Biology Group . 2023 April 10;4(4):665-677. doi: 10.37871/jbres1724.
Hepatoprotective Effect of Eulophia gracilis on Benzene-Induced Liver Toxicity in Wistar Rats
Olaniyi Solomon Ola1,2* and Oyeronke Adunni Odunola2
2Department of Biochemistry, Cancer Research and Molecular Biology Laboratories, College of Medicine, University of Ibadan, Ibadan
- Benzene
- Haematological disturbance
- Hepatic damage
- >Eulophia gracilis
Abstract
Benzene (BZ) is an organic solvent that can induce pathological disorders such as haematological disburbance and liver damage. Eulophia gracilis (EG) plant is a medicinal orchid explored traditionally to treat myriads of ailments including anemia and liver disease. The present study investigated the protective effect of Eulophia gracilis extract against Benzene-induced hepatotoxicity in haematologically disturbed rats. Male Wistar rats (100-120)g were randomized into four groups (n = 6 / group): I (control), II BZ-treated: 175 mg/kgbw benzene every alternate days for two weeks; III Co-treated with 175 mg/kgbw benzene and E. gracilis 200 mg/kgbw each day for two weeks and IV administered 200 mg/kg of E. gracilis for two weeks. BZ caused a significant reduction in PCV, Hb, RBC and altered white blood cell count. Similarly, activities of hepatic biomarker enzymes such as alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, and γ-glutamyl transferase were significantly increased in the BZ-treated group relative to control. Furthermore, BZ caused a significant decrease in the activities of hepatic arylesterase, superoxide dismutase, catalase and glutathione-S-transferase and levels of Ascorbic Acid (AA) and Glutathione (GSH). A significant increase in hepatic malondialdehyde content, altered liver ultrastructure and modulation of p53 and BCl-2 protein in the liver also accompanied benzene intoxication. However, coadministration of E. gracilis ameliorated the BZ-induced changes in haematological parameter and liver biomarkers and improved hepatic antioxidant biomarkers and ultrastructure. Overall, the results suggest that Eulophia gracilis protected against BZ-induced hepatic oxidative damage and haematological disturbance in rats.
Introduction
Benzene is a monocyclic aromatic hydrocarbons used as solvent in many industries especially as a feeder chemical in the manufacture of detergents, lubricants, rubber, dyes and pesticides. However, it constitutes an industrial and environmental contaminant that continues to raise significant medical concern as chronic exposure to benzene was associated with progressive decline of hematopoietic function that lead to various disorders like aplastic anemia, myelo-dysplastic syndrome and leukemia [1]. Therefore, benzene is a known leukemogen to humans and is classified as a Group 1 carcinogen [2,3]. In human beings, the main health risk linked to low levels of benzene exposure is acute myelogenous leukemia [4]. It was reported that exposure to benzene reduced the packed cell volume which is a reliable indication of the quantity of circulating erythrocytes and the degree of anemia or polycythemia and induced leukocytosis in animal model [5]. In modern medicine, patients with acute myelogenous leukemia most often present with nonspecific symptoms that begin gradually or abruptly and are the consequence of anaemia, leukocytosis, leucopoenia or leukocyte dysfunction, or thrombocytopenia [6]. Hematotoxicity and chronic toxicity of benzene is the result of a series of biotransformation events that initiate with the generation of reactive intermediates. These reactive metabolites form covalent adducts with diverse critical macromolecules such as proteins and nucleic acids in liver, kidney, spleen, and blood [7]. Oxidation of benzene in the liver by cytochrome P450 2E1 (CYP2E1) to benzene oxide and other reactive intermediates is an initial step in the bioactivation of benzene and is a prerequisite for cellular toxicity [8]. Benzene affects some enzyme activities in the liver and peripheral blood that can cause decrease in the activity of antioxidant enzymes and consequently oxidative stress [9]. Occupational exposure to benzene has been shown to cause liver damage in humans which may be alleviated by antioxidant molecules [10]. The association between occupational exposure to different chemical and liver injury has been well established. This is because liver is the primary organ for the biotransformation of chemicals and the main organ involved in the metabolism of toxins and xenobiotic agent within the body [11]. Moreover, biotransformation or metabolism of chemical agent was always linked to the disturbance of hepatocyte biochemistry and generation of reactive oxygen that are likely cause of oxidative damage in liver and hematopoietic system species [12].
Eulophia gracilis belongs to family of Orchidaceae found in swampy rocky area in Nigeria. It is one of the medicinal plants in Nigeria used in the treatment of different ailments such as liver disorder, tumor, diabetes and also utilized as aphrosidiac. Its involvement in traditional medicine has been linked to the presence of secondary metabolites such as glycoside, alkaloid, tannins, phlobatanins and flavonoid [13].
Thus, the aim of this study was to determine the possible hepatoprotective effect of Eulophia gracilis extract on Benzene-induced leukocytosis in rats using the antioxidant indices and markers of liver damages.
Materials and Methods
Chemicals and reagents
The following substances were employed in the study: Benzene (JHD Guangdong Guanghua Sci-Tech Co, Ltd, China), GSH, Thiobarbituric acid (TBA), epinephrine, 5’,5’-dithio-bis-2-nitrobenzoic acid, 1-chloro-2,4dinitrobenzene, para-nitrophenyl phosphate, and hydrogen peroxide (Sigma Chemical Company, London, UK). Assay kits for alanine transferases, aspartate transferases, gammaglutamyl transferase (Randox Laboratories Ltd, Antrim, UK). All the remaining substances used were of analytical grade.
Plant collection, identification and preparation
Fresh Eulophia gracilis plants were collected from rocky area in Oyo, Nigeria. The plant was identified and authenticated by plant taxonomist at Herbarium section of Department of Botany, University of Ibadan, Nigeria with UIH number 22528. The tubers were thoroughly washed with tap water, sliced into pieces and shed dried. The dried tuber were pulverized into powder form using electric grinder. 200 g of Eulophia gracilis fine powdered sample was extracted with 800 mL aqueous methanol (20:80) for 48 hours by cold marceration. The extract was evaporated under reduced pressure by using a rotary evaporator and further lyophilized using freeze-dryer machine.
Animal selection and care
A total of 24 adult male Wistar rats (100-120 g) were used in this experiment. They were obtained from University of Ibadan animal housing unit, Ibadan, Nigeria. The rats were then acclimatized for 2 weeks before the commencement of the study. During the course of the study, rats were housed in laboratory wire-meshed cages and supplied food and water ad libitum. Experimental animals were handled in compliance with international guidelines on animal use and care [14].
Experimental design
Twenty four rats were randomly assigned into four experimental groups (I-IV) of six animals each. The dose for Benzene (175 mg/kgbw of benzene mixture) was selected based on the available literature and dose for Eulophia gracilis (200 mg/kgbw) was arrived at from rationale on pilot in each treatment group and administered in one mL of distilled water by oral gavage using oral intubator. The animals in each group were treated accordingly as presented in table 1.
| Table 1: Experimental design. | |
| Treatment Groups | Treatment (2 Weeks) |
| I. Control (CTRL) | Distilled water |
| II. Benzene only (BZ) | 175 mg/kgbw of benzene mixture every two days for 2 weeks |
| III. Benzene and 200 mg Eulophia gracilis extract cotreatment group (BZ + 200 mg EG) | 175 mg/kgbw of benzene mixture every two days for 2 weeks + 200 mg/kg EG extract every day for 2 weeks |
| IV. 200 mg Eulophia gracilis extract alone group (200 mg EG) | 200 mg/kg EG extract every day for 2 weeks |
Collection of blood samples for haematological analysis and plasma preparation and animal sacrifice
Blood was collected from the retro orbital plexus of the animals into EDTA bottle and heparinized tubes for haematological analysis and Plasma Preparation respectively. The rats were then sacrificed by cervical dislocation. The blood samples were centrifuged for 5 min at 4000 rpm using a bench centrifuge (Analytica, Athens, Greece) to prepare the plasma. The clear supernatant was used for the estimation of liver function enzymes.
Preparation of post mitochondrial fraction of liver homogenate
Liver was excised from rat, blotted on filter paper, rinsed in 1.15% KCl and homogenized in 4 volumes of ice-cold 0.01 M potassium phosphate buffer (pH 7.4). The homogenates were centrifuged in a refrigerated centrifuge (Eppendorf, Stevenage, UK) at 12,500 g for 15 min and the supernatants, known as the Post Mitochondrial Fractions (PMF), were used for enzyme assays.
Measurement of haematological parameters
Percentage Packed Cell Volume (PCV), Red Blood Cell (RBC) count, haemoglobin concentration and White Blood Cell (WBC) count were determined following the procedure for full blood count using automated cell counter (Mudrain).
Biomarkers of liver function
Activities of GGT, alanine transaminase and aspartate transaminase were assayed using assay kits (RANDOX). GGT activity was evaluated by the principle described by Szasz GA [15]. ALT and AST activities were determine by principle developed by Reitman S, et al. [16]. ALP activity was determined in accordance with the principles of Tietz NW, et al. [17]. The p-nitrophenol formed by the hydrolysis of p-nitrophenyl phosphate confers a yellowish color to the reaction mixture and its intensity can be monitored at 405 nm to give a measure of enzyme activity.
Determination of non-enzymatic antioxidants and lipid peroxidation
Reduced glutathione (GSH) level was determined according to procedure of Jollow DJ, et al. [18] where GSH reacts with Ellman’s reagent to give 2-nitro-5-thiobenzoic acid, a chromophoric product, with a molar absorption at 412 nm. The absorbance was measured at a wavelength of 412 nm. Reduced GSH concentration in the liver homogenate was extrapolated from the standard curve for GSH. Ascorbic acid level was determined by the procedure of Jagota SK, et al. [19]. Folin Ciocalteu (Folin-phenol) reagent and ascorbic acid in biological samples react together to give a blue color, which absorbs maximally at 760 nm. Ascorbic acid (mg/mL) in the liver post-mitochondrial fraction was obtained from the standard curve for AA. Level of Lipid Peroxidation (LPO) was assessed following the method of Varshney R, et al. [20]. The principle involved the reaction between malondialdehyde, a known product of lipid peroxidation and Thiobarbituric acid to yield a stable pink chromophore which intensity is measured spectrophotometrically at 532 nm.
Assay of antioxidant enzymes
Superoxide Dismutase (SOD) activity in liver was determined by measuring the inhibition of autooxidation of epinephrine at pH 10.2 and 30°C following the procedure of Misra HP, et al. [21] as described by Magwere T, et al. [22]. SOD activity was expressed in U/mg protein. Catalase (CAT) activity was determined as described by Sinha BK. [23] based on the principle that dichromate in acetic acid is reduced to chromic acetate when heated in the presence of hydrogen peroxide (H2O2. The chromic acetate was measured spectrophotometrically at 570 nm and the amount of H2O2 remaining was extrapolated from the standard curve for H2O2. The activity of catalase was expressed as micromole of H2O2 consumed per minute per mg protein. Glutathione S-Transferase (GST) activity was determined as described by Habig WA, et al. [24] where 1-Chloro-2, 4-Dinitrobenzene (CDNB) was used as substrate. GST activity in post-mitochondrial fraction expressed in mol/min/mg protein. Activity of Glutathione Peroxidase (GPx) in liver homogenate was determined using procedure developed by Rotruck JT, et al. [24]. GPx activity was expressed as mg GSH/mg protein. Arylesterase activity was measured using phenylacetate as the substrate by the procedure of Junge W, et al. [26] where the rate of phenol generation was monitored at 270 nm wavelength at 30 seconds interval for 3 minutes. 1 unit of arylesterase activity is defined as the quantity of enzyme that disintegrated 1 milimole of phenylacetate substrate in 1 minute.
Statistical analysis
Data were expressed as the Mean±Standard (SD) for 6 rats in each group. Graphpad Prism 6.0.1 (Graphpad Software, La Jolla, CA) was used for statistical analysis and graphical constructions. The statistical significance of differences among experimental groups were determined by one-way analysis of variance. P values of < .05 were considered to be significant.
Results
Effect of Eulophia gracilis extract on benzene-induced changes in haematological parameters in wistar rats
Table 2 shows the effect of Eulophia gracilis extract on Benzene-induced changes in haematological parameters in wistar rats. PCV, Hb and RBC were drastically reduced in rats treated with Benzene when compared with control group as shown in table 1. However, white blood cells was greatly increased in rats treated with benzene when ompared with control group. The coadministration of Eulophia gracilis alongside with Benzene intoxication restored the amount of PCV, Hb, RBC and WBC to near the level of control group.
| Table 2: Effect of Eulophia gracilis on Benzene-induced changes in haematological parameters in wistar rats. | ||||
| Groups | PCV | HB | RBC | WBC |
| I. Control | 40.00 ± 6.09 | 13.03 ± 2.02 | 6.65 ± 1.20 | 3916.70 ± 160.72 |
| II. BZ only | 26.00 ± 4.36 | 8.17 ± 1.18 | 4.09 ± 0.65 | 5533.30 ± 1274.10 |
| (BZ + 200 mg EG) | 37.00 ± 2.83 | 12.40 ± 1.27 | 5.74 ± 0.66 | 3225.00 ± 671.75 |
| IV. 200 mg EG alone | 40.00 ± 3.61 | 13.13 ± 0.83 | 6.60 ± 0.64 | 4300.00 ± 1375.68 |
| Data represent the means ± SD for six rats in each group. PCV: Packed Cell Volume; HB: Heamoglobin; RBC: Red Blood Cell; WBC: White Blood Cell; BZ: Benzene; EG: Eulophia gracilis |
||||
Protective effects of Eulophia gracilis on benzene-induced changes in the plasma activities of ALT, AST, ALP, and g-GT in rats
The protective effects of Eulophia gracilis on Benzene-induced changes in the plasma activities of ALT, AST, ALP, and g-GT in rats is presented in table 3. Benzene significantly increased the activities of ALT, AST, ALP and GGT by 23.76%, 35.86%, 246.24% and 80.82% respectively, when compared with control (p < 0.05) as reprensented in table 2. However, treatment with aqueous methanolic extract of Eulophia gracilis significantly ameliorated the Benzene-induced increase in plasma activities of ALT, AST, ALP and γ-GT in rats.
| Table 3: Protective effects of Eulophia gracilis on Benzene-induced changes in the plasma activities of ALT, AST, ALP, and g-GT in rats. | ||||
| Treatment | ALT (U/L) | AST (U/L) | ALP (U/L) | g-GT (U/L) |
| I. CTRL | 83.21 ± 16.64 | 125.26 ± 18.38 | 2420.08 ± 568.72 | 13.66 ± 1.90 |
| II. BZ only | 102.98 ± 13.64 (23.76%)* | 170.18 ± 13.24 (35.86%)* | 8379.14 ± 1400.21 (246.24%)* | 24.70 ± 1.77 (80.82%)* |
| III. (BZ + 200 mg EG) | 73.78 ± 6.48# | 130.53 ± 18.38# | 4870.49 ± 678.14# | 13.66 ± 3.00# |
| IV. 200 mg EG alone | 71.78 ± 10.13 | 129.47 ± 17.69 | 3173.165 ± 565.00 | 11.35 ± 3.61 |
| Data represent the means ± SD for six rats in each group; *significantly different from the Control; #significantly different from Benzene group (p < 0.05). Values in parenthesis represent percentage (%) increase. |
||||
Influence of Eulophia gracilis extract on benzene-induced changes in hepatic biomarkers of oxidative stress in rats
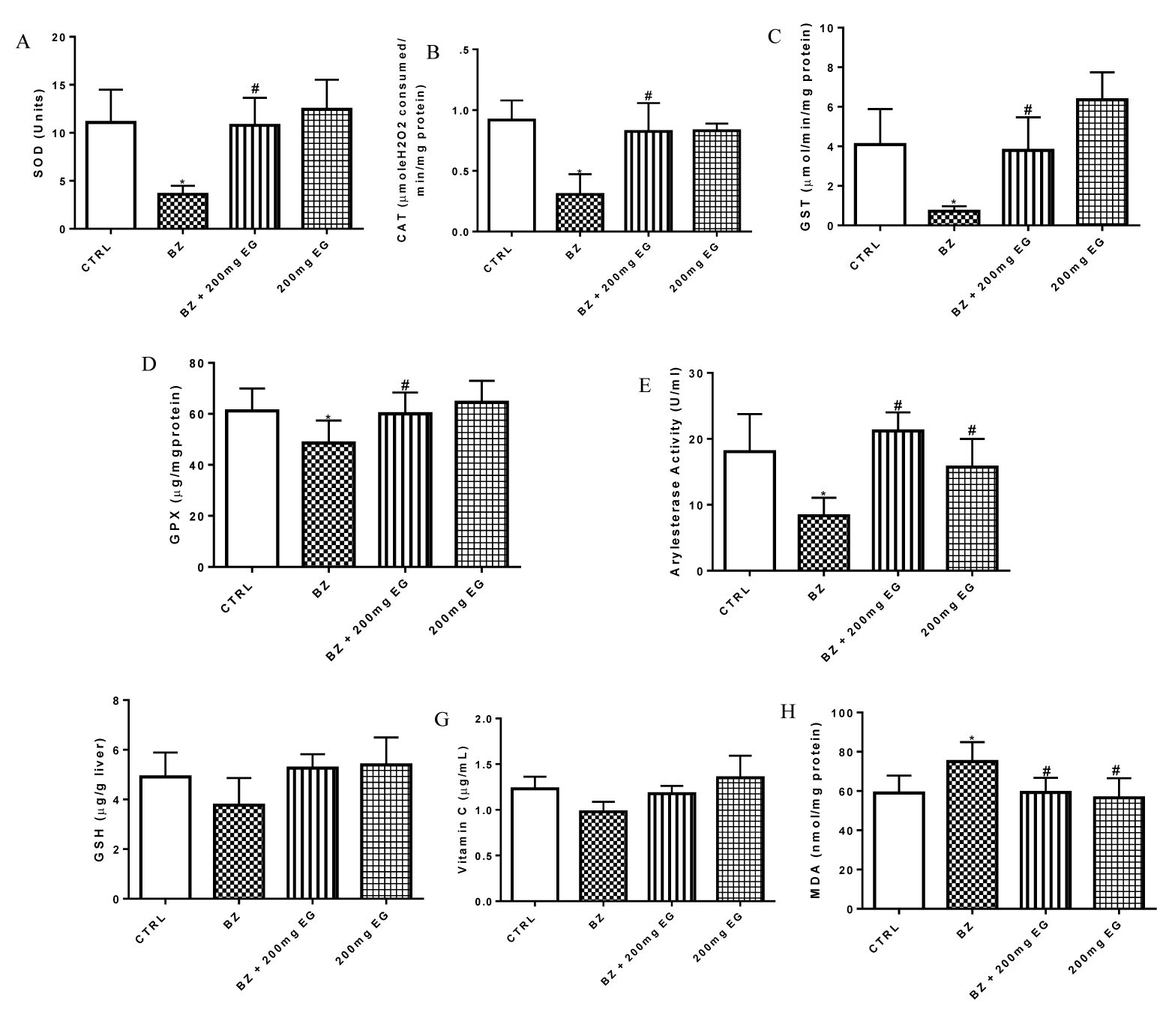
Figure 1 presents influence of Eulophia gracilis extract on benzene-induced changes in hepatic biomarkers of oxidative stress in rats. Hepatic levels of the nonenzymatic antioxidants: reduced glutathione and vitamin C were significantly reduced (p < 0.05) after administration of BZ (Figures 1F,G). Concurrently, a similar decrease was also observed in the hepatic activities of enzymatic antioxidants: SOD, CAT, GST, GPx and arylesterase (Figures 1A-E). However, cotreatment with Eulophia gracilis extract significantly ameliorated the levels of GSH and AA and the activities of liver SOD, CAT, GPx, GST and arylesterase in rats especially at 200 mg/kg weight dose. MDA is a stable product of lipid peroxidation consequent to oxidative stress. Compared to the control group, BZ induced a significant (p < 0.05) increase in the hepatic lipid peroxidation as depicted by the MDA level in figure 1H). Cotreatment with EG, however, ameliorated this increase in liver MDA levels.
The influence of Eulophia gracilis extract on benzene-induced alteration in architecture of rats liver
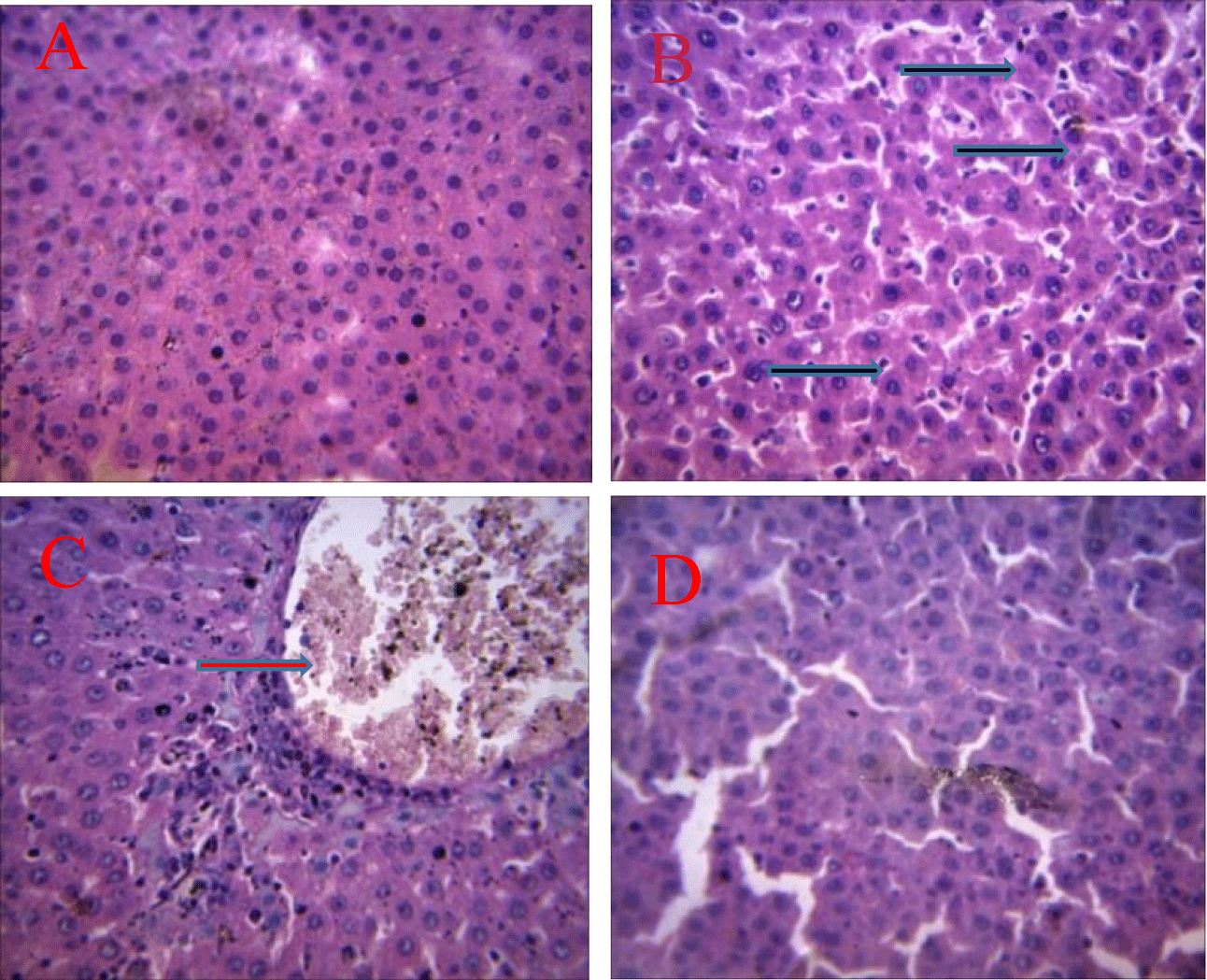
The pictures in plate 1 presents the influence of Eulophia gracilis extract on Benzene-induced alteration in architecture of rats liver. The picture in plate 1A shows normal histology of the liver cell with no visible lesions seen. Similarly administration of 200 mg/kgbw of Eulophia gracilis into rats does not have remarkable effect on the architectural structure of liver as there is no visible leision observed (E). In contrast, exposure of rats to 175 mg/kgbw benzene by intravenous administration once in two days for two weeks resulted in a mild diffuse kupffer cell hyperplasia in (B). Coadministration of 200 mg/kgbw of Eulophia gracilis extract for 14 days into animals exposed to benzene intoxication resulted into a mild to moderate portal congestion, with mild periportal cellular infiltration in rats.
Influence of Eulophia gracilis extract on p53 and bcl-2 expression in liver cell of benzene-induced toxicity in rats
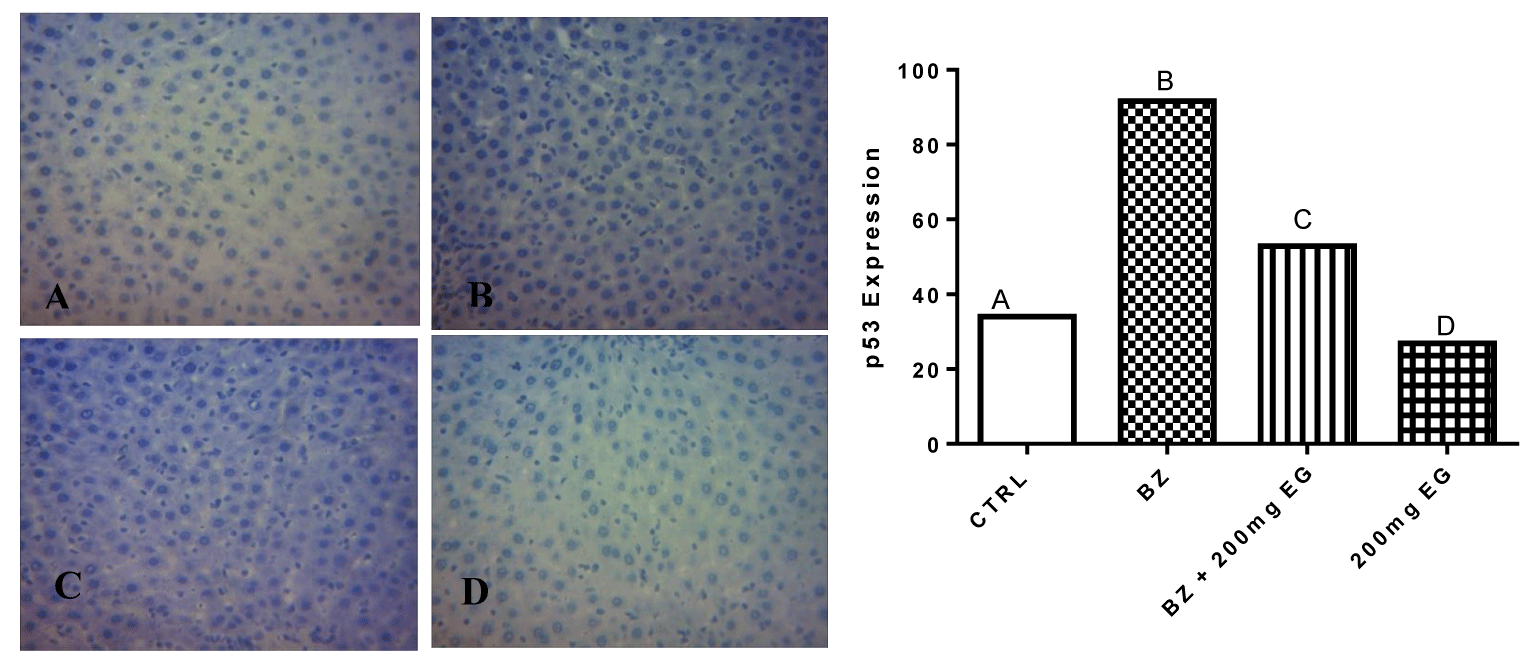
Figures 2,3 present the influence of Eulophia gracilis Extract on p53 and BCl-2 expression in Liver cell of benzene-induced toxicity in rats respectively. Exposure of rats to benzene intoxication resulted into increased expression of proapoptotic p53 protein as shown in figure 3. However, coadministration with the plant extract modified the expression.
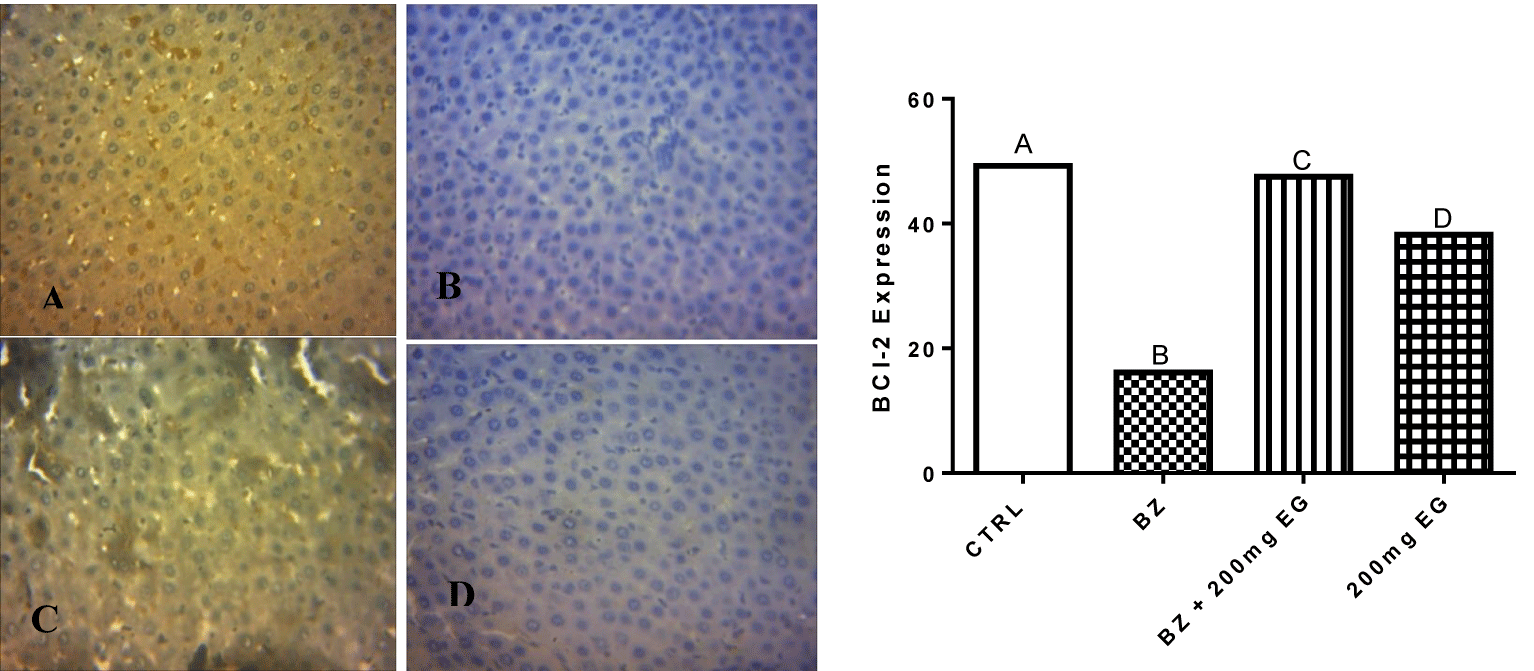
Figure 3 shows the influence of Eulophia gracilis extract on BCl-2 expression in Liver cell of benzene-induced toxicity in rats. The expression of BCl-2 protein in liver was reduced following the intravenous administration of Benzene mixture. However, treatment with Eulophia gracilis extract restored the expression of this anti-apoptotic protein to a level near that of control group.
Discussion
The association between occupational exposure to benzene and liver injury has been well established. This is because biotransformation or metabolism of this organic chemical was linked to the disturbance of hepatocyte biochemistry and generation of reactive oxygen species that likely caused oxidative damage in liver and hematopoietic system [13]. Liver was previously reported to be one of the important target organs affected by benzene vapour toxicity [27]. The increased activities of plasma ALT, AST, ALP and g-GT has been related to hepatic oxidative damage [27,28]. Plasma activities of liver function biomarkers such as ALT, AST, ALP and γ-GT were significantly increased relative to control group on exposure to benzene in this present work. It was earlier reported that benzene intoxication enhanced cellular lipid peroxidation that affect the permeability barrier of the biological membrane of the cell [29]. Therefore, the significant increase in LPO of the liver homogenate and liver function enzymes observed in this study supported the earlier findings of other researchers. The result of this study indicated that benzene was probably metabolized in the liver to reactive species which interacted with the tissues to induce lipid peroxidation. The observed increase in the activities of plasma ALT, AST, ALP and γ-GT is likely due to peroxidation of biomembrane lipid that causes leakage of cellular components including these cytosolic transaminases and γ-GT which is a membrane bound enzyme [30]. However, administration of Eulophia gracilis plant extract restored the cellular integrity of liver cell as observed in significant reduction of liver function enzymes and MDA levels in animal supplemented with extract when compared to animal exclusively treated with benzene.
The connection between chemical-induced hepatotoxicity and oxidative stress had been established by several studies [31-33]. This therefore prompted the consideration of the effect of the extract on major enzymatic and non-enzymatic antioxidant systems of rats. Activities of enzymatic antioxidants such as SOD, CAT, GPx and GST are vital to the maintenance of the cellular redox balance [34]. In this study, benzene significantly reduced the activity of SOD, CAT, GPx and GST in the liver of benzene treated rats. SOD catalytic activity effected the dismutation of superoxide radical to hydrogen peroxide and dioxygen after which catalase catalyzed the conversion of the hydrogen peroxide formed in this process and other cellular processes to molecular oxygen and water [35]. Significant reduction in the activities of SOD and CAT by benzene may expose the liver to oxidative stress [17]. Decreased activity of SOD in liver is an indication of oxidative stress [36]. Similarly, reduction in the activity of catalase in the liver of benzene treated rats may have resulted from accumulation of superoxide anion radical due to reduction in hepatic superoxide dismutase activity [37]. GST enzyme is a vital component of the antioxidant defense mechanism that is found in most tissues [38,39] and it is actively involved in the detoxification of ingested xenobiotics in the liver [38,40]. However, supplementation of benzene-intoxicated rats with extract of Eulophia gracilis boosted the activities SOD, CAT, GPX and GST in rats as shown by the results of this work.
Moreover, non-enzymatic antioxidant molecules like AA and GSH also play a crucial role in cellular redox balance. Ascorbic acid is a water soluble vitamin that functions in the aqueous environments of the body and take part in the regeneration of tocopherol from tocopherol radicals in membranes and lipoproteins [41,42]. A glutathione is a tripeptide antioxidant molecule that acts as a cofactor for several enzymes such as Glutathione Peroxidase (GPx) and glutathione-S-transferase which possess antioxidant properties [43]. Disturbance in the cellular redox status of AA and GSH has been reported to enhance oxidative stress and tissue injury [44]. In this study, a decrease in the levels of GSH and ascorbic acid were observed in animals following benzene exposure. This observation agreed with previous findings in both human being and animal models [45]. The decrease in these antioxidant molecules may be due to their utilization in preventing cellular damage from free radicals as vitamin C was shown to be an effective antioxidant molecule against lipid peroxidation initiated by a peroxyl radical [46,47]. and GSH in cells can be consumed during the condensation reaction that yields phenylmercapturic acid leading to its depletion [48]. More importantly, reactive metabolite of benzene biotransformation, p-benzoquinone can readily deplete GSH content in the cells resulting to depletion GSH in an ROS independent manner [49,50]. However, treatment with Eulophia gracilis extract significantly improved the levels of ascorbic acid and GSH in rats which supports the previous report that plant rich in polyphenolic compounds may attenuate Benzene-Induced Oxidative Stress [51].
The presence of oxidative stress with concomitant severe inflammatory response had been used to characterize chronic liver diseases [52]. Paraoxonase is an enzyme that is produced in liver which possess both paraoxonase-1 and arylesterase activity and has antioxidant effect [53,54]. It is widely distributed in the body but its higher activity is found in blood and liver [55,56]. It catalyzes the hydrolysis of paraoxon, aromatic esters, lipid peroxides and circulates in plasma bound to High-Density Lipoproteins (HDL) protecting lipoproteins against oxidative modification by reducing the accumulation of peroxidation products [57,58]. Experimental study showed that PON1 over-expression offers protection against the development of liver disease [59]. However, Low PON1 levels was related to enhanced sensitivity to the development of liver damage [60]. Therefore, serum PON1 and arylesterase activities were suggested as useful markers of liver function status [58,61]. This present study showed a significant reduction in arylesterase activity after benzene exposure relative to control group. This result is in agreement with previously report that associated decrease in serum PON1 activity to liver diseases [62,63]. This might be mediated by enhanced lipid peroxidation induced by benzene metabolite in the liver. This is because the ability of PON to protect against oxidation is usually accompanied by an inactivation of the enzyme [64]. However, administration of the plant extract raised the activity of arylesterase in this study relative to animal treated exclusively with benzene. This result agreed to previous finding that paraoxonase activity was preserved by antioxidant suggesting the antioxidant potential of the plant extract [64].
The induction of oxidative stress and alteration in liver biomarkers in rats exposed to the benzene corroborated the histopathological lesions observed in this study. The histopathological result of liver organ cross section showed a diffuse kupffer cell hyperplasia in the liver of benzene exclusively treated group as compare with no visible lesion observed in control group. These observations indicated marked changes in the overall histoarchitecture of liver organ in response to benzene. These changes could be due to benzene vapor toxic effects primarily by the generation of reactive oxygen species causing damage to the various membrane components of the cell. These histopathological results support the earlier results previously reported by other investigators [65,66]. However, administration of the extract ameliorated histopathological lesions in livers of animal supplemented with the extract therefore improved the liver architecture.
Various hepatocarcinogens had been shown to induce high levels of p53 protein in the rat liver [67]. In most cell types the levels of wild type p53 are reported to be low [68]. Exposure of normal cells to certain genotoxic compounds like cytostatic drug and benzo[a]pyrene (B[a] P*) induced high levels of p53 in normal cells [69]. This is probably due to recognition of DNA damage by a DNA activated protein kinase that subsequently leads to phosphorylation and stabilisation of the wide type p53 protein [70,71]. The significant increase in level of p53 protein expression found in animal group exposed to benzene corroborated the findings of other researchers [72,73]. Free radical-induced lipid peroxide has been associated with formation of DNA adduct which is the mutagenic pirimedopurinone adduct of deoxyguanosine [74]. Moreover DNA damage was implicated in expression of p53 in order to blocks cell cycle, and repairs the damaged DNA [67]. In this study administration of the extract reduced the expression of p53 in supplemented animal group relative to the animals treated only with benzene. This likely suggested the genoprotective role of this extract mediated via free radical scavenging activity and therefore prevented lipid peroxidation and subsequent DNA damage as observed in this study.
Some studies have reported that benzene induced apoptosis which is a potential toxic effect of benzene that results in proliferation, cytotoxicity and diseases [75-77]. It was well accepted that apoptosis can be modulated or enhanced by oxidative stress [78]. Benzene was reported to induce ROS generation that damaged cell ultrastructures and caused cell death [79]. Bcl-2 overexpression was suggested to disturb the normally physiologic surveillance in genomic stability and caused cells to become more susceptible to genotoxic agents-induced genetic mutation [80]. However in this study, low expression of Bcl-2 protein was observed in animal exposed to benzene which may be due to adaptive responses to the benzene toxicant. Bcl-2 is an antiapoptotic protein whose dissociation from beclin1 –bcl2 complex promote autophagy. The administration of the extract however nearly normalizes the expression level of bcl-2 in supplemented animal group when compared to control group.
Conclusion
Overall, aqueous methanolic extract of Eulophia gracilis protected against benzene-induced hepatotoxicity and haematological influence in Wistar rats.
Declarations
Authors’ contributions
OSO and OAO designed the work. OSO performed the experiments on animals. OSO and OAO collected, analyzed, and interpreted the data and wrote the manuscript. The authors read and approved the final manuscript.
Funding
No funding source.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
The study was approved by The Research Ethics Committee, Faculty of Natural Sciences, Ajayi Crowther University, Oyo with approval number: FNS/ERC/2017/021
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- Sonoda T, Nagata Y, Mori M, Ishida T, Imai K. Meta-analysis of multiple myeloma and benzene exposure. J Epidemiol. 2001 Nov;11(6):249-54. doi: 10.2188/jea.11.249. PMID: 11769942.
- Medeiros Vinci R, Jacxsens L, Van Loco J, Matsiko E, Lachat C, de Schaetzen T, Canfyn M, Van Overmeire I, Kolsteren P, De Meulenaer B. Assessment of human exposure to benzene through foods from the Belgian market. Chemosphere. 2012 Aug;88(8):1001-7. doi: 10.1016/j.chemosphere.2012.03.044. Epub 2012 Apr 5. PMID: 22483726.
- Benzene - IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. France, Lyon: International Agency for Research on Cancer; 1987. p.1-42.
- Snyder R. Leukemia and benzene. Int J Environ Res Public Health. 2012 Aug;9(8):2875-93. doi: 10.3390/ijerph9082875. Epub 2012 Aug 14. PMID: 23066403; PMCID: PMC3447593.
- Akanni EO, Folarin OR, Igbeneghu C. Tumour suppressive and organ protective effects of aqueous andrographis paniculata leaves extract on benzene induced leukaemia bearing rats. Annual Research & Review in Biology. 2014;4(7):1070-1079.doi: 10.9734/ARRB/2014/6886.
- Sharma M, Porte SM. Role of Ayurveda in management of leukemia (Raktarbuda). IJPSR. 2016;7(2):520-530. doi: 10.13040/IJPSR.0975-8232.7(2).520-30.
- Martínez-Velázquez M, Maldonado V, Ortega A, Meléndez-Zajgla J, Albores A. Benzene metabolites induce apoptosis in lymphocytes. Exp Toxicol Pathol. 2006 Aug;58(1):65-70. doi: 10.1016/j.etp.2006.03.010. Epub 2006 May 18. PMID: 16713212.
- Snyder R. Leukemia and benzene. Int J Environ Res Public Health. 2012 Aug;9(8):2875-93. doi: 10.3390/ijerph9082875. Epub 2012 Aug 14. PMID: 23066403; PMCID: PMC3447593.
- Scandalios JG. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res. 2005 Jul;38(7):995-1014. doi: 10.1590/s0100-879x2005000700003. Epub 2005 Jul 4. PMID: 16007271.
- Hegazy RM, Kamel HFM. Oxidant Hepatic &/or Haem. Injury on fuel-station workers exposed to benzene vapor, possible protection of antioxidants. American Journal of Medicine and Medical Sciences. 2014;4(2):35-46.
- Krewski D, Snyder R, Beatty P, Granville G, Meek B, Sonawane B. Assessing the health risks of benzene: A report on the benzene state of the science Workshops. J Toxicol Environ Health. 2000;61:307-38. doi: 10.1080/00984100050166325.
- Fernandez-Checa JC, Kaplowitz N. Hepatic mitochondrial glutathione: transport and role in disease and toxicity. Toxicol Appl Pharmacol. 2005 May 1;204(3):263-73. doi: 10.1016/j.taap.2004.10.001. PMID: 15845418.
- Ola OS. Preliminary proximate analysis, chemical composition and phytoconstituents of Eulophia gracilis orchid. International Journal of Sciences: Basic and Applied Research. 2017;36(8):215-222.
- National Research Council. Guide for the care and use of laboratory animals. 8th ed. Washington, DC, USA: The National Academies Press; 2011.
- Szasz GA. Kinetic photometric method for serum - glutamyl transpeptidase. Clin Chem. 1969;15,124-136.
- Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957 Jul;28(1):56-63. doi: 10.1093/ajcp/28.1.56. PMID: 13458125.
- Tietz NW, Pruden EL, Siggaard-Andersen O. Liver function, in Tietz Textbook of Clinical Chemistry. Burtis AC, Ashwood ER, editors. WB Saunders, London, UK: 1994. p.1354-1374.
- Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11(3):151-69. doi: 10.1159/000136485. PMID: 4831804.
- Jagota SK, Dani HM. A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal Biochem. 1982 Nov 15;127(1):178-82. doi: 10.1016/0003-2697(82)90162-2. PMID: 7165085.
- Varshney R, Kale RK. Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int J Radiat Biol. 1990 Nov;58(5):733-43. doi: 10.1080/09553009014552121. PMID: 1977818.
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972 May 25;247(10):3170-5. PMID: 4623845.
- Magwere T, Naik YS, Hasler JA. Effects of chloroquine treatment on antioxidant enzymes in rat liver and kidney. Free Radic Biol Med. 1997;22(1-2):321-7. doi: 10.1016/s0891-5849(96)00285-7. PMID: 8958157.
- Sinha BK. Metabolic activation of procarbazine. Evidence for carbon-centered free-radical intermediates. Biochem Pharmacol. 1984 Sep 1;33(17):2777-81. doi: 10.1016/0006-2952(84)90695-6. PMID: 6431996.
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130-9. PMID: 4436300.
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973 Feb 9;179(4073):588-90. doi: 10.1126/science.179.4073.588. PMID: 4686466.
- Junge W, Klees H. 1,2-Arylesterase. Methods Enzym Anal. 1984;4:8-14.
- Uboh FE, Akpanabiatu MI, Eteng MU, Ebong PE, Umoh IB. Toxicological effects of exposure to gasoline vapour in male and female rats. Internet J Toxicol. 2008;4.
- Uboh FE, Akpanabiatu MI, Ebong PE, Eyong EU, Eka OU. Evaluation of toxicological implication of inhalation exposure to kerosene and petrol fumes in rats. Acta Biol Szeged. 2005;49:19-22.
- Uboh FE, Akpanabiatu MI, Ekaidem IS, Ebong PE, Umoh IB. Effect of inhalation exposure to gasoline fumes on sex hormones profile in Wistar albino rats. Acta Endocrinol. 2007;4:23-30.
- Al-Olayan EM, El-Khadragy MF, Aref AM, Othman MS, Kassab RB, Abdel Moneim AE. The potential protective effect of Physalis peruviana L. against carbon tetrachloride-induced hepatotoxicity in rats is mediated by suppression of oxidative stress and downregulation of MMP-9 expression. Oxid Med Cell Longev. 2014;2014:381413. doi: 10.1155/2014/381413. Epub 2014 Apr 27. PMID: 24876910; PMCID: PMC4020166.
- El-Shakour A, El-Ebiarie AS, Ibrahim YH, Abdel Moneim AE, El-Mekawy AM. Effect of benzene on oxidative stress and the functions of liver and kidney in rats. J Environ Occup Sci. 2015;4(1).
- Oh JM, Jung YS, Jeon BS, Yoon BI, Lee KS, Kim BH, Oh SJ, Kim SK. Evaluation of hepatotoxicity and oxidative stress in rats treated with tert-butyl hydroperoxide. Food Chem Toxicol. 2012 May;50(5):1215-21. doi: 10.1016/j.fct.2012.01.031. Epub 2012 Feb 1. PMID: 22326806.
- Yamamoto T, Kikkawa R, Yamada H, Horii I. Identification of oxidative stress-related proteins for predictive screening of hepatotoxicity using a proteomic approach. J Toxicol Sci. 2005 Aug;30(3):213-27. doi: 10.2131/jts.30.213. PMID: 16141655.
- Notas G, Koutroubakis IE, Kouroumalis EA. Oxidants and antioxidants in liver disease, in Antioxidants: New Research. Panglossi HV, editor. 2012. p.2-48.
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002 Jan;82(1):47-95. doi: 10.1152/physrev.00018.2001. PMID: 11773609.
- Yousef MI, Omar SA, El-Guendi MI, Abdelmegid LA. Potential protective effects of quercetin and curcumin on paracetamol-induced histological changes, oxidative stress, impaired liver and kidney functions and haematotoxicity in rat. Food Chem Toxicol. 2010 Nov;48(11):3246-61. doi: 10.1016/j.fct.2010.08.034. Epub 2010 Sep 4. PMID: 20804811.
- Boone L, Meyer D, Cusick P, Ennulat D, Bolliger AP, Everds N, Meador V, Elliott G, Honor D, Bounous D, Jordan H. Selection and interpretation of clinical pathology indicators of hepatic injury in preclinical studies. Vet Clin Pathol. 2005 Sep;34(3):182-8. doi: 10.1111/j.1939-165x.2005.tb00041.x. PMID: 16134065.
- Morgenstern R, Zhang J, Johansson K. Microsomal glutathione transferase 1: mechanism and functional roles. Drug Metab Rev. 2011 May;43(2):300-6. doi: 10.3109/03602532.2011.558511. PMID: 21495795.
- Sherratt PJ, Hayes JD. Glutathione s-transferases in enzyme systems that metabolise drugs and other xenobiotics. Ioannides C, editor. Chichester: John Wiley & Sons; 2002. p.219-252.
- Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008 Aug;10(8):1343-74. doi: 10.1089/ars.2007.1957. PMID: 18522489; PMCID: PMC2932530.
- Al-Helaly LA, Ahmed TY. Antioxidants and some biochemical parameters in workers exposed to petroleum station pollutants in mosul city, Iraq. International Research Journal of Environment Sciences. 2014;3(1):31-37.
- Chávez J, Cano C, Souki A, Bermúdez V, Medina M, Ciszek A, Amell A, Vargas ME, Reyna N, Toledo A, Cano R, Suárez G, Contreras F, Israili ZH, Hernández-Hernández R, Valasco M. Effect of cigarette smoking on the oxidant/antioxidant balance in healthy subjects. Am J Ther. 2007 Mar-Apr;14(2):189-93. doi: 10.1097/01.psp.0000249918.19016.f6. PMID: 17414589.Chen YY. [Effects of benzene on lipid peroxidation and the activity of relevant enzymes in human]. Zhonghua Yu Fang Yi Xue Za Zhi. 1992 Nov;26(6):336-8. Chinese. PMID: 1303347.Smith MT, Yager JW, Steinmetz KL, Eastmond DA. Peroxidase-dependent metabolism of benzene's phenolic metabolites and its potential role in benzene toxicity and carcinogenicity. Environ Health Perspect. 1989 Jul;82:23-9. doi: 10.1289/ehp.898223. PMID: 2551665; PMCID: PMC1568105.
- Bratton SB, Lau SS, Monks TJ. The putative benzene metabolite 2,3, 5-tris(glutathion-S-yl)hydroquinone depletes glutathione, stimulates sphingomyelin turnover, and induces apoptosis in HL-60 cells. Chem Res Toxicol. 2000 Jul;13(7):550-6. doi: 10.1021/tx0000015. PMID: 10898586.Brunmark A, Cadenas E. Reductive addition of glutathione to p-benzoquinone, 2-hydroxy-p-benzoquinone, and p-benzoquinone epoxides. Effect of the hydroxy- and glutathionyl substituents on p-benzohydroquinone autoxidation. Chem Biol Interact. 1988;68(3-4):273-98. doi: 10.1016/0009-2797(88)90021-x. PMID: 3214888..
- Emara AM, El-Bahrawy H. Green tea attenuates benzene-induced oxidative stress in pump workers. J Immunotoxicol. 2008 Jan;5(1):69-80. doi: 10.1080/15476910802019029. PMID: 18382860.Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol. 2001 Aug;35(2):297-306. doi: 10.1016/s0168-8278(01)00142-8. PMID: 11580156.Afolabi OK, Wusu AD, Ogunrinola OO, Abam EO, Babayemi DO, Dosumu OA, Onunkwor OB, Balogun EA, Odukoya OO, Ademuyiwa O. Paraoxonase 1 activity in subchronic low-level inorganic arsenic exposure through drinking water. Environ Toxicol. 2016 Feb;31(2):154-62. doi: 10.1002/tox.22030. Epub 2014 Aug 1. PMID: 25082665.
- Takaeidi MR, Jahangiri A, Khodayar MJ, Siahpoosh A, Yaghooti H, Rezaei S, Salecheh M, Mansourzadeh Z. The Effect of Date Seed (Phoenix dactylifera) Extract on Paraoxonase and Arylesterase Activities in Hypercholesterolemic Rats. Jundishapur J Nat Pharm Prod. 2014 Feb;9(1):30-4. doi: 10.17795/jjnpp-10368. Epub 2014 Feb 15. PMID: 24644436; PMCID: PMC3957140.
- Keskin M, Dolar E, Dirican M, Kiyici M, Yilmaz Y, Gurel S, Nak SG, Erdinc S, Gulten M. Baseline and salt-stimulated paraoxonase and arylesterase activities in patients with chronic liver disease: relation to disease severity. Intern Med J. 2009 Apr;39(4):243-8. doi: 10.1111/j.1445-5994.2009.01793.x. PMID: 19402863.
- Rao MN, Marmillot P, Gong M, Palmer DA, Seeff LB, Strader DB, Lakshman MR. Light, but not heavy alcohol drinking, stimulates paraoxonase by upregulating liver mRNA in rats and humans. Metabolism. 2003 Oct;52(10):1287-94. doi: 10.1016/s0026-0495(03)00191-4. PMID: 14564680.
- Litvinov D, Mahini H, Garelnabi M. Antioxidant and anti-inflammatory role of paraoxonase 1: implication in arteriosclerosis diseases. N Am J Med Sci. 2012 Nov;4(11):523-32. doi: 10.4103/1947-2714.103310. PMID: 23181222; PMCID: PMC3503369.
- Kilic SS, Aydin S, Kilic N, Erman F, Aydin S, Celik I. Serum arylesterase and paraoxonase activity in patients with chronic hepatitis. World J Gastroenterol. 2005 Dec 14;11(46):7351-4. doi: 10.3748/wjg.v11.i46.7351. PMID: 16437641; PMCID: PMC4725136.
- Zhang C, Peng W, Jiang X, Chen B, Zhu J, Zang Y, Zhang J, Zhu T, Qin J. Transgene expression of human PON1 Q in mice protected the liver against CCl4-induced injury. J Gene Med. 2008 Jan;10(1):94-100. doi: 10.1002/jgm.1128. PMID: 18044792.
- Ustundag B, Bahcecioglu IH, Sahin K, Duzgun S, Koca S, Gulcu F, Ozercan IH. Protective effect of soy isoflavones and activity levels of plasma paraoxonase and arylesterase in the experimental nonalcoholic steatohepatitis model. Dig Dis Sci. 2007 Aug;52(8):2006-14. doi: 10.1007/s10620-006-9251-9. Epub 2007 Apr 10. PMID: 17420940.
- Ferré N, Camps J, Prats E, Vilella E, Paul A, Figuera L, Joven J. Serum paraoxonase activity: a new additional test for the improved evaluation of chronic liver damage. Clin Chem. 2002 Feb;48(2):261-8. PMID: 11805006.Ferré N, Marsillach J, Camps J, Mackness B, Mackness M, Riu F, Coll B, Tous M, Joven J. Paraoxonase-1 is associated with oxidative stress, fibrosis and FAS expression in chronic liver diseases. J Hepatol. 2006 Jul;45(1):51-9. doi: 10.1016/j.jhep.2005.12.018. Epub 2006 Feb 6. PMID: 16510204.Marsillach J, Ferré N, Vila MC, Lligoña A, Mackness B, Mackness M, Deulofeu R, Solá R, Parés A, Pedro-Botet J, Joven J, Caballeria J, Camps J. Serum paraoxonase-1 in chronic alcoholics: relationship with liver disease. Clin Biochem. 2007 Jun;40(9-10):645-50. doi: 10.1016/j.clinbiochem.2007.01.020. Epub 2007 Feb 3. PMID: 17335791.Aviram M, Rosenblat M, Billecke S, Erogul J, Sorenson R, Bisgaier CL, Newton RS, La Du B. Human serum paraoxonase (PON 1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free Radic Biol Med. 1999 Apr;26(7-8):892-904. doi: 10.1016/s0891-5849(98)00272-x. PMID: 10232833.
- Abu El-Saad AM, Al-Kahtani MA, Abdel-Moneim AM. N-acetylcysteine and meso-2,3-dimercaptosuccinic acid alleviate oxidative stress and hepatic dysfunction induced by sodium arsenite in male rats. Drug Des Devel Ther. 2016 Oct 20;10:3425-3434. doi: 10.2147/DDDT.S115339. PMID: 27799742; PMCID: PMC5076801.
- Mehrzadi S, Fatemi I, Malayeri AR, Khodadadi A, Mohammadi F, Mansouri E, Rashno M, Goudarzi M. Ellagic acid mitigates sodium arsenite-induced renal and hepatic toxicity in male Wistar rats. Pharmacol Rep. 2018 Aug;70(4):712-719. doi: 10.1016/j.pharep.2018.02.007. Epub 2018 Feb 5. PMID: 29935397.
- van Gijssel HE, Maassen CB, Mulder GJ, Meerman JH. p53 protein expression by hepatocarcinogens in the rat liver and its potential role in mitoinhibition of normal hepatocytes as a mechanism of hepatic tumour promotion. Carcinogenesis. 1997 May;18(5):1027-33. doi: 10.1093/carcin/18.5.1027. PMID: 9163691.
- Keskin M, Dolar E, Dirican M, Kiyici M, Yilmaz Y, Gurel S, Nak SG, Erdinc S, Gulten M. Baseline and salt-stimulated paraoxonase and arylesterase activities in patients with chronic liver disease: relation to disease severity. Intern Med J. 2009 Apr;39(4):243-8. doi: 10.1111/j.1445-5994.2009.01793.x. PMID: 19402863.
- Hess R, Plaumann B, Lutum AS, Haessler C, Heinz B, Fritsche M, Brandner G. Nuclear accumulation of p53 in response to treatment with DNA-damaging agents. Toxicol Lett. 1994 Jun;72(1-3):43-52. doi: 10.1016/0378-4274(94)90008-6. PMID: 8202955.
- Milne DM, Campbell DG, Caudwell FB, Meek DW. Phosphorylation of the tumor suppressor protein p53 by mitogen-activated protein kinases. J Biol Chem. 1994 Mar 25;269(12):9253-60. PMID: 7510706.Anderson CW. DNA damage and the DNA-activated protein kinase. Trends Biochem Sci. 1993 Nov;18(11):433-7. doi: 10.1016/0968-0004(93)90144-c. PMID: 8291090.
- Weaver CV, Liu SP, Lu JF, Lin BS. The effects of benzene exposure on apoptosis in epithelial lung cells: localization by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) and the immunocytochemical localization of apoptosis-related gene products. Cell Biol Toxicol. 2007 May;23(3):201-20. doi: 10.1007/s10565-006-0165-2. Epub 2006 Dec 14. PMID: 17171516.
- Heijne WH, Slitt AL, van Bladeren PJ, Groten JP, Klaassen CD, Stierum RH, van Ommen B. Bromobenzene-induced hepatotoxicity at the transcriptome level. Toxicol Sci. 2004 Jun;79(2):411-22. doi: 10.1093/toxsci/kfh128. Epub 2004 Mar 31. PMID: 15056800.Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000 Mar;21(3):361-70. doi: 10.1093/carcin/21.3.361. PMID: 10688856.
- Chen Y, Sun P, Guo X, Gao A. MiR-34a, a promising novel biomarker for benzene toxicity, is involved in cell apoptosis triggered by 1,4-benzoquinone through targeting Bcl-2. Environ Pollut. 2017 Feb;221:256-265. doi: 10.1016/j.envpol.2016.11.072. Epub 2016 Dec 8. PMID: 27939626.Chen Y, Sun P, Bai W, Gao A. MiR-133a regarded as a potential biomarker for benzene toxicity through targeting Caspase-9 to inhibit apoptosis induced by benzene metabolite (1,4-Benzoquinone). Sci Total Environ. 2016 Nov 15;571:883-91. doi: 10.1016/j.scitotenv.2016.07.071. Epub 2016 Jul 15. PMID: 27425441.
- Qian S, Han Y, Shi Y, Xu W, Zhu Y, Jiang S, Chen Y, Yu Z, Zhang S, Yang Y, Yu K, Zhang S. Benzene induces haematotoxicity by promoting deacetylation and autophagy. J Cell Mol Med. 2019 Feb;23(2):1022-1033. doi: 10.1111/jcmm.14003. Epub 2018 Nov 8. PMID: 30411500; PMCID: PMC6349156.
- Uzma N, Kumar BS, Hazari MA. Exposure to benzene induces oxidative stress, alters the immune response and expression of p53 in gasoline filling workers. Am J Ind Med. 2010 Dec;53(12):1264-70. doi: 10.1002/ajim.20901. PMID: 20886531.
- Chen Y, Zhang W, Guo X, Ren J, Gao A. The crosstalk between autophagy and apoptosis was mediated by phosphorylation of Bcl-2 and beclin1 in benzene-induced hematotoxicity. Cell Death Dis. 2019 Oct 10;10(10):772. doi: 10.1038/s41419-019-2004-4. PMID: 31601785; PMCID: PMC6787223.
- Abu El-Saad AM, Al-Kahtani MA, Abdel-Moneim AM. N-acetylcysteine and meso-2,3-dimercaptosuccinic acid alleviate oxidative stress and hepatic dysfunction induced by sodium arsenite in male rats. Drug Des Devel Ther. 2016 Oct 20;10:3425-3434. doi: 10.2147/DDDT.S115339. PMID: 27799742; PMCID: PMC5076801.
- Mehrzadi S, Fatemi I, Malayeri AR, Khodadadi A, Mohammadi F, Mansouri E, Rashno M, Goudarzi M. Ellagic acid mitigates sodium arsenite-induced renal and hepatic toxicity in male Wistar rats. Pharmacol Rep. 2018 Aug;70(4):712-719. doi: 10.1016/j.pharep.2018.02.007. Epub 2018 Feb 5. PMID: 29935397.
- van Gijssel HE, Maassen CB, Mulder GJ, Meerman JH. p53 protein expression by hepatocarcinogens in the rat liver and its potential role in mitoinhibition of normal hepatocytes as a mechanism of hepatic tumour promotion. Carcinogenesis. 1997 May;18(5):1027-33. doi: 10.1093/carcin/18.5.1027. PMID: 9163691.
- Rämet M, Castrén K, Järvinen K, Pekkala K, Turpeenniemi-Hujanen T, Soini Y, Pääkkö P, Vähäkangas K. p53 protein expression is correlated with benzo[a]pyrene-DNA adducts in carcinoma cell lines. Carcinogenesis. 1995 Sep;16(9):2117-24. doi: 10.1093/carcin/16.9.2117. PMID: 7554063.
- Hess R, Plaumann B, Lutum AS, Haessler C, Heinz B, Fritsche M, Brandner G. Nuclear accumulation of p53 in response to treatment with DNA-damaging agents. Toxicol Lett. 1994 Jun;72(1-3):43-52. doi: 10.1016/0378-4274(94)90008-6. PMID: 8202955.
- Milne DM, Campbell DG, Caudwell FB, Meek DW. Phosphorylation of the tumor suppressor protein p53 by mitogen-activated protein kinases. J Biol Chem. 1994 Mar 25;269(12):9253-60. PMID: 7510706.
- Anderson CW. DNA damage and the DNA-activated protein kinase. Trends Biochem Sci. 1993 Nov;18(11):433-7. doi: 10.1016/0968-0004(93)90144-c. PMID: 8291090.
- Weaver CV, Liu SP, Lu JF, Lin BS. The effects of benzene exposure on apoptosis in epithelial lung cells: localization by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) and the immunocytochemical localization of apoptosis-related gene products. Cell Biol Toxicol. 2007 May;23(3):201-20. doi: 10.1007/s10565-006-0165-2. Epub 2006 Dec 14. PMID: 17171516.
- Heijne WH, Slitt AL, van Bladeren PJ, Groten JP, Klaassen CD, Stierum RH, van Ommen B. Bromobenzene-induced hepatotoxicity at the transcriptome level. Toxicol Sci. 2004 Jun;79(2):411-22. doi: 10.1093/toxsci/kfh128. Epub 2004 Mar 31. PMID: 15056800.
- Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000 Mar;21(3):361-70. doi: 10.1093/carcin/21.3.361. PMID: 10688856.
- Chen Y, Sun P, Guo X, Gao A. MiR-34a, a promising novel biomarker for benzene toxicity, is involved in cell apoptosis triggered by 1,4-benzoquinone through targeting Bcl-2. Environ Pollut. 2017 Feb;221:256-265. doi: 10.1016/j.envpol.2016.11.072. Epub 2016 Dec 8. PMID: 27939626.
- Chen Y, Sun P, Bai W, Gao A. MiR-133a regarded as a potential biomarker for benzene toxicity through targeting Caspase-9 to inhibit apoptosis induced by benzene metabolite (1,4-Benzoquinone). Sci Total Environ. 2016 Nov 15;571:883-91. doi: 10.1016/j.scitotenv.2016.07.071. Epub 2016 Jul 15. PMID: 27425441.
- Qian S, Han Y, Shi Y, Xu W, Zhu Y, Jiang S, Chen Y, Yu Z, Zhang S, Yang Y, Yu K, Zhang S. Benzene induces haematotoxicity by promoting deacetylation and autophagy. J Cell Mol Med. 2019 Feb;23(2):1022-1033. doi: 10.1111/jcmm.14003. Epub 2018 Nov 8. PMID: 30411500; PMCID: PMC6349156.
- Uzma N, Kumar BS, Hazari MA. Exposure to benzene induces oxidative stress, alters the immune response and expression of p53 in gasoline filling workers. Am J Ind Med. 2010 Dec;53(12):1264-70. doi: 10.1002/ajim.20901. PMID: 20886531.
- Chen Y, Zhang W, Guo X, Ren J, Gao A. The crosstalk between autophagy and apoptosis was mediated by phosphorylation of Bcl-2 and beclin1 in benzene-induced hematotoxicity. Cell Death Dis. 2019 Oct 10;10(10):772. doi: 10.1038/s41419-019-2004-4. PMID: 31601785; PMCID: PMC6787223.
- Kuo ML, Shiah SG, Wang CJ, Chuang SE. Suppression of apoptosis by Bcl-2 to enhance benzene metabolites-induced oxidative DNA damage and mutagenesis: A possible mechanism of carcinogenesis. Mol Pharmacol. 1999 May;55(5):894-901. PMID: 10220568.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.