Medicine Group . 2022 December 31;3(12):1579-1588. doi: 10.37871/jbres1639.
Non-Ventilatory Co-Factors of Ventilator-Induced Lung Injury
Lauren Thornton DO1* and John J Marini MD2
2Regions Hospital, St. Paul, MN USA
Introduction
Numerous experimental studies demonstrate key aspects of mechanical ventilation which initiate or exacerbate acute lung injury, characterized by proteinaceous edema, inflammation, and hemorrhage. The great majority of these investigations into Ventilator-Induced Lung Injury (VILI) have focused on the characteristics of mechanical ventilation directly regulated by the clinician, including the individual tidal cycle-tidal volume, inspiratory flow rate, driving Pressure and End-Expiratory airway Pressure (PEEP). More recently, the impact of frequency has been recognized by tracking the inflation energy expended per minute, an inclusive variable termed ‘power’ [1]. Although these mechanical features are of unquestioned importance, other characteristics of the clinical environment have been shown to modify the intensity and/or nature of any resulting damage. While most research has focused mainly on approaches to minimize VILI over the last decades, largely ignoring these other injury co-factors, we believe a renewed focus on these background characteristics will help improve patient outcomes. Indeed, many facets of the COVID pandemic highlighted the need for a more holistic approach to lung injury.
In the largest and perhaps the most widely cited clinical trial of ventilator strategy to prevent acute lung injury, smaller tidal volumes (6 ml/kg predicted body weight, PBW) were associated with reduced mortality as compared to unnaturally large ones (12 ml/kg PBW) [2]. This result was attributed to the generally lower peak alveolar pressures and reduced mechanical stresses associated with smaller tidal volumes. Analysis of the data pooled from both trial groups not only demonstrated a positive correlation between plateau pressure and mortality rate, but also revealed its monotonic, linear nature—without an obvious break point down to pressures that are considerably lower than those that are feasible to use in the management of Acute Respiratory Distress Syndrome (ARDS) [3]. Although such an association does not confirm plateau pressure as the sole causative variable, it implies that there may be no “safe” plateau pressure below which mortality cannot be influenced by further pressure reduction. Because this relationship seems unexplainable on the basis of trans-pulmonary pressure and stretch alone, it underscores the need to identify any cofactors that influence VILI expression.
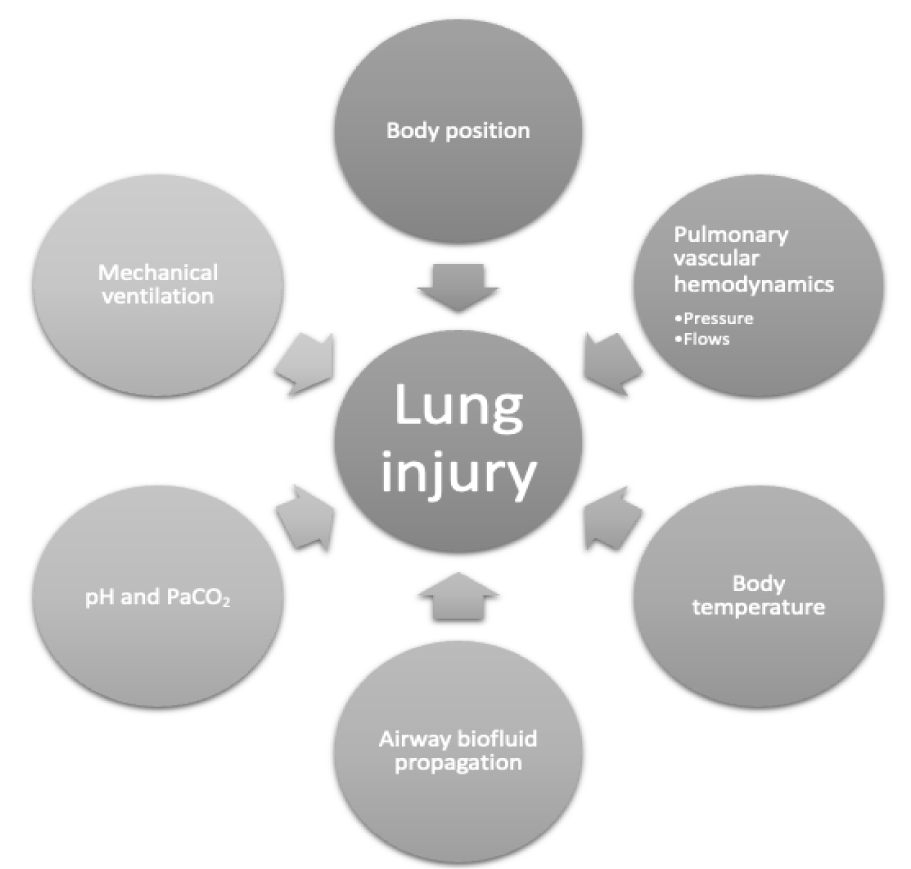
Focusing on lower tidal volumes, lower plateau pressures, and higher PEEP settings during the early phase of injury has helped to decrease ARDS -associated length of Intensive Care Unit (ICU) stay and to curtail the mortality rate [4]. We raise awareness here to the potential for lung injury to be modified during mechanical ventilation by such non-ventilatory factors as body position, acid-base status, pulmonary vascular dynamics, body temperature, concomitant pathologies, and pharmacologic agents. In this discussion, we review a selected subset of these non-ventilatory factors and propose mechanisms for their actions, summarized in figure 1. Our objective is to focus on those amenable to modification at the bedside.
Pathophysiology of Ventilation-Induced Lung Injury (VILI)
Although VILI is a complex process initiated and propagated through several mechanisms, generally speaking, damage that results from excessive mechanical stress can be classified into two broad categories: structural and inflammatory. The injury process is initiated by repeated cycles of intolerable mechanical stress that depend on the magnitude, frequency and cumulative effect of this stimulus. Repetitive over-stretching and cyclic recruitment-derecruitment of collapsed areas that are exposed periodically to high pressure favor injury that disrupts the integrity of the epithelial membrane [5].
Simultaneously, these mechanical forces activate intracellular signaling cascades within epithelial cells. This activation culminates in recruitment of polymorphonuclear leukocytes, production of pro-inflammatory cytokines, vasodilatation and alveolar edema—collectively a process termed ‘biotrauma’ [6]. Once formed, alveolar edema has two opposing effects. On one hand, completely flooded alveoli are theoretically subject to lower shearing stresses than atelectatic units, as the gas-liquid interface is eliminated and alveolar dimensions increase. On the other hand, the increased weight of the edematous lung may promote small airway compression and accentuate the tendency for tidal opening and closure of dependent units to occur [7]. Overt breaks in the blood-gas barrier may arise when the applied mechanical force is extreme. In experiments, these microscopic breaks, or “stress fractures”, may arise very quickly after high pressure ventilatory cycles are initiated and involve both the cellular membranes of individual cells as well as larger tears that traverse intracellular boundaries. The presence of alveolar hemorrhage implies breaks of sufficient size to allow erythrocyte passage through them.
Because stresses severe enough to disrupt structural elements of the matrix are most likely to be amplified at the junctions of aerated and non-aerated tissues, hemorrhagic edema due to materials failure tends to form preferentially in gravitationally dependent regions. In such zones, inflammation may be a secondary epi-phenomenon to physical disruption of the barrier, rather than a primary event initiated by repetitive mechanical signaling. Whatever the stimulus, the potential for mechanical ventilation to induce systemic cytokine release and for a protective lung ventilation strategy to attenuate this response have been well demonstrated in the clinical context [8].
Prone Position and Lung Injury
The trans-pulmonary pressure that determines tissue stress is not only a function of plateau pressure but also of the local (interstitial) pressure that surrounds the alveolus. Although interstitial pressure undoubtedly varies from site to site, by convention it is approximated by the local pleural pressure along any given transverse plane [9]. Pleural pressure, in turn, is a function of the relative elastances of the lung and chest wall (and in the prone position, of the surface against which the thorax rests, as well). The conformations of the chest wall and lung influence regional pleural pressures, and these are affected by body position. Edema from acute injury imposes lung tissue weight upon more gravitationally dependent zones, accentuating the gradient of pleural pressure (and inversely of transpulmonary pressure) that exists in the supine healthy subject [10]. Computed tomography has made clear that lung collapse is most prevalent in the dependent lung zones, whatever the body position [11]. When supine, the weight of the heart and mediastinal contents makes an important contribution to atelectasis, as they are cradled by the lower lobes that partially support them [12]. Conceptually, tissue forces (stresses) are focused at the interface between open and closed lung units. Judged solely on this basis, the tendency for VILI should be greatest in the most dependent and mid-positioned lung zones. In fact, when high plateau pressures are used with insufficient PEEP to keep the high-risk dependent units continually patent, VILI does prevail in the dorsal regions of large, supine experimental animals—even of those with initially healthy lungs [13,14]. Other co-factors, such as secretion retention and microvascular pressure (discussed below) may also contribute to this regional predilection for injury, but these are likely to be of secondary importance.
Although the supine position has traditionally been favored for the care of the critically ill, there are important reasons to question this practice in patients with ARDS. The great majority of mammals—including our closest primate relatives—orient themselves in the prone position, and healthy humans normally spend only a small fraction of their time supine. Anatomically, the shapes of the lung and the chest wall are more closely matched in the prone position, so that the gradients of pleural and transpulmonary pressure are attenuated. Overall, the resting aerated lung volume changes little after proning, while the distribution of that volume is markedly affected. Moreover, the airways drain more effectively in the prone position, and the weight of the heart is borne by the anterior chest wall rather than by the lungs, reducing further the tendency for dependent atelectasis to form. Less tissue collapse and reduced heterogeneity of regional transpulmonary pressures is likely to account for improved ventilation-perfusion matching in the prone position [14]. To a lesser degree, these observations apply to normal lungs as well as to acutely injured ones.
Experimental studies demonstrate that injurious ventilatory patterns produce less damage when the animal is oriented prone [13,14] and the positional reduction in injury is experienced primarily in the gravitationally dependent regions. Early clinical trials suggested advantages from prone positioning despite relatively short durations of application per day. These included improved oxygenation [15,16] and improved efficiency of carbon dioxide elimination [17], likely due to the combination of better ventilation/perfusion matching and reduced atelectasis. Initial studies showed non-statistically significant declines in mortality for those with severe ARDS [18]. However, the first trial to demonstrate improved mortality (both 28 and 90 day) was that of Guerin, et al. [19] who utilized prone positioning early in the course of severe ARDS for at least 16 consecutive hours per day, along with accepted ‘lung protective ventilation’ (6 mg/kg PBW and targeted plateau pressure< 30 cmH2O). The COVID-19 pandemic provided additional support for the benefits of prone positioning, including reduced need for intubation in non-ventilated patients [20] and reduced mortality in proned, intubated ones [21].
Co2, Acidosis and Lung Injury
Not only does the stress associated with high pressure ventilatory cycles incite injury, but also an association of hyperventilation and hypocapnia with worsened lung injury has been suggested. In fact, hypocapnia appears to mediate parenchymal injury by altering surfactant function and by increasing permeability in airway and parenchymal microvessels [22]. Accepting a lower minute ventilation level reduces the energy and power delivered by mechanical ventilation, but invariably results in more carbon dioxide (Co2) retention and hypercapnia relative to baseline. Doing so may reduce VILI risk simply by reducing cumulative stress and strain. Indeed, the obligate hypoventilation associated with lung protective strategies of ventilation has been termed “permissive” hypercapnia for this reason. It has been proposed, however, that hypercapnia and resultant acidosis itself may promote increased survival in ARDS [23,24].
If this contention proves true, is it hypercapnia or any associated acidosis that confers benefit? Laboratory data indicate that acidosis may confer protective adaptation in the context of cellular stress, thereby benefitting acute organ injury [25]. In the setting of ARDS, hypercapnic acidosis may alter the activity of polymorphonuclear phagocytes and influence numerous subcellular mechanisms [26-30]. The bulk of current evidence suggests that any beneficial effects of hypercapnic acidosis in acute lung injury more closely relate to the acidosis, rather than to the elevated partial pressure of Co2 per se. Although benefit-confirming clinical evidence remains sparse, laboratory data suggest that lower pH may favor cellular functioning, down-regulate inflammatory responses, improve cardiac function, and maintain or reactivate hypoxic pulmonary vasoconstriction to improve ventilation/perfusion matching [26,31-36].
In some clinical work, patients with ARDS (both ventilated and non-ventilated) have been reported to have higher mortality rates in moderate ARDS after experiencing sustained early hypocapnia (study days 1-2). Importantly, those with normocapnia and hypocapnia were less likely to have protective mechanical ventilatory strategies employed than the hypercapnic group [24]. The hypercapnic group was found to experience neither harm nor benefit with regards to mortality. It is noteworthy that other clinical work indicates that severe hypercapnia (>50mmHg), rather than being protective, has been associated with higher ICU mortality in ARDS patients [37].
In summary, it remains unsettled whether hypercapnia in isolation exerts therapeutic benefit for inflamed tissue when dissociated from the low tidal volume and ventilating frequencies that simultaneously reduce cumulative tissue stress while promoting atelectasis.
Vascular Pressure and Lung Injury
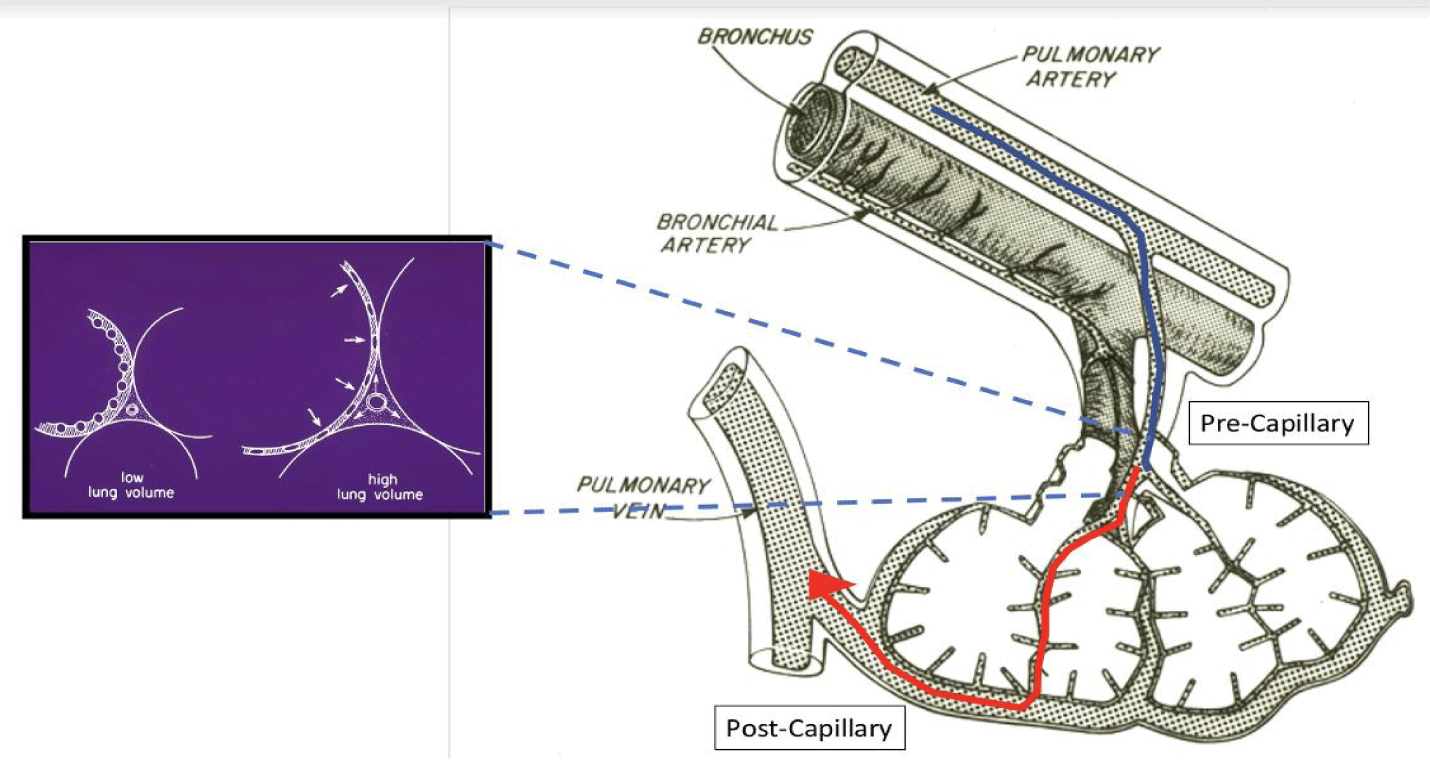
The pulmonary vascular tree can be considered as a series of three segments: arterial, intermediate or middle (which includes alveolar capillaries and contiguous microvessels), and venous, as visualized in figure 2. Under normal conditions the arterial and venous segments (which are extra-alveolar) contribute most to overall pulmonary vascular resistance, while the compliant intermediate segment is influenced primarily by alveolar pressures. As a consequence, it undergoes the greatest change in overall vascular resistance during the ventilation cycle [38].
Different animal model experiments collectively underscore the potential for deleterious interactions to occur between lung volumes and pulmonary hemodynamics [39-42]. Increased blood flows and vascular pressures within the intermediate segment influence the gradient of trans-alveolar vascular pressure and result in greater severity of VILI that result from an unchanging downstream pressure and an adverse pattern of ventilation. In keeping with this concept, ventilation with negative pressure may cause lung damage more severe than equivalent ventilation by positive pressure, implicating involvement of increased blood flow in ventilation-related damage [43]. Moreover, experimental rats given dopamine to increase cardiac output suffer increased albumin leak when ventilated with high pressure, suggesting that a major portion of the protective effect of PEEP in the setting of high pressure ventilation may be due to its reduction of pulmonary perfusion [44].
Intriguing experiments indicate that lowering post-capillary pressure increases the edema and filtration coefficient resulting from a fixed pattern of ventilation and upstream pre-capillary pressure [45]. In other words, vascular pressures as well as the characteristics of the tidal cycle appear to be fundamental to the genesis of VILI. In addition, the minute ventilation, the number of the stress cycles of a potentially damaging character that occur per unit time, or their cumulative number, might be important from the viewpoint of small vessel trauma as well as the airspace energy per minute (‘power’) already discussed [39-41]. The effects of respiratory frequency and vascular pressures on VILI are not mediated primarily by pulsatile vascular pressure in the absence of lung motion, but rather by a phenomenon related to cyclic modulation of the vascular microenvironment induced by ventilation itself [40-42].
At first consideration, the fact that lowering post-capillary pressure might accentuate the edema resulting from VILI might seem paradoxical; in other disease settings reducing capillary pressure often confers benefit on lung functioning. Lower capillary and venous pressures limit exudation of protein-rich fluid, which may inactivate surfactant and further alter membrane permeability by increasing surface tension. Furthermore, edematous lungs tend to collapse under their own weight and to develop dependent atelectasis. When tidal airway pressures are high enough, such compression may lead to cyclic opening and collapse, and amplified shear stresses. Such conditions could account for the preferentially dependent distribution of VILI, as discussed above [44]. At the same time, flooding the alveoli of dependent regions can reduce regional mechanical stress by preventing their tidal expansion and collapse. In fact, excessive reduction of capillary pressure may promote vascular de-recruitment, alter ventilation-perfusion matching, and contribute to vascular stress, as during positive pressure ventilation it tends to impose the ‘zone two’ conditions under which alveolar pressure exceeds pulmonary venous pressure [46]. This extension of zone two can amplify the increase in vascular resistance caused by inflation and augment tidal intramural pressure changes and stresses within the vessels located upstream from the narrowed and/or collapsed vessels. Consequently, alveolar collapse that occurs in the course of mechanical inflation tends to redistribute flow toward extra-alveolar vessels, thereby increasing their transmural pressure and rates of fluid filtration.
During positive pressure ventilation, the majority of capillaries embedded within the alveolar wall are compressed by the expansion of adjoining alveoli. At the same time, lung expansion decreases interstitial pressure, which increases the transmural pressure of the vessels in the intermediate segment of small, fragile vessels (Figure 2). Raising pre-capillary and/or reducing post-capillary microvascular pressures simultaneously, increases the pressure gradient and energy dissipated across this middle segment of the pulmonary microvasculature. These actions appear to worsen edema and/or accentuate barrier injury when airway mechanical stresses are sufficiently high [40]. On the other hand, cyclic opening and closure of the microvessels may amplify shearing forces and stretching of the vascular endothelium (similar to that undergone by the alveolar wall), with the potential to initiate inflammation-mediated tissue breakdown. By promoting alveolar vascular collapse and amplifying extra-alveolar vascular stress, excessive reductions in lung volume and microvascular pressure have the potential to contribute to VILI [39].
If raising pre-capillary microvascular pressure and reducing post-capillary pressure might both amplify VILI, how can pre-capillary microvascular pressure be reduced without compromising systemic organ perfusion or lowering pulmonary post-capillary pressures excessively? Blood flow and oxygen consumption are linked variables. It follows that reducing tissue demands for oxygen also reduces the blood flow through the lung, which apart from lowering the ventilating power need also decreases both upstream microvascular pressure and the luminal transcapillary pressure gradient. Although more studies are clearly necessary to determine the exact relationship between vascular pressure and lung injury, it would seem prudent to diminish unnecessary demands for ventilation and cardiac output; the direct clinical implication is that conditions of agitation, high fever, pain and elevated work of breathing should be avoided if potentially damaging alveolar pressures must be applied.
Extensive acute lung injury occurring in the COVID-19 pandemic has renewed interest in the role of the pulmonary vasculature in its pathogenesis. SARS-CoV-2 promotes endothelial dysfunction, vasoregulatory dysfunction, vascular leak, and microthrombi [47]. Ackermann, et al. [48] reviewed autopsy findings in COVID-19 ARDS lungs and compared them to those of influenza associated respiratory failure. They found COVID-19 infected lungs were unique in the extensive presence of intracellular SARS-CoV-2 and disruption of endothelial cell membranes. These findings were associated with widespread vascular thrombosis with microangiopathy and ‘intussusceptive angiogenesis’. Interestingly, the lung weight of COVID-ARDS was significantly less than influenza-related ARDS. Moreover, significantly increased numbers of inflammatory CD3+ t-cells within the pre-and post-capillary vessel walls suggested ‘angiocentric inflammation’. Also notable, COVID-19 lungs revealed nine times the alveolar capillary microthrombi compared to influenza. The occlusion of alveolar capillaries appears to have contributed to profound hypoxia and hypercarbia of COVID-19 ARDS, irrespective of the level of parenchymal injury [48].
Temperature - Preconditioning and Lung Injury
Maintenance of appropriate thermoregulation is essential for normal cellular functioning. Beyond certain defined physiologic limits, enzymatic performance may be either induced or impaired, distorting the mechanisms of normal homeostasis. Extremes of temperature may culminate in protein degradation or overt cellular disruption. The pace at which temperature aberrations are accomplished strongly impacts their ultimate physiologic effects. Slowly cooling into the range of tolerable hypothermia slows metabolic processes sufficiently to allow tolerance to certain high risk surgical procedures. It has also been demonstrated that induced hypothermia of modest proportions may improve the prognosis of cardiac arrest [49].
Preconditioning is a process whereby cells or tissues exposed to a sub-lethal stimulus are transiently protected from a subsequent noxious stress. When preconditioning is conducted using a stressful stimulus (e.g. high temperatures) applied for a tolerable interval, Heat Shock Proteins (HSP) are elaborated [50-52]. These HSPs act as molecular chaperones against injury for certain classes of vital intracellular proteins, preventing their premature folding and denaturation and allowing normal protein assembly and interactions to proceed under otherwise adverse environmental conditions. Thermal preconditioning attenuates VILI-associated decreases in lung compliance, reduces the production of inflammatory cytokines, and increases the percentage of large surfactant aggregates (the active form) [53]. Following thermal exposure, full expression of HSP requires hours to develop. It has been demonstrated that experimental animals that have been pre-stressed by heat exposure are more resistant to the adverse effects of such noxious influences as endotoxin when later exposed to it [54]. In this context, the protective effects of pre-stressing could theoretically attenuate VILI, as well. This possibility, however, currently remains unproven.
This concept of preconditioning has been beneficial in other aspects of lung injury. Gradual imposition of a purely mechanical ventilation stress may improve tolerance to it. This principle has been convincingly shown experimentally for recruiting maneuvers [55], large tidal volumes [56], increments of PEEP [57] and higher frequency [58].
In this context, the pace and timing of non-ventilatory interventions may also influence their actions. Although appropriate heat pre-conditioning may be lung protective, heat exposure occurring simultaneously with high pressure ventilation accentuates rather than attenuates VILI [59]. Presumably, the heat shock class of proteins has not yet been elaborated during simultaneous exposure to heat and mechanical stress, whereas destructive inflammatory enzymes are up-regulated, edema clearance mechanisms are overwhelmed, and/or metabolic demands outstrip the delivery of vital energy substrates. Moderate cooling of experimental animals also appears to afford a VILI-protective effect when the mechanical stress is applied simultaneously with the cooling challenge [59].
Clinical studies have demonstrated that moderate to extreme hypothermia attenuates the adverse response in models of VILI induced purely by mechanical forces and in models characterized by pre-existing inflammation [59]. In animal models, rats undergoing high pressure ventilation showed less expression of systemic inflammatory markers (serum chemokines and cytokines) when performed under hypothermia than normo or hyperthermia [60]. Additional animal models have shown lower inflammatory markers (neutrophil counts and IL-1beta levels) in bronchoalveolar lavage fluid as well as lower lung weight ratios and histologic acute lung injury scores when ventilated under hypothermic conditions (27°C) [61].
While awaiting confirmation in patients, such observations illustrate the potential for thermal manipulation to reduce the risk of VILI. Although clearly of unproven benefit and certainly not yet advocated for clinical application, preventing hyperthermia when high alveolar pressures are necessary or even inducing mild hypothermia may eventually prove viable options to reinforce our approach to lung protective ventilation.
Airway Bio-Fluids and Injury Propagation
Inactivation of pre-formed surfactant results from exposure to the proteinaceous and often mediator-rich edema fluid that forms after breakdown of the alveolar-capillary barrier. Resulting alveolar instability predisposes to creation of high-stress parenchymal foci and to VILI. Seen from this vantage point, preventing transfer of noxious fluids to well-functioning lung units should help to preserve gas exchange and limit the injury process.
Perhaps in part for this reason, the lung has evolved as a segmented structure organized as parallel compartments connected by a branching common corridor. Because of this compartmentalized nature, the lung is well designed to confine regional damage to isolated lobes or segments. Furthermore, the spiraling array of segmental openings ensures that at any given time the feeder channels of some lung sectors are less gravitationally dependent than others, whatever the spatial orientation of the lung might be. Such geometry offers advantages when attempting to confine potentially damaging bio-fluids to their sites of origin and impede propagation of an initially regionalized injury [62,63].
In ARDS, bio-fluids initially fill the interstitial compartment and the alveolar airspaces once occupied by air (percentages of each depend on severity and phase of the inflammatory process). The edematous phase of biofluid mobility is short lived; however, in the initial hours of ARDS development the sheer volume of such fluid can potentially overwhelm anatomic defenses. Indeed, most experienced clinicians have occasionally encountered rapid and poorly explained patient deterioration shortly after intubation. One might speculate that in such cases deep breathing and forceful exhalation are coupled with ineffective airway clearance just prior to initiating ventilator support. Post-intubation, deeper sedation, endotracheal intubation and adverse gravitational biases associated with unfavorable body positioning encourage propagation of injury via the airway passages.
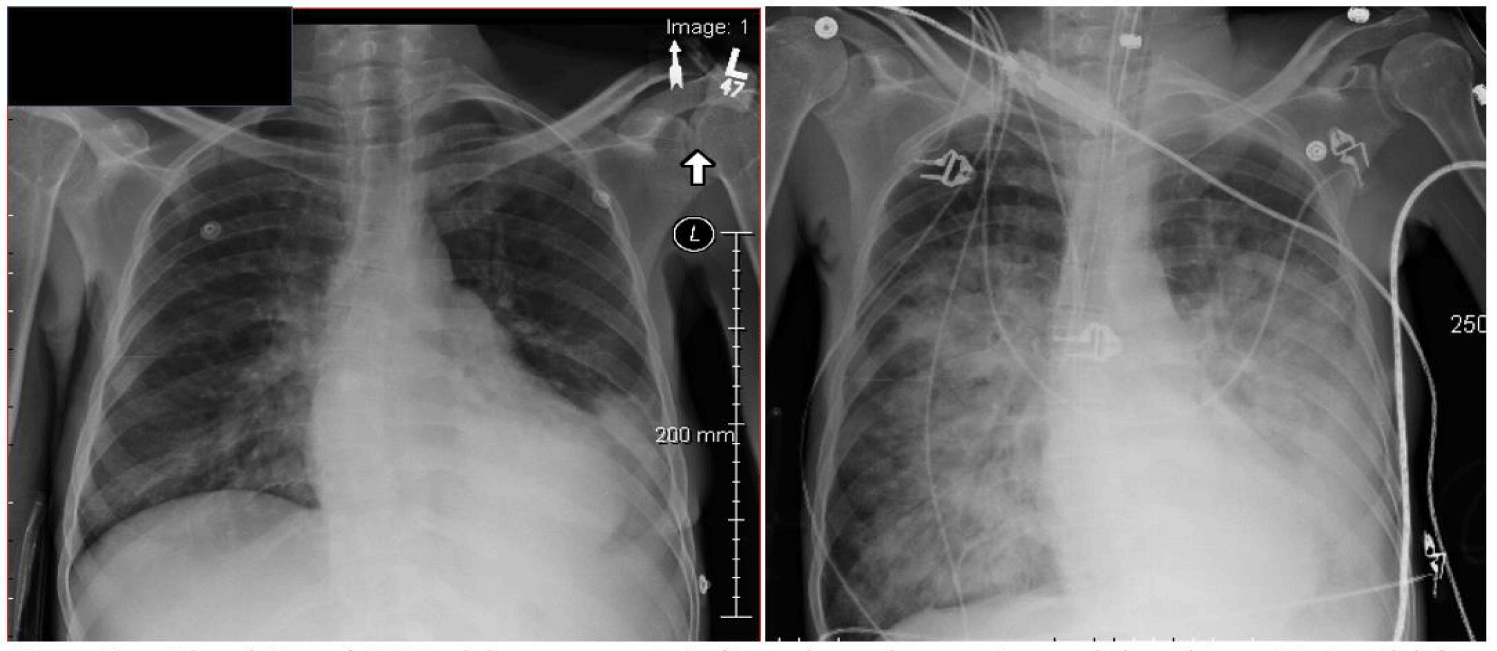
While the resting functional residual capacity of an adult normally exceeds 2000 mL, the tracheo-bronchial tree accommodates little more than 150 mL, with only a minor fraction of that capacity residing in conducting airways <2 mm in diameter. Although liquid secretions are not commonly seen to enter the endotracheal tube (especially when PEEP is applied), it is not difficult to envision how the acutely flooded alveoli could extend injury rapidly, sector to sector by distributing mobile proteinaceous fluids through the airway network to previously unaffected lung units, as illustrated in figure 3.
Conclusion
VILI is undoubtedly a dynamic and complex process that depends on considerably more than the augmented mechanical ventilation strategies targeting ‘safe’ airway pressures, tidal volumes, and mechanical power, which justifiably have received the most investigative attention over the last several decades. Yet, many laboratory experiments and observations have helped us to better understand the multiple cofactors that modulate VILI expression and have suggested novel therapeutic options. This review highlights those aspects of lung injury which may be readily modified by a bedside clinician. Not described are other factors which deserve further investigation regarding vulnerability to VILI development, such as the roles of age and gender [64,65]. Renewed research interest and closer attention to such non-mechanical co-factors of lung injury may further reduce the iatrogenic morbidity associated with the ventilation of patients with acute respiratory failure.
References
- Gattinoni L, Tonetti T, Cressoni M, Cadringher P, Herrmann P, Moerer O, Protti A, Gotti M, Chiurazzi C, Carlesso E, Chiumello D, Quintel M. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med. 2016 Oct;42(10):1567-1575. doi: 10.1007/s00134-016-4505-2. Epub 2016 Sep 12. PMID: 27620287.
- Brower RG, Matthay M, Schoenfeld D. Meta-analysis of acute lung injury and acute respiratory distress syndrome trials. Am J Respir Crit Care Med. 2002 Dec 1;166(11):1515-7. doi: 10.1164/ajrccm.166.11.340. PMID: 12450936.
- Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000 May 4;342(18):1301-8. doi: 10.1056/NEJM200005043421801. PMID: 10793162.
- Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998 Feb 5;338(6):347-54. doi: 10.1056/NEJM199802053380602. PMID: 9449727.
- Ricard JD, Dreyfuss D, Saumon G. Ventilator-induced lung injury. Eur Respir J. 2003;22(S42); 2s-9s.
- Ricard JD, Dreyfuss D. Cytokines during ventilator-induced lung injury: a word of caution. Anesth Analg. 2001;93;251-252.
- Martynowicz MA, Minor TA, Walters BJ, Hubmayr RD. Regional expansion of oleic acid-injured lungs. Am J Respir Crit Care Med. 1999 Jul;160(1):250-8. doi: 10.1164/ajrccm.160.1.9808101. PMID: 10390408.
- Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999 Jul 7;282(1):54-61. doi: 10.1001/jama.282.1.54. PMID: 10404912.
- Nunn JF. Distribution of pulmonary ventilation and perfusion. Chapter 8 in: Nunn’s Applied Respiratory Physiology-4th Ed., 1993;156-197.
- Pelosi P, Tubiolo D, Mascheroni D, Vicardi P, Crotti S, Valenza F, Gattinoni L. Effects of the prone position on respiratory mechanics and gas exchange during acute lung injury. Am J Respir Crit Care Med. 1998 Feb;157(2):387-93. doi: 10.1164/ajrccm.157.2.97-04023. PMID: 9476848.
- Gattinoni L, Pelosi P, Crotti S, Valenza F. Effects of positive end-expiratory pressure on regional distribution of tidal volume and recruitment in adult respiratory distress syndrome. Am J Respir Crit Care Med. 1995 Jun;151(6):1807-14. doi: 10.1164/ajrccm.151.6.7767524. PMID: 7767524.
- Marini JJ, Culver BH, Butler J. Mechanical effect of lung distention with positive pressure on cardiac function. Am Rev Respir Dis. 1981 Oct;124(4):382-6. doi: 10.1164/arrd.1981.124.4.382. PMID: 7027851.
- Broccard A, Shapiro RS, Schmitz LL, Adams AB, Nahum A, Marini JJ. Prone positioning attenuates and redistributes ventilator-induced lung injury in dogs. Crit Care Med. 2000 Feb;28(2):295-303. doi: 10.1097/00003246-200002000-00001. PMID: 10708156.
- Johansson MJ, Wiklund A, Flatebø T, Nicolaysen A, Nicolaysen G, Walther SM. Positive end-expiratory pressure affects regional redistribution of ventilation differently in prone and supine sheep. Crit Care Med. 2004 Oct;32(10):2039-44. doi: 10.1097/01.ccm.0000142395.82277.6f. PMID: 15483412.
- Gattinoni L, Tognoni G, Pesenti A, Taccone P, Mascheroni D, Labarta V, Malacrida R, Di Giulio P, Fumagalli R, Pelosi P, Brazzi L, Latini R; Prone-Supine Study Group. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001 Aug 23;345(8):568-73. doi: 10.1056/NEJMoa010043. PMID: 11529210.
- Chatte G, Sab JM, Dubois JM, Sirodot M, Gaussorgues P, Robert D. Prone position in mechanically ventilated patients with severe acute respiratory failure. Am J Respir Crit Care Med. 1997 Feb;155(2):473-8. doi: 10.1164/ajrccm.155.2.9032181. PMID: 9032181.
- Gattinoni L, Vagginelli F, Carlesso E, Taccone P, Conte V, Chiumello D, Valenza F, Caironi P, Pesenti A; Prone-Supine Study Group. Decrease in PaCo2 with prone position is predictive of improved outcome in acute respiratory distress syndrome. Crit Care Med. 2003 Dec;31(12):2727-33. doi: 10.1097/01.CCM.0000098032.34052.F9. PMID: 14668608.
- Mancebo J, Fernández R, Blanch L, Rialp G, Gordo F, Ferrer M, Rodríguez F, Garro P, Ricart P, Vallverdú I, Gich I, Castaño J, Saura P, Domínguez G, Bonet A, Albert RK. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006 Jun 1;173(11):1233-9. doi: 10.1164/rccm.200503-353OC. Epub 2006 Mar 23. PMID: 16556697.
- Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M, Chatellier D, Jaber S, Rosselli S, Mancebo J, Sirodot M, Hilbert G, Bengler C, Richecoeur J, Gainnier M, Bayle F, Bourdin G, Leray V, Girard R, Baboi L, Ayzac L; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013 Jun 6;368(23):2159-68. doi: 10.1056/NEJMoa1214103. Epub 2013 May 20. PMID: 23688302.
- Ehrmann S, Li J, Ibarra-Estrada M, Perez Y, Pavlov I, McNicholas B, Roca O, Mirza S, Vines D, Garcia-Salcido R, Aguirre-Avalos G, Trump MW, Nay MA, Dellamonica J, Nseir S, Mogri I, Cosgrave D, Jayaraman D, Masclans JR, Laffey JG, Tavernier E; Awake Prone Positioning Meta-Trial Group. Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial. Lancet Respir Med. 2021 Dec;9(12):1387-1395. doi: 10.1016/S2213-2600(21)00356-8. Epub 2021 Aug 20. PMID: 34425070; PMCID: PMC8378833.
- Shelhamer MC, Wesson PD, Solari IL, Jensen DL, Steele WA, Dimitrov VG, Kelly JD, Aziz S, Gutierrez VP, Vittinghoff E, Chung KK, Menon VP, Ambris HA, Baxi SM. Prone Positioning in Moderate to Severe Acute Respiratory Distress Syndrome Due to COVID-19: A Cohort Study and Analysis of Physiology. J Intensive Care Med. 2021 Feb;36(2):241-252. doi: 10.1177/0885066620980399. PMID: 33380236; PMCID: PMC7780273.
- Tobin MJ. Mechanical ventilation. N Engl J Med. 1994;330;1056-1061.
- Laffey JG, O'Croinin D, McLoughlin P, Kavanagh BP. Permissive hypercapnia--role in protective lung ventilatory strategies. Intensive Care Med. 2004 Mar;30(3):347-56. doi: 10.1007/s00134-003-2051-1. Epub 2004 Jan 14. PMID: 14722644.
- Madotto F, Rezoagli E, McNicholas BA, Pham T, Slutsky AS, Bellani G, Laffey JG; LUNG SAFE Investigators and the ESICM Trials Group. Patterns and Impact of Arterial Co2 Management in Patients With Acute Respiratory Distress Syndrome: Insights From the LUNG SAFE Study. Chest. 2020 Nov;158(5):1967-1982. doi: 10.1016/j.chest.2020.05.605. Epub 2020 Jun 24. PMID: 32589951.
- Laffey JG, Kavanagh BP. Carbon dioxide and the critically ill--too little of a good thing? Lancet. 1999 Oct 9;354(9186):1283-6. doi: 10.1016/S0140-6736(99)02388-0. PMID: 10520649.
- Laffey JG, Tanaka M, Engelberts D, Luo X, Yuan S, Tanswell AK, Post M, Lindsay T, Kavanagh BP. Therapeutic hypercapnia reduces pulmonary and systemic injury following in vivo lung reperfusion. Am J Respir Crit Care Med. 2000 Dec;162(6):2287-94. doi: 10.1164/ajrccm.162.6.2003066. PMID: 11112153.
- Adding LC, Agvald P, Persson MG, Gustafsson LE. Regulation of pulmonary nitric oxide by carbon dioxide is intrinsic to the lung. Acta Physiol Scand. 1999 Oct;167(2):167-74. doi: 10.1046/j.1365-201x.1999.00585.x. PMID: 10571553.
- Shibata K, Cregg N, Engelberts D, Takeuchi A, Fedorko L, Kavanagh BP. Hypercapnic acidosis may attenuate acute lung injury by inhibition of endogenous xanthine oxidase. Am J Respir Crit Care Med. 1998 Nov;158(5 Pt 1):1578-84. doi: 10.1164/ajrccm.158.5.9804039. PMID: 9817711.
- Takeshita K, Suzuki Y, Nishio K, Takeuchi O, Toda K, Kudo H, Miyao N, Ishii M, Sato N, Naoki K, Aoki T, Suzuki K, Hiraoka R, Yamaguchi K. Hypercapnic acidosis attenuates endotoxin-induced nuclear factor-[kappa]B activation. Am J Respir Cell Mol Biol. 2003 Jul;29(1):124-32. doi: 10.1165/rcmb.2002-0126OC. Epub 2003 Feb 6. PMID: 12600832.
- Holmes JM, Duffner LA, Kappil JC. The effect of raised inspired carbon dioxide on developing rat retinal vasculature exposed to elevated oxygen. Curr Eye Res. 1994 Oct;13(10):779-82. doi: 10.3109/02713689409047013. PMID: 7842726.
- Broccard AF, Hotchkiss JR, Vannay C, Markert M, Sauty A, Feihl F, Schaller MD. Protective effects of hypercapnic acidosis on ventilator-induced lung injury. Am J Respir Crit Care Med. 2001 Sep 1;164(5):802-6. doi: 10.1164/ajrccm.164.5.2007060. PMID: 11549536.
- Sinclair SE, Kregenow DA, Lamm WJ, Starr IR, Chi EY, Hlastala MP. Hypercapnic acidosis is protective in an in vivo model of ventilator-induced lung injury. Am J Respir Crit Care Med. 2002 Aug 1;166(3):403-8. doi: 10.1164/rccm.200112-117OC. PMID: 12153979.
- Laffey J, Engelberts D, Kavanach. Buffering hypercapnic acidosis worsens acute lung injury. Am J respir Crit Care Med. 2000;161;141-146.
- Lang JD Jr, Chumley P, Eiserich JP, Estevez A, Bamberg T, Adhami A, Crow J, Freeman BA. Hypercapnia induces injury to alveolar epithelial cells via a nitric oxide-dependent pathway. Am J Physiol Lung Cell Mol Physiol. 2000 Nov;279(5):L994-1002. doi: 10.1152/ajplung.2000.279.5.L994. PMID: 11053037.
- Zhu S, Basiouny KF, Crow JP, Matalon S. Carbon dioxide enhances nitration of surfactant protein A by activated alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2000 May;278(5):L1025-31. doi: 10.1152/ajplung.2000.278.5.L1025. PMID: 10781434.
- Gores GJ, Nieminen AL, Fleishman KE, Dawson TL, Herman B, Lemasters JJ. Extracellular acidosis delays onset of cell death in ATP-depleted hepatocytes. Am J Physiol. 1988 Sep;255(3 Pt 1):C315-22. doi: 10.1152/ajpcell.1988.255.3.C315. PMID: 3421314.
- Nin N, Muriel A, Peñuelas O, Brochard L, Lorente JA, Ferguson ND, Raymondos K, Ríos F, Violi DA, Thille AW, González M, Villagomez AJ, Hurtado J, Davies AR, Du B, Maggiore SM, Soto L, D'Empaire G, Matamis D, Abroug F, Moreno RP, Soares MA, Arabi Y, Sandi F, Jibaja M, Amin P, Koh Y, Kuiper MA, Bülow HH, Zeggwagh AA, Anzueto A, Sznajder JI, Esteban A; VENTILA Group. Severe hypercapnia and outcome of mechanically ventilated patients with moderate or severe acute respiratory distress syndrome. Intensive Care Med. 2017 Feb;43(2):200-208. doi: 10.1007/s00134-016-4611-1. Epub 2017 Jan 20. PMID: 28108768; PMCID: PMC5630225.
- Marini JJ, Hotchkiss J, Broccard A. Bench-to-bedside review: Microvascular and airspace linkage in ventilator-induced lung injury. Critical Care. 2003;7:435-444.
- Broccard AF, Hotchkiss JR, Suzuki S, Olson D, Marini JJ. Effects of mean airway pressure and tidal excursion on lung injury induced by mechanical ventilation in an isolated perfused rabbit lung model. Crit Care Med. 1999 Aug;27(8):1533-41. doi: 10.1097/00003246-199908000-00022. PMID: 10470761.
- Broccard AF, Hotchkiss JR, Kuwayama N, Olson DA, Jamal S, Wangensteen DO, Marini JJ. Consequences of vascular flow on lung injury induced by mechanical ventilation. Am J Respir Crit Care Med. 1998 Jun;157(6 Pt 1):1935-42. doi: 10.1164/ajrccm.157.6.9612006. PMID: 9620930.
- Hotchkiss JR Jr, Blanch L, Murias G, Adams AB, Olson DA, Wangensteen OD, Leo PH, Marini JJ. Effects of decreased respiratory frequency on ventilator-induced lung injury. Am J Respir Crit Care Med. 2000 Feb;161(2 Pt 1):463-8. doi: 10.1164/ajrccm.161.2.9811008. PMID: 10673186.
- Hotchkiss JR Jr, Blanch L, Naveira A, Adams AB, Carter C, Olson DA, Leo PH, Marini JJ. Relative roles of vascular and airspace pressures in ventilator-induced lung injury. Crit Care Med. 2001 Aug;29(8):1593-8. doi: 10.1097/00003246-200108000-00016. PMID: 11505134.
- Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med. 1998 Jan;157(1):294-323. doi: 10.1164/ajrccm.157.1.9604014. PMID: 9445314.
- Dreyfuss D, Saumon G. Role of tidal volume, FRC, and end-inspiratory volume in the development of pulmonary edema following mechanical ventilation. Am Rev Respir Dis. 1993 Nov;148(5):1194-203. doi: 10.1164/ajrccm/148.5.1194. PMID: 8239153. 45.
- Broccard A, Vannay Ch, Feihl F, Schaller M. Impact of low pulmonary vascular pressure on ventilator induced lung injury. Crit Care Med. 2002;30(10);2183-2190.
- West JB. Regional differences in gas exchange in the lung of erect man. J Appl Physiol. 1962;17:893-901.
- Potus F, Mai V, Lebret M, Malenfant S, Breton-Gagnon E, Lajoie AC, Boucherat O, Bonnet S, Provencher S. Novel insights on the pulmonary vascular consequences of COVID-19. Am J Physiol Lung Cell Mol Physiol. 2020 Aug 1;319(2):L277-L288. doi: 10.1152/ajplung.00195.2020. Epub 2020 Jun 17. PMID: 32551862; PMCID: PMC7414237.
- Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020 Jul 9;383(2):120-128. doi: 10.1056/NEJMoa2015432. Epub 2020 May 21. PMID: 32437596; PMCID: PMC7412750.
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002 Feb 21;346(8):557-63. doi: 10.1056/NEJMoa003289. PMID: 11856794.
- McCormick PH, Chen G, Tlerney S, Kelly CJ, Bouchier-Hayes DJ. Clinically relevant thermal preconditioning attenuates ischemia-reperfusion injury. J Surg Res. 2003 Jan;109(1):24-30. doi: 10.1016/s0022-4804(02)00035-5. PMID: 12591231.
- Yoo CG, Lee S , Lee CT. Anti-inflammatory effect of HSP induction. J Immu. 2000;164;5416.
- Koh Y, Lim CM, Kim MJ, Shim TS, Lee SD, Kim WS, Kim DS, Kim WD. Heat shock response decreases endotoxin-induced acute lung injury in rats. Respirology. 1999 Dec;4(4):325-30. doi: 10.1046/j.1440-1843.1999.00200.x. PMID: 10612564.
- Ribeiro SP, Rhee K, Tremblay L, Veldhuizen R, Lewis JF, Slutsky AS. Heat stress attenuates ventilator-induced lung dysfunction in an ex vivo rat lung model. Am J Respir Crit Care Med. 2001 May;163(6):1451-6. doi: 10.1164/ajrccm.163.6.9908076. PMID: 11371417.
- Villar J, Ribeiro SP, Mullen JB, Kuliszewski M, Post M, Slutsky AS. Induction of the heat shock response reduces mortality rate and organ damage in a sepsis-induced acute lung injury model. Crit Care Med. 1994 Jun;22(6):914-21. PMID: 8205824.
- Silva PL, Moraes L, Santos RS, Samary C, Ramos MB, Santos CL, Morales MM, Capelozzi VL, Garcia CS, de Abreu MG, Pelosi P, Marini JJ, Rocco PR. Recruitment maneuvers modulate epithelial and endothelial cell response according to acute lung injury etiology. Crit Care Med. 2013 Oct;41(10):e256-65. doi: 10.1097/CCM.0b013e31828a3c13. PMID: 23887231.
- Felix NS, Samary CS, Cruz FF, Rocha NN, Fernandes MVS, Machado JA, Bose-Madureira RL, Capelozzi VL, Pelosi P, Silva PL, Marini JJ, Rocco PRM. Gradually Increasing Tidal Volume May Mitigate Experimental Lung Injury in Rats. Anesthesiology. 2019 May;130(5):767-777. doi: 10.1097/ALN.0000000000002630. PMID: 30870161.
- Fernandes MVS, Rocha NN, Felix NS, Rodrigues GC, Silva LHA, Coelho MS, Fonseca ACF, Teixeira ACGM, Capelozzi VL, Pelosi P, Silva PL, Marini JJ, Rocco PRM. A more gradual positive end-expiratory pressure increase reduces lung damage and improves cardiac function in experimental acute respiratory distress syndrome. J Appl Physiol (1985). 2022 Feb 1;132(2):375-387. doi: 10.1152/japplphysiol.00613.2021. Epub 2021 Dec 23. PMID: 34941443.
- Xavier PH, Fernandes Fonseca AC, Gonçalves LA, de Sousa GC, Coelho da Silva M, de Magalhães Sacramento RF, Dos Santos Samary C, Medeiros M, Cruz FF, Capelozzi VL, Felix NS, Pelosi P, Marini JJ, Rocco PRM, Silva PL. Lung injury is induced by abrupt increase in respiratory rate but prevented by recruitment maneuver in mild acute respiratory distress syndrome in rats. Anesthesiology. 2022 Dec 26. doi: 10.1097/ALN.0000000000004479. Epub ahead of print. PMID: 36571572.
- Suzuki S, Hotchkiss JR, Toshimichi T, Olson D, Adams AB, Marini JJ. Effect of core body temperature on ventilator-induced lung injury. Crit Care Med. 2004;32(1):144-149.
- Akinci OI, Celik M, Mutlu GM, Martino JM, Tugrul S, Ozcan PE, Yilmazbayhan D, Yeldandi AV, Turkoz KH, Kiran B, Telci L, Cakar N. Effects of body temperature on ventilator-induced lung injury. J Crit Care. 2005 Mar;20(1):66-73. doi: 10.1016/j.jcrc.2004.11.001. PMID: 16015518.
- Hong SB, Koh Y, Lee IC, Kim MJ, Kim WS, Kim DS, Kim WD, Lim CM. Induced hypothermia as a new approach to lung rest for the acutely injured lung. Crit Care Med. 2005 Sep;33(9):2049-55. doi: 10.1097/01.ccm.0000178186.37167.53. PMID: 16148479.
- Marini JJ, Gattinoni L. Propagation prevention: a complementary mechanism for "lung protective" ventilation in acute respiratory distress syndrome. Crit Care Med. 2008 Dec;36(12):3252-8. doi: 10.1097/CCM.0b013e31818f0e68. PMID: 18936705.
- Marini JJ. Can we prevent the spread of focal lung inflammation? Crit Care Med. 2010 Oct;38(10 Suppl):S574-81. doi: 10.1097/CCM.0b013e3181f20f01. PMID: 21164400.
- Setzer F, Oschatz K, Hueter L, Schmidt B, Schwarzkopf K, Schreiber T. Susceptibility to ventilator induced lung injury is increased in senescent rats. Crit Care. 2013 May 27;17(3):R99. doi: 10.1186/cc12744. PMID: 23710684; PMCID: PMC4056597.
- Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM. The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010 Oct;38(10):1947-53. doi: 10.1097/CCM.0b013e3181ef4460. PMID: 20639743.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.