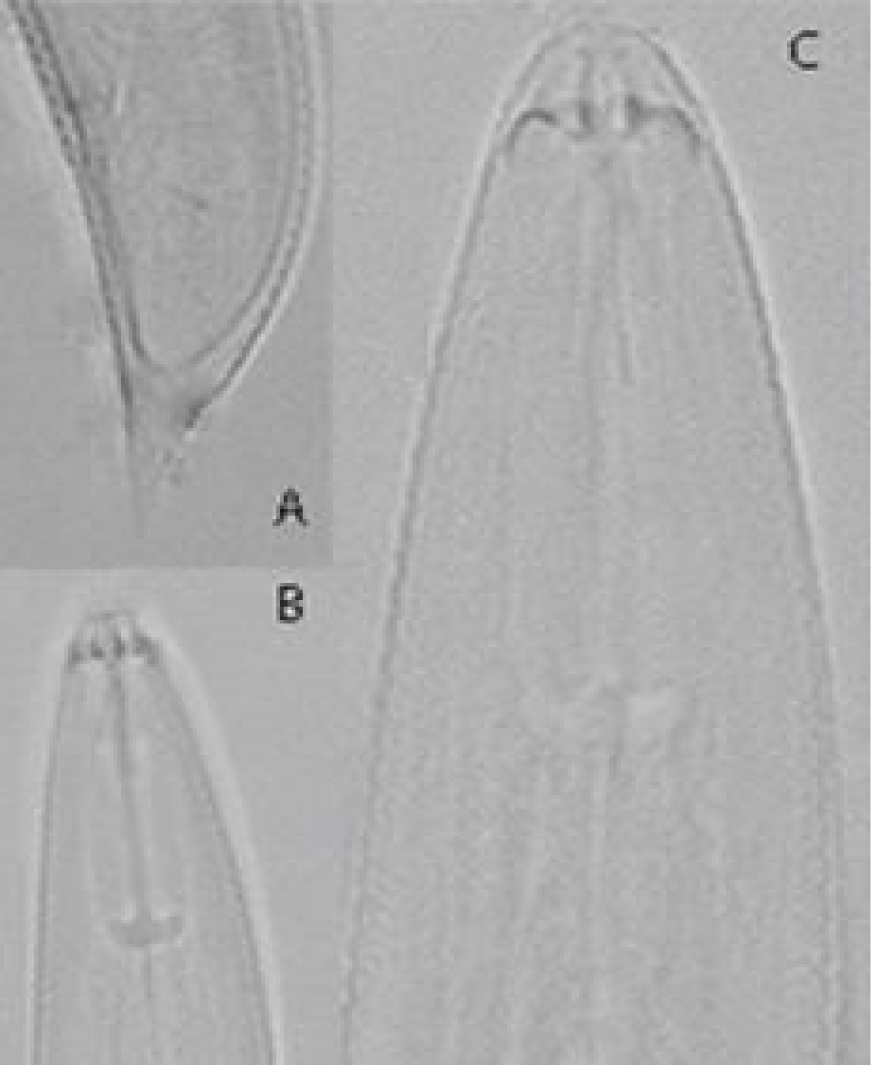
H. morassi (Figures 3,4 ) (Table 1) [14]
Female: The body assumes an open spiral when relaxed with heat. Cuticle striation distinct, 1.2 μm apart near mid body. Lateral fields with four. Lip region truncate, continuous, marked with four indistinct annules. Spear robust with knobs anteriorly cupped measuring 3.5 μm. Orifice of dorsal oesophageal gland 9 μm behind the stylet knobs. Metacorpus well developed 9-11 μm long and 6-8 μm wide filling half of the corresponding body width. Nerve ring 75 μm, excretory pore 90 μm from anterior end. Hemizonid not seen. Vulva a transverse slit. Vagina at right angle to body axis extending to about two-thirds of body width into the body. Spermatheca spheroidal, empty. Tail short, dorsally convex with blunt to sharply conoid terminal projection. Tail annules 15 on ventral side. Phasmids located 5-10 annules anterior to anus.
Male: Not found.
Remarks: According to the morphological characters and morphometric data given in the original description, there were no differences between the Iranian population of H. morassi and the original description. H. morassi is similar to H. elegans [16] and H. abunaamai [8]. It differs from H. elegans by body lengh (550-620 vs. 440-445μm) and stylet length (18-20 vs. 21-24 μm). It also differs from H. abunaamai by stylet length (18-20 vs. 24-27 μm), hemizonid (absent vs. present).
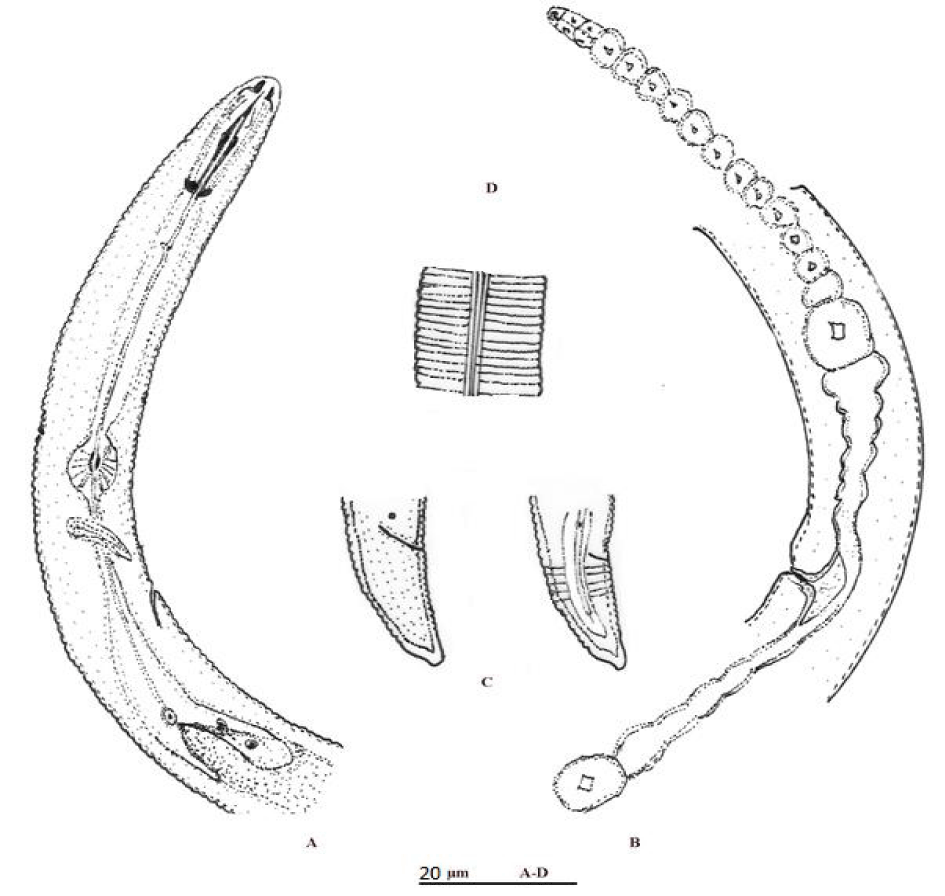
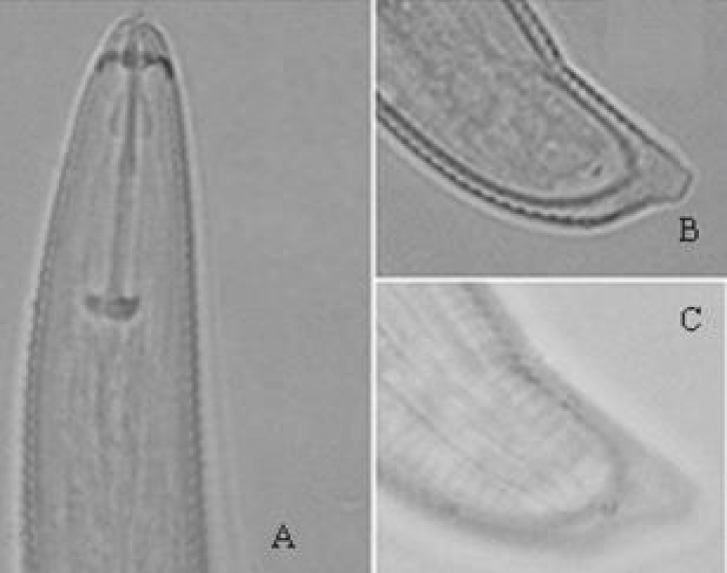
H. pisi (Figures 5,6) (Table 1) [15]
Female: Body spiral when relaxed. Lip region conical, with three or four inconspicuous annules. Stylet knobs anteriorly flat. Orifice of dorsal esophageal gland duct 14 μm posterior to base of stylet knobs. Cuticule annules coarse, 1.9 μm wide in middle part of body. Lateral fields about one-eighth diameter. Excretory pore somewhat anterior to level of anterior end of esophageal glands. Hemizonid anterior to excretory pore (one or three cuticular rings). Phasmids distin ct, situated three cuticular rings anterior or behind anus. Vulva in form of transverse slit, with vulval membrane. Ovaries paired, straight. Spermatheca rounded, empty. Oocytes in single row. Tail narrows from dorsal side with small ventral outgrowth, 12 annules in ventral side.
Male: Not found.
Remarks: According to the morphological characters and morphometric data given in the original description, there were no differences between the Iranian population of H. pisi and the original description. This species is close to H. canadensis [20]. H. pisi differentiated from H. canadensis by values for indexes “O” and “C” (48-56 vs. 25 and 33-41 vs. 36-66, respevtively).
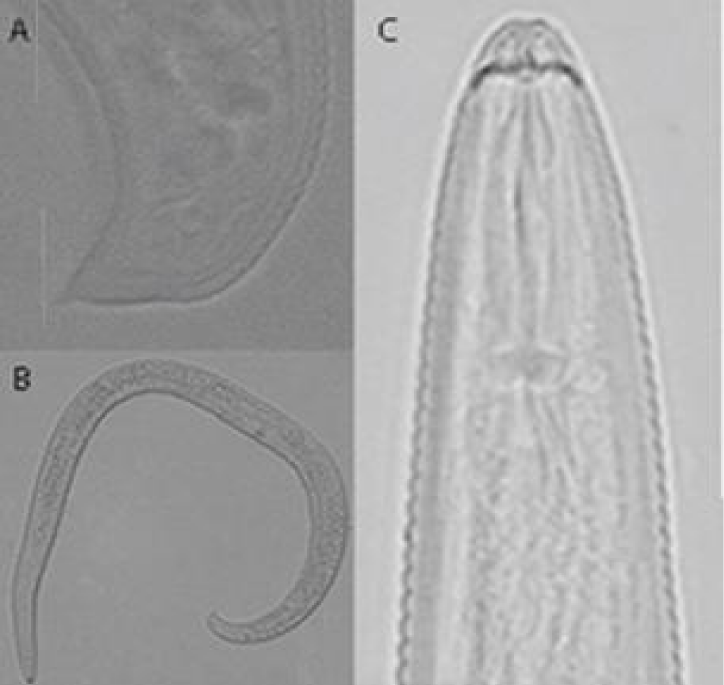
H. thornei (Figures 7,8) (Table 1) [16]
Female: Body usually in form of spiral. Stylet knobs anteriorly concave. Orifice of dorsal esophageal gland duct 10-12 μm posterior to base of stylet knobs. Lateral field with four incisures, reducing to three anterior to level of bulb of matacarpus, areolation of field totally absent. Hemizonid 1-2 annules anterior to excretory pore. Ovaries paired, straight. Oocytes arranged in a single row, except in short zone of division. Spermatheca present. Vulva a transverse slit. Phasmids distinct, 12-15 μm from tip of tail. Tail mucronate.
Male: Not found.
Remarks: According to the morphological characters and morphometric data given in the original description, there were no differences between the Iranian population of H.thornei and the original description. H. thornei close to H. minutus [21] and H. mucronatus [22]. It differs from H. minutus by body length (600-750 vs. 350-430), stylet length (24-38 vs. 19.5-22.4), stylet knobs (anteriorly concave vs anteriorly flat) and differs from H. mucronatus by body length (600-750 vs. 490-590), stylet length (24-38 vs. 21-23), stylet knobs (anteriorly concave vs. cup shape).
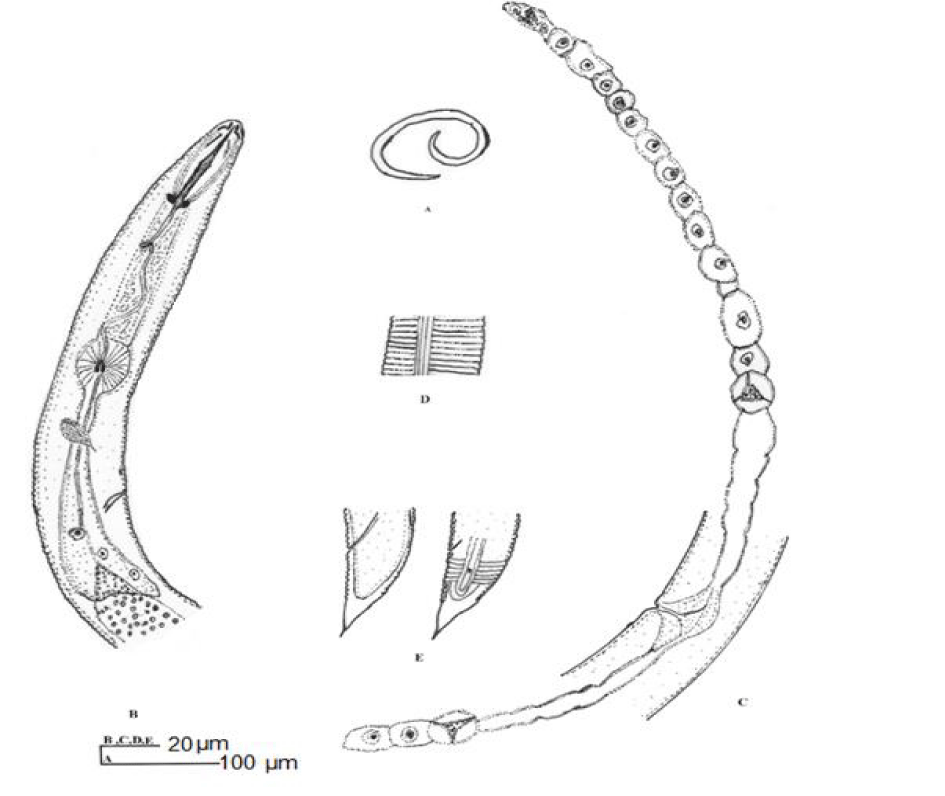
H. urobelus (Figures 9,10) (Table 1) [17]
Female: Body spirally curved when relaxed by heat. Lip region hemispherical, bearing five annules. Lateral field with four incisures. Stylet knobs flattened on anterior surfaces. Excretory pore 97 pm posterior from head end. Hemizonid indistinct, three annules anterior to excretory pore. Ovaries paired, outstretched. Spermatheca axial. Tail cylindrical dorsally convex-conoid with 11 annules on ventral side, tapering to a prominent, pointed ventral projection bearing a short mucro or without mucro (variation in tail tip). Phasmids four and seven annules anterior to anal level.
Male: Not found.
Remarks: According to the morphological characters and morphometric data given in the original description, there were no differences between the Iranian population of H. urobelus and the original description. Helicotylenchus urobelus is most similar to H. erythrinae [23]. H. urobelus differs from H. erythrinae in having a larger body length (650 vs. 480-610 μm), c factor (35-40 vs. 27-34), number of head annules (5 vs. 4) and position of the phasmids (4-7 annules anterior vs. 4 annules anterior to 2 annules posterior to anal level).
References
- Filip'ev IN. The classification of the free-living nematodes and their relation to the parasitic nematodes. Smithsonian Miscellaneous Collection. 1934;89:1-63.
- Steiner G. Helicotylenchus, a new genus of plant-parasitic nematodes and its relationship to Rotylenchus Filipjev. Proceedings of the Helminthological Society of Washington. 1945;12:34-38.
- Bannon JH, Inserra RN. Helicotylenchus species as crop damaging parasitic nematodes. Nematology Circular 165. Florida Department of Agriculture and Consumer Services. 1989. doi: 10.32473/edis-in973-2017.
- Subbotin SA, Inserra RN, Marais M, Mullin P, Powers TO, Roberts PA, Van den berg E, Yeats GW, Baldwin JG. Diversity and phylogenetic relationships within the spiral nematodes of Helicotylenchus (Tylenchida: Hoplolaimidaei) as inferred from analysis of the D2-D3 expansion segments of 28S rRNA gene sequences. Nematology. 2011;13(3):333-345.
- Cobb NA. Nematodes, mostly Australian and Fijian. Linnean Society of New South Wales, Macleay Memorial. 1893; 252-303.
- Steiner G. Freilebende Nematodes aus der Schweiz. 1. Teil einer vorlaufigen Mitteilung. Arcrnvesfi1r Hydrobiologie und Planktonkunde. 1914;9:259-276.
- Perry VG, Darling HM, Thorne G. Anatomy, taxonomy and control of certain spiral nematodes attacking blue grass in Wisconsin. University of Wisconsin, Agricultural Experiment Station, Research Bulletin. 1959;207.
- Siddiqi MR. On the genus Helicotylenchus (Nematoda: Tylenchida), with descriptions of nine new species. Nematologica. 1972;18(1):74-91. doi: 10.1163/187529275X00077.
- Mulk MM, Siddiqi MR. Three new species of Hoplolaimid nematodes from South America. Indian Journal of Nematology. 1982;12(1):124-131.
- Sher SA. Revision of the Hoplolaiminae (Nematoda). VI. Helicotylenchus. Nematologica. 1966;12:1-56.
- Tarjan AC. Two new mucronate-tailed spiral nematodes (Helicotylenchus: Hoplolaiminae). Nematologica. 1964;10(2):185-191.
- Eroshenko AS, Thanh NV. New species of soil nematodes from Vietnam. Zoologicheskii zhurnal. 1981.
- Khan SH, Basir MA. Two new species of the genus Helicotylenchus (Nematoda: Hoplolaimidae) from India. In Pro. helminth Soc Wash. 1964;31:199-202.
- Darekar KS, Khan E. Two new species of Helicotylenchus (Tylenchida: Nematoda) from Maharashtra, India. Nematologia Mediterranea. 1980;8(1):1-7.
- Swarup G, Sethi CL. Plant-parasitic nematodes of northwestern India. II. The genus Helicotylenchus. Bull Ent Loyola Coll. 1968;9:76-80.
- Roman J. Nematodes of Puerto Rico, the genus Helicotylenchus (Nematoda: Hoplolaiminae). In: Technical Paper, University of Puerto Rico Agricultural Experiment Station. No 41. 1965.
- Anderson RV. Helicotylenchus urobeles. .n.sp. (Nematoda: Hoplolaimidae) from New Brunswick, Canada. Canadian Journal of Zoology. 1978;56:1232-1234.
- Whitehead AG, Hemming JR. A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Annals of Applied Biology. 1965;55(1):25-38. doi: 10.1111/j.1744-7348.1965.tb07864.x.
- De grisse AT. Redescription ou modification de quelques techniques dansL’etude des nematodes phytoparasitaires. Mede. Rijks fak Landbou Weten Gent. 1969;34:351-369.
- Waseem M. Two new species of the genus Helicotylenchus (Nematoda: Hoplolaiminae). Canadian Journal of Zoology. 1961;39:505-509.
- Van Den Berg E, Cadet P. One new and some known plant parasitic nematode species from the French Caribbean (Nemata: Tylenchida). Revue de Nematologie. 1991;14:389-405.
- Siddiqi MR. Helicotylenchus mucronatus n.sp. and H. tunisiensis n. sp. (Nematoda: Hoplolaimidae). Nematologica. 1964;9:386-390.
- Zimmermann A. Eenige pathologische en physiologische waarnemingen over koffie. Mededeelingen uit 'Slands Plantentiun (Buitenzorg). 1904;67:89-92.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.