Medicine Group . 2022 December 24;3(12):1511-1521. doi: 10.37871/jbres1627.
Characteristics of the Gut Microbiota in Rheumatic Diseases and Promising Therapeutic Strategies
Shangui Zhang and Xuyan Yang*
- Gut microbiota
- Dysbiosis
- Rheumatoid arthritis
- Systemic lupus erythematosus
- Ankylosing spondylitis
- Fecal microbiota transplantation
Abstract
Over the past twenty years, the link between gut microbes and human autoimmune diseases has attracted extensive attention. Available studies have shown that alterations in gut microbiota are closely related to the development of autoimmune diseases. A variety of factors may influence gut microecology and lead to gut dysbiosis, which may influence systemic inflammatory or autoimmune responses through mechanisms such as aberrant microbial translocation, molecular mimicry, and regulatory T cells/ T helper 17 cells imbalance, then lead to autoimmune diseases. Rheumatic diseases are a group of chronic diseases involving bones and joints, their surrounding soft tissues, and other related tissues and organs. The etiology is varied and the pathogenesis is unclear, but most of them are closely related to autoimmune reactions. This review introduces the alterations of gut microbiota in several common rheumatic diseases and focuses on the promise of antibiotics, probiotics, and fecal microbiota transplantation in rheumatic diseases.
Abbreviations
RA: Rheumatoid Arthritis; HCs: Healthy Controls; PAD: Peptidyl Arginine Deiminase; Acpas: Anti-Citrullinated Protein Antibodies; SLE: Systemic Lupus Erythematosus; F/B: Firmicutes/Bacteroidetes; RG: Ruminococcus gnavus; LN: Lupus Nephritis; SLEDAI: SLE Disease Activity Index; ANA: Anti-Nuclear Antibody; GF: Germ-Free; SFB: Segmented Filamentous Bacteria; AS: Ankylosing Spondylitis; IBD: Inflammatory Bowel Disease; Treg: Regulatory T Cells; Th17: T Helper 17 Cells; E. gallinarum: Enterococcus gallinarum; IFN: Interferon; MLN: Mesenteric Lymph Nodes; GI: Gastrointestinal; IL: Interleukin; CIA: Collagen-Induced Arthritis; Tcrs: T-Cell Receptors; Scfas: Short-Chain Fatty Acids; L. reuteri: Lactobacillus reuteri; pdc: Plasmacytoid Dendritic Cell; APC: Antigen-Presenting Cell; Th1: T Helper 1 Cell; L. animalis: Lactobacillus animalis; TGF-b: Transforming Growth Factor b; FMT: Fecal Microbiota Transplantation; AIA: Adjuvant-Induced Arthritis; ESR: Erythrocyte Sedimentation Rate; CRP: C-Reactive Protein; T1D: Type 1 Diabetes; DAS-28: Disease Activity Score-28; RF: Rheumatoid Factor; HAQ-DI: Health Assessment Questionnaire Disability Index; SRI-4: SLE Responder Index-4
Introduction
Microbiota is defined as the ecological communities of commensal, symbiotic, and pathogenic microorganisms living in and on the human body [1]. Abundant and genetically diverse microorganisms are colonized in our body, whose total number is about 100 trillion, outnumbering human cells tenfold [2]. The human gut is the richest in microorganisms, whose number of genes is ~150 times larger than those of humans [3].
The relationship between microorganisms and human hosts is mutually beneficial, the gut microbiota provides us with fundamental protective, metabolic and immune capacities, such as food processing, vitamin synthesis, maintenance of host nutrition and energy balance, and regulation of immunity. In turn, the human body provides a suitable environment and essential nutrients for them [4,5]. Many investigations have demonstrated that gut microbiota is crucial in the normal development and maturation of the immune system [6-12], and gut microbiota dysbiosis can lead to immune dysregulation and various autoimmune diseases [13-19].
Rheumatic diseases are a group of autoimmune disorders of unknown pathogenesis, and genetic and environmental factors (including microbes) are possible reasons. Currently, numerous studies have explored the role of microbiota in autoimmune diseases and demonstrated an evident correlation between them. The review aims to explore gut microbiota alterations in several common rheumatic diseases and the prospects for microbiologically relevant therapeutic strategies.
Gut Microbiota and Rheumatic Diseases
Rheumatoid arthritis (RA)
RA is a chronic, systemic autoimmune disorder with erosive, symmetrical polyarthritis as the main clinical manifestation, which can eventually lead to joint deformity and loss of function. The etiology of RA is not fully understood, and it is currently thought to be related to genetic and environmental factors.
Many studies have demonstrated a correlation between periodontal disease and RA. Periodontal disease is more prevalent and more symptomatic in RA patients compared with Healthy Controls (HCs) [20]. Symptoms of RA also decrease after treatment for periodontal disease [21]. Porphyromonas gingivalis, a periodontopathic bacterium, can produce Peptidyl Arginine Deiminase (PAD), an enzyme that can convert arginine to citrulline [22,23]. Interestingly, under certain conditions, the citrullination of proteins is associated with the production of Anti-Citrullinated Protein Antibodies (ACPAs) [24,25]. Therefore, P. gingivalis may induce the production of ACPAs, which are highly specific for RA.
Currently, numerous research has confirmed the existence of gut dysbiosis in RA patients. Research shows that RA patients have a reduced microbial diversity than HCs, which is linked to autoantibody levels and disease duration. And the microbial profile of RA is characterized by the reduction of abundant taxa and expansion of rare taxa and Actinobacteria [26]. In new-onset untreated RA patients, the abundance of Prevotella was increased and the number of Bacteroides was decreased, and Prevotella (Prevotella copri) was closely related to the disease [17]. In addition, a metagenomic analysis demonstrated that Lactobacillus csalivarius was overabundant, while Haemophilus spp. were depleted in RA fecal samples [27]. Furthermore, it was found that both Prevotella copri and Collinsella, which were enriched in RA patients, exacerbated disease severity in mouse model of arthritis, indicating that these two bacteria may be involved in the development of RA [26,28].
Systemic Lupus Erythematosus (SLE)
SLE is a systemic autoimmune disease that mainly affects women of childbearing age, characterized by the formation of immune complexes and pathogenic autoantibodies. The pathogenesis of SLE remains elusive, however, increasing evidence suggests that gut dysbiosis is involved in SLE development.
Hevia, et al. [29] conducted the first human study, analyzing stool samples from 20 SLE patients and 20 HCs, and found that SLE patients had a lower Firmicutes/Bacteroidetes (F/B) ratio. Later, a larger human study including 45 SLE patients and 48 HCs also observed a decreased F/B ratio in SLE patients. At the genus level, a prevalence of Rhodococcus, Prevotella, Eggerthella, Flavonifractor, Eubacterium, and Klebsiella and a decline of Dialister and Pseudobutyrivibrio were observed and suggested a gut microbiota profile for SLE patients [30]. Recently, a study demonstrated that SLE patients had a fivefold increase of Ruminococcus gnavus (RG) in the gut compared with controls, and expansion of RG was positively correlated with overall disease activity and was most prominent in those with Lupus Nephritis (LN) [31]. In addition, it was found that in patients with SLE, the abundance of Clostridium species ATCC BAA-442 was positively correlated with the SLE Disease Activity Index (SLEDAI) score [32].
A previous study in lupus-prone mice observed an enrichment of Lachnospiraceae and a higher abundance of Lachnospiraceae was linked to the earlier onset of lupus and more severe symptoms [33]. Furthermore, an unexpected connection was found between gut microbiota and Anti-Nuclear Antibody (ANA). Germ-free (GF) lymphotoxin-deficient mice mono-colonized with Segmented Filamentous Bacteria (SFB) were found to produce more ANAs than lymphotoxin-deficient controls mono-colonized with Escherichia coli, which demonstrated that ANA production is influenced by gut commensals, particularly increased colonization of SFB. This study indicates that neonatal colonization of the gut can influence systemic autoimmunity in adult life [34].
Ankylosing Spondylitis (AS)
AS is a spondyloarthropathy that mainly affects the axial skeleton and is characterized by inflammatory low back pain. In severe cases, spinal stiffness and deformity may occur. In China, AS has a prevalence of about 0.20-0.40%, with almost 80% of patients being young adults [35,36]. Regarding pathogenesis, it is currently believed that it is mainly due to the combined effect of genetic and environmental factors.
HLA-B27 is a major genetic risk factor for AS [37]. About 90% of AS patients are HLA-B27 positive, however, in all HLA-B27 positive individuals, less than 5% are affected [38]. Studies suggest that HLA-B27 may induce the development of AS by affecting the gut microbiota. A study conducted in transgenic rats found that HLA-B27 was correlated with alterations in the cecal microbiota [39]. A subsequent study observed significantly different microbiota composition between HLA-B27-positive and HLA-B27-negative healthy individuals, indicating that genetic background may affect gut microbial composition [40].
Research has demonstrated that gut microbiota is involved in the pathogenesis of AS. For example, HLA-B27 transgenic rats did not develop inflammation of the gut and joints in a GF environment, however, when they were exposed to normal gut bacteria, colitis and arthritis would appear [41]. In addition, in AS patients, the distinctive fecal microbiota feature is associated with levels of fecal calprotectin, a marker of gut inflammation [42].
Currently, increasing studies have shown the presence of gut dysbiosis in AS patients. An earlier study found a significant increase in the proportion of sulfate-reducing bacteria and a decrease in the abundance of Clostridium leptum in the stools of patients with AS compared with HCs [43]. Recently, A study identified terminal ileum dysbiosis in AS patients. Specifically, compared with HCs, Lachnospiraceae, Bacteroidaceae, Porphyromonadaceae, Rikenellaceae, and Ruminococcaceae increased in abundance, while Veillonellaceae and Prevotellaceae decreased in abundance [44]. Similar to alterations observed in SLE [31], Breban, et al. [40] found that AS patients have a 2-fold to 3-fold increase in RG abundance compared with HCs, which was significant and paralleled with disease activity in patients having an Inflammatory Bowel Disease (IBD) history. In addition, Chen, et al. [45] revealed that AS patients with different phenotypes have specific gut microbiota alterations separately. They found that Prevotella_2 was more abundant in axial AS patients, while Collinsella, Streptococcus, and Comamonas were more enriched in peripheral AS patients. In this study, an 8 genera-based model (a classification model based on gut microbial characteristics) could accurately distinguish AS patients from HCs, which might be instructive in future clinical diagnosis.
Mechanisms of gut dysbiosis related to autoimmune diseases
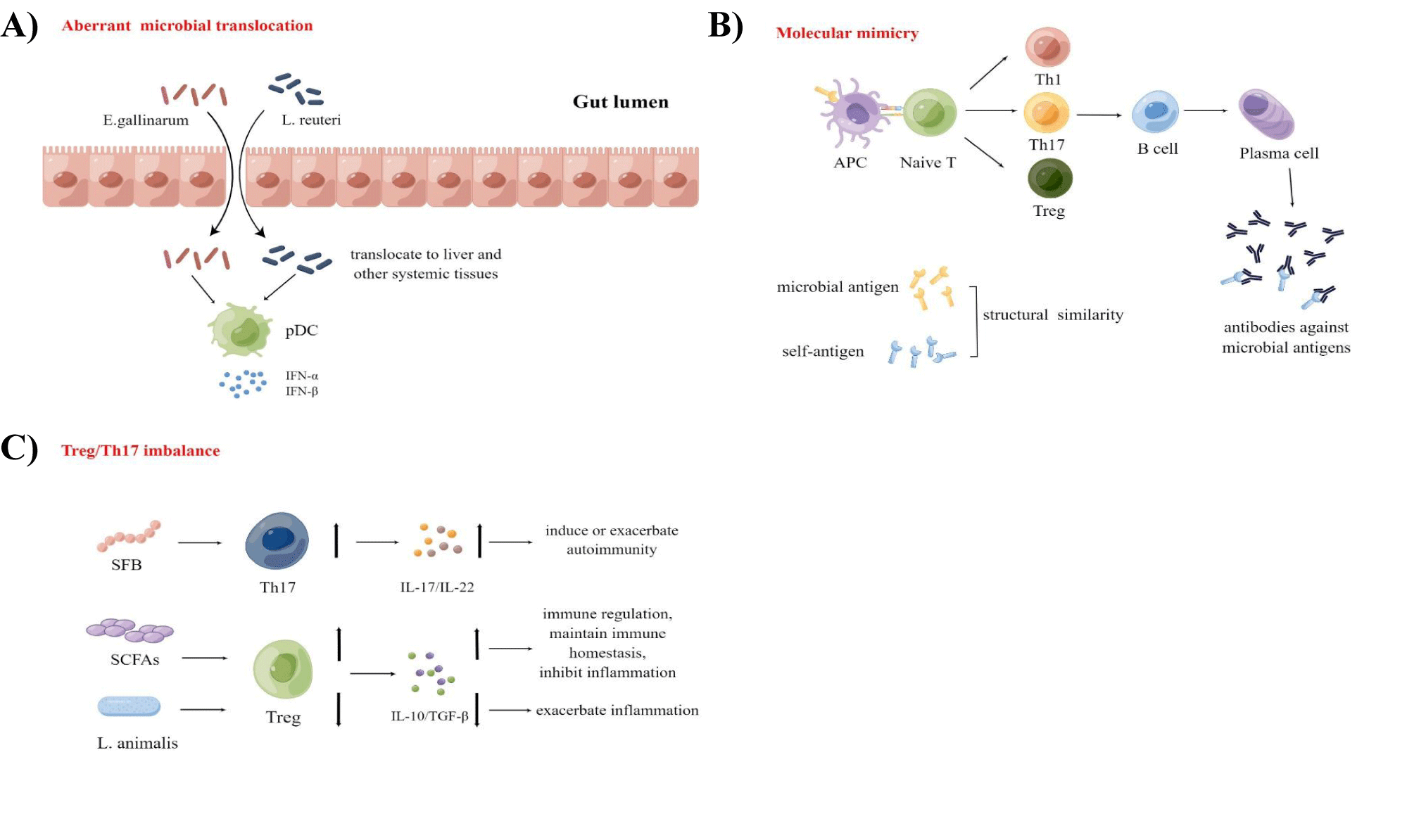
Recently, increasing studies have demonstrated that gut microbiota dysbiosis may induce autoimmune disease via certain mechanisms, which mainly include aberrant microbial translocation, molecular mimicry, and regulatory T cells/ T helper 17 cells (Treg/Th17) imbalance (Figure 1).
Aberrant microbial translocation
An intact gut barrier helps prevent the over-activation of the immune system, maintaining the balance between gut microbes and host immunity. Gut commensals and their components translocate to other tissues or organs outside the gut when the gut barrier is compromised, triggering autoimmunity by interacting with the immune system abnormally [46] (Figure 1A).
Recently, Enterococcus gallinarum (E. gallinarum), a gut pathobiont, was detected in liver biopsies of patients with SLE or autoimmune hepatitis, but not in healthy individuals [47]. The study demonstrated that E. gallinarum translocated from the gut into the liver and other systemic tissues, then interacted with the host immune system, activating the type I Interferon (IFN) pathway and inducing the production of autoantibodies [47]. Moreover, in mono-colonized and autoimmune-prone mice, pathobiont translocation could induce autoantibodies and cause mortality. However, using a vaccine against E. gallinarum could prevent translocation, reduce autoantibodies and increase survival in mice [47]. Additionally, Lactobacillus reuteri, which is enriched in the gut of lupus-prone mice, can also translocate to the liver, spleen, and Mesenteric Lymph Nodes (MLN), then engage type I IFN pathways and aggravate lupus-like symptoms [48]. The above findings suggest that enteric pathogens can translocate to organs outside the gut and trigger or promote autoimmunity.
Molecular mimicry
In the development of autoimmunity, molecular mimicry is considered an important mechanism due to the sequence similarity between certain microbial peptides and autoantigens [46] (Figure 1B).
Many commensals present in the skin, oral cavity, and Gastrointestinal (GI) tract were found to express orthologs of the human Ro60 autoantigen, an RNA-binding autoantigen targeted in SLE [49,50]. In SLE patients, commensals could trigger autoimmune responses through cross-reactivity of T cells (which were commensal-reactive) with Ro60 autoantigen. For example, Bacteroides thetaiotaomicron can induce lupus-like symptoms via molecular mimicry, because it is the ortholog of human autoantigen Ro60 [49]. In addition, Roseburia intestinalis can induce autoimmunity via molecular mimicry as well, due to the homology of its peptide with β2-glycoprotein I [51].
A recent study revealed that in SLE patients, the expansion of RG was positively correlated with increased disease activity and LN [31]. In eight strains of RG, RG2 cell wall lipoglycans have antigenic properties, which could react with native DNA, and trigger an anti-ds DNA antibody response [31]. The findings above indicate that SLE and LN may be triggered or aggravated by molecular mimicry between RG strain and native DNA molecules [52]. Recently, a stool analysis using metagenomic shotgun sequencing found that in untreated AS patients, gut microbiota was disturbed, and some enriched species may trigger autoimmunity through molecular mimicry. Specifically, AS-enriched species include Acidaminococcus fermentans, Parabacteroides distasonis, Prevotella copri, Eubacterium siraeum, and Bacteroides coprophilus. Bacterial peptides of these species could induce the increase of IFN-γ producing cells via mimicking type II collagen [53].
Treg/Th17 imbalance
The imbalance between pro-inflammatory Th17 cells and anti-inflammatory Treg cells is also one of the important mechanisms involved in autoimmunity development, with a reduced Treg/Th17 cell ratio leading to an exacerbation of autoimmunity [46] (Figure 1C).
Recently, increasing investigations have shown that rheumatic diseases are related to Treg/Th17 imbalance. For example, selective depletion of Treg cells leads to the worsening of delayed-type hypersensitivity arthritis in C57BL/6 mice, which could be counteracted by an anti-interleukin-17 (IL-17) monoclonal antibody, which was used for IL-17 blockade [54]. IL-17 is a major pro-inflammatory cytokine, produced mainly by Th17 cells [55]. Furthermore, multiple investigations have shown that gut microbiota is linked to Treg/Th17 imbalance. Maeda, et al. [28] transferred feces from RA patients (with altered gut microbiota) to GF arthritis-prone SKG mice and found that gut dysbiosis activated autoreactive T cells in mice, increased intestinal Th17 cells, and caused joint inflammation. Under GF conditions, both Th17 cells in the gut and the severity of arthritis were reduced in the K/BxN arthritis mouse model, but these could be restored after ingestion of SFB [16]. Additionally, in Collagen-Induced Arthritis (CIA) models, SFB could trigger lung autoimmunity by inducing Gut-Lung Axis Th17 cells expressing dual T-cell Receptors (TCRs) [56].
Among the gut microbial metabolites, Short-Chain Fatty Acids (SCFAs) derived from the breakdown of dietary fiber by bacteria, are the most studied and considered important in Treg/Th17 balance. SCFAs can enhance intestinal integrity and inhibit intestinal inflammation through mechanisms such as Treg cell induction [57]. A recent study showed that administration of butyrate (one of the SCFAs) in the CIA mouse model suppressed arthritis by increasing systematic Treg cells, decreasing Th17 cells, and inhibiting inflammatory cytokine expression [58].
Microbial-related therapeutic strategies
In recent years, probiotics, antibiotics, as well as Fecal Microbiota Transplantation (FMT) have shown good prospects in the treatment of rheumatic diseases by regulating gut microbiota and promoting the balance of gut microecology.
Probiotics
Probiotics are living microorganisms that can bring health benefits to the host when administered in sufficient quantities [59. Studies have revealed that in RA patients, Lactobacillus casei 01 and Lactobacillus acidophilus supplementation have improved disease activity and inflammatory status [60,61]. Faecalibacterium, a butyrate-producing bacteria, can reduce the occurrence of RA by maintaining the integrity of gut epithelium and anti-inflammatory properties [62-64]. In Adjuvant-Induced Arthritis (AIA) rat model, Lactobacillus casei (ATCC334) ameliorated gut dysbiosis, markedly inhibited the induction of arthritis, and protected bones from damage [65]. In addition, Prevotella histicola, a unique commensal bacterium, administered enterally could suppress HLA-DQ8 mice arthritis via mucosal regulation [66]. In SLE patients, it was found that Lactobacillus spp. depletion was greatest before disease onset, suggesting that Lactobacillus may be involved in preventing SLE [67]. A previous study in young, female lupus-prone mice also observed a significant decrease in lactobacilli. The study also showed that retinoic acid could restore lactobacilli in lupus-prone mice and improve symptoms, which suggests that intaking of dietary supplement retinoic acid and probiotic lactobacilli may be promising in relieving inflammatory flares in SLE patients [33]. In the early days, Jenks and colleagues [68] examined the influence of orally administered probiotics on patients with active spondyloarthritis. However, in this study, probiotics did not demonstrate a noticeable benefit over placebo. At present, the research on probiotics in AS is not mature enough, and further research is needed to explore the impact of probiotics on AS.
Antibiotics
In the early stage, the Netherlands and the United States conducted three large, double-blind, placebo-controlled studies to realize the impact of minocycline on RA patients [69-71]. Two of the studies targeted RA patients with a long history [69,70], and the third study targeted early RA patients [71]. All three studies showed that minocycline was superior to placebo in several laboratory and clinical parameters, including hemoglobin level, Erythrocyte Sedimentation Rate (ESR), and joint swelling and tenderness. There is no study on the treatment of SLE patients with antibiotics at present, but there are relevant animal experiments. Studies conducted in MRL/LPR mice and NZB/WF1 lupus mice found that treatment with broad-spectrum antibiotics or vancomycin could clear harmful bacteria in the gut, improve the gut barrier function, and thus improve lupus-like symptoms [47,72]. In addition, antibiotic treatment can enrich probiotics and alleviate Treg/Th17 imbalance in lupus mice [72]. However, two other studies showed that antibiotic treatment had no significant effect on gut microbiota and lupus progression in lupus mice [73], and even aggravated lupus-like disease in mice [74] A previous study has shown that after 12 weeks of treatment with moxifloxacin, AS patients had a significant attenuation in inflammatory symptoms and a marked reduction in the mean of ESR and C-Reactive Protein (CRP) [75]. Recently, a study demonstrated that rifaximin significantly attenuated symptoms of AS mice and down-regulated inflammatory factors. Rifaximin also changed the gut microbiota composition, increasing the ratio of Bacteroidetes/Firmicutes, and selectively enhancing some probiotic populations, including Lactobacillus [76].
In summary, antibiotics may be a novel treatment strategy for patients with rheumatic diseases, but a large number of clinical studies are still needed for further discussion.
FMT
Nowadays, the therapeutic potential of FMT in autoimmune diseases is constantly being investigated, since gut dysbiosis is a key characteristic of most autoimmune diseases.
FMT is the transfer of fecal bacteria from a healthy donor into the recipient's GI tract to alter the recipient's microbial composition and provide health benefits [77]. Earlier studies demonstrated that recurrent Clostridium difficile infections can be effectively treated with FMT. Recently, a randomized controlled trial demonstrated that in patients with new-onset Type 1 Diabetes (T1D), FMT could halt the decrease of endogenous insulin production and stabilize residual beta cell function [78]. Recently, A case report [79] has shown that a young patient with a 5-year history of RA was admitted to the hospital for active RA flare and was successfully cured with FMT, which manifested as a decrease in Disease Activity Score-28 (DAS-28) and Rheumatoid Factor (RF) and improvement of the health assessment questionnaire Disability Index (HAQ-DI). For now, no more data is available regarding the effect of FMT on RA patients, however, relevant clinical trials are in progress. Recently, an explorer clinical trial evaluated the safety and efficacy of FMT in SLE patients [80]. The results demonstrated that FMT recipients had no serious adverse effects and 42.12% of patients reached the SLE Responder Index-4 (SRI-4) primary outcome. Moreover, SCFAs-producing bacteria were significantly increased in FMT recipients, while inflammation-related bacteria decreased. For now, no data is available regarding the effect of FMT on AS patients.
The results above indicate that FMT may be a safe and feasible treatment option for patients with rheumatic diseases. However, to fully evaluate FMT's safety and efficacy, larger randomized trials are needed.
Conclusion
At present, microbiology-related research is very popular in medical disciplines. Although there have been many studies in this area, the real causal relationship between microorganisms and the occurrence and development of disease is still unclear. The pathogenesis of rheumatic diseases is considered to be associated with genetic and environmental factors. Studies have shown that there is a close relationship between gut microbiota and rheumatic diseases. Several mechanisms have been proposed to explain the role of gut microbiota in rheumatic diseases, such as aberrant microbial translocation, molecular mimicry, and Treg/Th17 imbalance. However, these cannot fully explain the pathogenesis of rheumatic diseases, and related researches are still in progress.
Antibiotics, probiotics, FMT, and other therapeutic strategies through the manipulation of gut microbiota have great therapeutic potential in rheumatic diseases. These treatments may help improve the symptoms of patients, reduce the occurrence of serious complications, and even prevent the occurrence of diseases. However, these are still in the primary stage and need further exploration.
Acknowledgment
Figures were created by Figdraw.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Lederberg J. Infectious history. Science. 2000 Apr 14;288(5464):287-93. doi: 10.1126/science.288.5464.287. PMID: 10777411.
- Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107-33. doi: 10.1146/annurev.mi.31.100177.000543. PMID: 334036.
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010 Mar 4;464(7285):59-65. doi: 10.1038/nature08821. PMID: 20203603.
- Littman DR, Pamer EG. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe. 2011 Oct 20;10(4):311-23. doi: 10.1016/j.chom.2011.10.004. PMID: 22018232; PMCID: PMC3202012.
- Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: Gut microbiota: the neglected endocrine organ. Mol Endocrinol. 2014 Aug;28(8):1221-38. doi: 10.1210/me.2014-1108. PMID: 24892638.
- Bouskra D, Brézillon C, Bérard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008 Nov 27;456(7221):507-10. doi: 10.1038/nature07450. PMID: 18987631.
- Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004 Jun;4(6):478-85. doi: 10.1038/nri1373. PMID: 15173836.
- Crabbé PA, Bazin H, Eyssen H, Heremans JF. The normal microbial flora as a major stimulus for proliferation of plasma cells synthesizing IgA in the gut. The germ-free intestinal tract. Int Arch Allergy Appl Immunol. 1968 34(4):362-75. doi: 10.1159/000230130. PMID: 4176641.
- Ivanov, II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009 Oct 30;139(3):485-98. doi: 10.1016/j.cell.2009.09.033. PMID: 19836068.
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov, II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011 Jan 21;331(6015):337-41. doi: 10.1126/science.1198469. PMID: 21205640.
- Hooijkaas H, Benner R, Pleasants JR, Wostmann BS. Isotypes and specificities of immunoglobulins produced by germ-free mice fed chemically defined ultrafiltered "antigen-free" diet. Eur J Immunol. 1984 Dec;14(12):1127-30. doi: 10.1002/eji.1830141212. PMID: 6083871.
- Bauer H, Horowitz RE, Levenson SM, Popper H. The response of the lymphatic tissue to the microbial flora. Studies on germfree mice. Am J Pathol. 1963 Apr;42(4):471-83. doi. PMID: 13966929.
- Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, Punit S, Karlsson M, Bry L, Glickman JN, Gordon JI, Onderdonk AB, Glimcher LH. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010 Sep 16;8(3):292-300. doi: 10.1016/j.chom.2010.08.004. PMID: 20833380.
- Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012 Oct 4;490(7418):55-60. doi: 10.1038/nature11450. PMID: 23023125.
- Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008 Oct 23;455(7216):1109-13. doi: 10.1038/nature07336. PMID: 18806780.
- Wu HJ, Ivanov, II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010 Jun 25;32(6):815-27. doi: 10.1016/j.immuni.2010.06.001. PMID: 20620945.
- Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, Rostron T, Cerundolo V, Pamer EG, Abramson SB, Huttenhower C, Littman DR. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013 Nov 5;2:e01202. doi: 10.7554/eLife.01202. PMID: 24192039.
- Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2011 Mar 15;108 Suppl 1(Suppl 1):4615-22. doi: 10.1073/pnas.1000082107. PMID: 20660719.
- Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011 Oct 26;479(7374):538-41. doi: 10.1038/nature10554. PMID: 22031325.
- Wolff B, Berger T, Frese C, Max R, Blank N, Lorenz HM, Wolff D. Oral status in patients with early rheumatoid arthritis: a prospective, case-control study. Rheumatology (Oxford). 2014 Mar;53(3):526-31. doi: 10.1093/rheumatology/ket362. PMID: 24273047.
- Al-Katma MK, Bissada NF, Bordeaux JM, Sue J, Askari AD. Control of periodontal infection reduces the severity of active rheumatoid arthritis. J Clin Rheumatol. 2007 Jun;13(3):134-7. doi: 10.1097/RHU.0b013e3180690616. PMID: 17551378.
- McGraw WT, Potempa J, Farley D, Travis J. Purification, characterization, and sequence analysis of a potential virulence factor from Porphyromonas gingivalis, peptidylarginine deiminase. Infect Immun. 1999 Jul;67(7):3248-56. doi: 10.1128/iai.67.7.3248-3256.1999. PMID: 10377098.
- Rosenstein ED, Greenwald RA, Kushner LJ, Weissmann G. Hypothesis: the humoral immune response to oral bacteria provides a stimulus for the development of rheumatoid arthritis. Inflammation. 2004 Dec;28(6):311-8. doi: 10.1007/s10753-004-6641-z. PMID: 16245073.
- Liao F, Li Z, Wang Y, Shi B, Gong Z, Cheng X. Porphyromonas gingivalis may play an important role in the pathogenesis of periodontitis-associated rheumatoid arthritis. Med Hypotheses. 2009 Jun;72(6):732-5. doi: 10.1016/j.mehy.2008.12.040. PMID: 19246161.
- Hitchon CA, Chandad F, Ferucci ED, Willemze A, Ioan-Facsinay A, van der Woude D, Markland J, Robinson D, Elias B, Newkirk M, Toes RM, Huizinga TW, El-Gabalawy HS. Antibodies to Porphyromonas gingivalis are associated with anticitrullinated protein antibodies in patients with rheumatoid arthritis and their relatives. J Rheumatol. 2010 Jun;37(6):1105-12. doi: 10.3899/jrheum.091323. PMID: 20436074.
- Chen J, Wright K, Davis JM, Jeraldo P, Marietta EV, Murray J, Nelson H, Matteson EL, Taneja V. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016 Apr 21;8(1):43. doi: 10.1186/s13073-016-0299-7. PMID: 27102666.
- Zhang X, Zhang D, Jia H, Feng Q, Wang D, Liang D, Wu X, Li J, Tang L, Li Y, Lan Z, Chen B, Li Y, Zhong H, Xie H, Jie Z, Chen W, Tang S, Xu X, Wang X, Cai X, Liu S, Xia Y, Li J, Qiao X, Al-Aama JY, Chen H, Wang L, Wu QJ, Zhang F, Zheng W, Li Y, Zhang M, Luo G, Xue W, Xiao L, Li J, Chen W, Xu X, Yin Y, Yang H, Wang J, Kristiansen K, Liu L, Li T, Huang Q, Li Y, Wang J. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 2015 Aug;21(8):895-905. doi: 10.1038/nm.3914. PMID: 26214836.
- Maeda Y, Kurakawa T, Umemoto E, Motooka D, Ito Y, Gotoh K, Hirota K, Matsushita M, Furuta Y, Narazaki M, Sakaguchi N, Kayama H, Nakamura S, Iida T, Saeki Y, Kumanogoh A, Sakaguchi S, Takeda K. Dysbiosis Contributes to Arthritis Development via Activation of Autoreactive T Cells in the Intestine. Arthritis Rheumatol. 2016 Nov;68(11):2646-61. doi: 10.1002/art.39783. PMID: 27333153.
- Hevia A, Milani C, López P, Cuervo A, Arboleya S, Duranti S, Turroni F, González S, Suárez A, Gueimonde M, Ventura M, Sánchez B, Margolles A. Intestinal dysbiosis associated with systemic lupus erythematosus. mBio. 2014 Sep 30;5(5):e01548-14. doi: 10.1128/mBio.01548-14. PMID: 25271284.
- He Z, Shao T, Li H, Xie Z, Wen C. Alterations of the gut microbiome in Chinese patients with systemic lupus erythematosus. Gut Pathog. 2016 8:64. doi: 10.1186/s13099-016-0146-9. PMID: 27980687.
- Azzouz D, Omarbekova A, Heguy A, Schwudke D, Gisch N, Rovin BH, Caricchio R, Buyon JP, Alekseyenko AV, Silverman GJ. Lupus nephritis is linked to disease-activity associated expansions and immunity to a gut commensal. Ann Rheum Dis. 2019 Jul;78(7):947-56. doi: 10.1136/annrheumdis-2018-214856. PMID: 30782585.
- Chen BD, Jia XM, Xu JY, Zhao LD, Ji JY, Wu BX, Ma Y, Li H, Zuo XX, Pan WY, Wang XH, Ye S, Tsokos GC, Wang J, Zhang X. An Autoimmunogenic and Proinflammatory Profile Defined by the Gut Microbiota of Patients With Untreated Systemic Lupus Erythematosus. Arthritis Rheumatol. 2021 Feb;73(2):232-43. doi: 10.1002/art.41511. PMID: 33124780.
- Zhang H, Liao X, Sparks JB, Luo XM. Dynamics of gut microbiota in autoimmune lupus. Appl Environ Microbiol. 2014 Dec;80(24):7551-60. doi: 10.1128/aem.02676-14. PMID: 25261516.
- Van Praet JT, Donovan E, Vanassche I, Drennan MB, Windels F, Dendooven A, Allais L, Cuvelier CA, van de Loo F, Norris PS, Kruglov AA, Nedospasov SA, Rabot S, Tito R, Raes J, Gaboriau-Routhiau V, Cerf-Bensussan N, Van de Wiele T, Eberl G, Ware CF, Elewaut D. Commensal microbiota influence systemic autoimmune responses. Embo j. 2015 Feb 12;34(4):466-74. doi: 10.15252/embj.201489966. PMID: 25599993.
- Dean LE, Jones GT, MacDonald AG, Downham C, Sturrock RD, Macfarlane GJ. Global prevalence of ankylosing spondylitis. Rheumatology (Oxford). 2014 Apr;53(4):650-7. doi: 10.1093/rheumatology/ket387. PMID: 24324212.
- Gan FY, Fei YY, Li MT, Wang Q, Xu D, Hou Y, Zeng XF, Zhang FC. The characteristics of patients having ankylosing spondylitis associated with Takayasu's arteritis. Clin Rheumatol. 2014 Mar;33(3):355-8. doi: 10.1007/s10067-013-2444-7. PMID: 24310108.
- Brewerton DA, Hart FD, Nicholls A, Caffrey M, James DC, Sturrock RD. Ankylosing spondylitis and HL-A 27. Lancet. 1973 Apr 28;1(7809):904-7. doi: 10.1016/s0140-6736(73)91360-3. PMID: 4123836.
- Yang M, Xu M, Pan X, Hu Z, Li Q, Wei Y, Zhang Y, Rong J, Zhai J, He P, Hu S, Song H, Wu H, Zhan F, Liu S, Gao G, Liu Z, Li Y, Shen L, Huang A, Lin Z, Liao Z, Cao S, Wei Q, Li Q, Lv Q, Qi J, Li T, Jin O, Pan Y, Gu J. Epidemiological comparison of clinical manifestations according to HLA-B*27 carrier status of Chinese ankylosing spondylitis patients. Tissue Antigens. 2013 Nov;82(5):338-43. doi: 10.1111/tan.12186. PMID: 24131020.
- Lin P, Bach M, Asquith M, Lee AY, Akileswaran L, Stauffer P, Davin S, Pan Y, Cambronne ED, Dorris M, Debelius JW, Lauber CL, Ackermann G, Baeza YV, Gill T, Knight R, Colbert RA, Taurog JD, Van Gelder RN, Rosenbaum JT. HLA-B27 and human β2-microglobulin affect the gut microbiota of transgenic rats. PLoS One. 2014 9(8):e105684. doi: 10.1371/journal.pone.0105684. PMID: 25140823.
- Breban M, Tap J, Leboime A, Said-Nahal R, Langella P, Chiocchia G, Furet JP, Sokol H. Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Ann Rheum Dis. 2017 Sep;76(9):1614-22. doi: 10.1136/annrheumdis-2016-211064. PMID: 28606969.
- Taurog JD, Richardson JA, Croft JT, Simmons WA, Zhou M, Fernández-Sueiro JL, Balish E, Hammer RE. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994 Dec 1;180(6):2359-64. doi: 10.1084/jem.180.6.2359. PMID: 7964509.
- Klingberg E, Magnusson MK, Strid H, Deminger A, Ståhl A, Sundin J, Simrén M, Carlsten H, Öhman L, Forsblad-d'Elia H. A distinct gut microbiota composition in patients with ankylosing spondylitis is associated with increased levels of fecal calprotectin. Arthritis Res Ther. 2019 Nov 27;21(1):248. doi: 10.1186/s13075-019-2018-4. PMID: 31771630.
- Stebbings S, Munro K, Simon MA, Tannock G, Highton J, Harmsen H, Welling G, Seksik P, Dore J, Grame G, Tilsala-Timisjarvi A. Comparison of the faecal microflora of patients with ankylosing spondylitis and controls using molecular methods of analysis. Rheumatology (Oxford). 2002 Dec;41(12):1395-401. doi: 10.1093/rheumatology/41.12.1395. PMID: 12468819.
- Costello ME, Ciccia F, Willner D, Warrington N, Robinson PC, Gardiner B, Marshall M, Kenna TJ, Triolo G, Brown MA. Brief Report: Intestinal Dysbiosis in Ankylosing Spondylitis. Arthritis Rheumatol. 2015 Mar;67(3):686-91. doi: 10.1002/art.38967. PMID: 25417597.
- Chen Z, Qi J, Wei Q, Zheng X, Wu X, Li X, Liao Z, Lin Z, Gu J. Variations in gut microbial profiles in ankylosing spondylitis: disease phenotype-related dysbiosis. Ann Transl Med. 2019 Oct;7(20):571. doi: 10.21037/atm.2019.09.41. PMID: 31807552.
- Zhang X, Chen BD, Zhao LD, Li H. The Gut Microbiota: Emerging Evidence in Autoimmune Diseases. Trends Mol Med. 2020 Sep;26(9):862-73. doi: 10.1016/j.molmed.2020.04.001. PMID: 32402849.
- Manfredo Vieira S, Hiltensperger M, Kumar V, Zegarra-Ruiz D, Dehner C, Khan N, Costa FRC, Tiniakou E, Greiling T, Ruff W, Barbieri A, Kriegel C, Mehta SS, Knight JR, Jain D, Goodman AL, Kriegel MA. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science. 2018 Mar 9;359(6380):1156-61. doi: 10.1126/science.aar7201. PMID: 29590047.
- Zegarra-Ruiz DF, El Beidaq A, Iñiguez AJ, Lubrano Di Ricco M, Manfredo Vieira S, Ruff WE, Mubiru D, Fine RL, Sterpka J, Greiling TM, Dehner C, Kriegel MA. A Diet-Sensitive Commensal Lactobacillus Strain Mediates TLR7-Dependent Systemic Autoimmunity. Cell Host Microbe. 2019 Jan 9;25(1):113-27.e6. doi: 10.1016/j.chom.2018.11.009. PMID: 30581114.
- Greiling TM, Dehner C, Chen X, Hughes K, Iñiguez AJ, Boccitto M, Ruiz DZ, Renfroe SC, Vieira SM, Ruff WE, Sim S, Kriegel C, Glanternik J, Chen X, Girardi M, Degnan P, Costenbader KH, Goodman AL, Wolin SL, Kriegel MA. Commensal orthologs of the human autoantigen Ro60 as triggers of autoimmunity in lupus. Sci Transl Med. 2018 Mar 28;10(434). doi: 10.1126/scitranslmed.aan2306. PMID: 29593104.
- Szymula A, Rosenthal J, Szczerba BM, Bagavant H, Fu SM, Deshmukh US. T cell epitope mimicry between Sjögren's syndrome Antigen A (SSA)/Ro60 and oral, gut, skin and vaginal bacteria. Clin Immunol. 2014 May-Jun;152(1-2):1-9. doi: 10.1016/j.clim.2014.02.004. PMID: 24576620.
- Ruff WE, Dehner C, Kim WJ, Pagovich O, Aguiar CL, Yu AT, Roth AS, Vieira SM, Kriegel C, Adeniyi O, Mulla MJ, Abrahams VM, Kwok WW, Nussinov R, Erkan D, Goodman AL, Kriegel MA. Pathogenic Autoreactive T and B Cells Cross-React with Mimotopes Expressed by a Common Human Gut Commensal to Trigger Autoimmunity. Cell Host Microbe. 2019 Jul 10;26(1):100-13.e8. doi: 10.1016/j.chom.2019.05.003. PMID: 31227334.
- Kim JW, Kwok SK, Choe JY, Park SH. Recent Advances in Our Understanding of the Link between the Intestinal Microbiota and Systemic Lupus Erythematosus. Int J Mol Sci. 2019 Sep 30;20(19). doi: 10.3390/ijms20194871. PMID: 31575045.
- Zhou C, Zhao H, Xiao XY, Chen BD, Guo RJ, Wang Q, Chen H, Zhao LD, Zhang CC, Jiao YH, Ju YM, Yang HX, Fei YY, Wang L, Shen M, Li H, Wang XH, Lu X, Yang B, Liu JJ, Li J, Peng LY, Zheng WJ, Zhang CY, Zhou JX, Wu QJ, Yang YJ, Su JM, Shi Q, Wu D, Zhang W, Zhang FC, Jia HJ, Liu DP, Jie ZY, Zhang X. Metagenomic profiling of the pro-inflammatory gut microbiota in ankylosing spondylitis. J Autoimmun. 2020 Feb;107:102360. doi: 10.1016/j.jaut.2019.102360. PMID: 31806420.
- Atkinson SM, Hoffmann U, Hamann A, Bach E, Danneskiold-Samsøe NB, Kristiansen K, Serikawa K, Fox B, Kruse K, Haase C, Skov S, Nansen A. Depletion of regulatory T cells leads to an exacerbation of delayed-type hypersensitivity arthritis in C57BL/6 mice that can be counteracted by IL-17 blockade. Dis Model Mech. 2016 Apr;9(4):427-40. doi: 10.1242/dmm.022905. PMID: 26822477.
- Qu N, Xu M, Mizoguchi I, Furusawa J, Kaneko K, Watanabe K, Mizuguchi J, Itoh M, Kawakami Y, Yoshimoto T. Pivotal roles of T-helper 17-related cytokines, IL-17, IL-22, and IL-23, in inflammatory diseases. Clin Dev Immunol. 2013 2013:968549. doi: 10.1155/2013/968549. PMID: 23956763.
- Bradley CP, Teng F, Felix KM, Sano T, Naskar D, Block KE, Huang H, Knox KS, Littman DR, Wu HJ. Segmented Filamentous Bacteria Provoke Lung Autoimmunity by Inducing Gut-Lung Axis Th17 Cells Expressing Dual TCRs. Cell Host Microbe. 2017 Nov 8;22(5):697-704.e4. doi: 10.1016/j.chom.2017.10.007. PMID: 29120746.
- Gill PA, van Zelm MC, Muir JG, Gibson PR. Review article: short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment Pharmacol Ther. 2018 Jul;48(1):15-34. doi: 10.1111/apt.14689. PMID: 29722430.
- Hui W, Yu D, Cao Z, Zhao X. Butyrate inhibit collagen-induced arthritis via Treg/IL-10/Th17 axis. Int Immunopharmacol. 2019 Mar;68:226-33. doi: 10.1016/j.intimp.2019.01.018. PMID: 30660077.
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014 Aug;11(8):506-14. doi: 10.1038/nrgastro.2014.66. PMID: 24912386.
- Vaghef-Mehrabany E, Alipour B, Homayouni-Rad A, Sharif SK, Asghari-Jafarabadi M, Zavvari S. Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition. 2014 Apr;30(4):430-5. doi: 10.1016/j.nut.2013.09.007. PMID: 24355439.
- Zamani B, Golkar HR, Farshbaf S, Emadi-Baygi M, Tajabadi-Ebrahimi M, Jafari P, Akhavan R, Taghizadeh M, Memarzadeh MR, Asemi Z. Clinical and metabolic response to probiotic supplementation in patients with rheumatoid arthritis: a randomized, double-blind, placebo-controlled trial. Int J Rheum Dis. 2016 Sep;19(9):869-79. doi: 10.1111/1756-185x.12888. PMID: 27135916.
- Arvonen M, Berntson L, Pokka T, Karttunen TJ, Vähäsalo P, Stoll ML. Gut microbiota-host interactions and juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2016 Jul 22;14(1):44. doi: 10.1186/s12969-016-0104-6. PMID: 27448997.
- van Dijkhuizen EHP, Del Chierico F, Malattia C, Russo A, Pires Marafon D, Ter Haar NM, Magni-Manzoni S, Vastert SJ, Dallapiccola B, Prakken B, Martini A, De Benedetti F, Putignani L. Microbiome Analytics of the Gut Microbiota in Patients With Juvenile Idiopathic Arthritis: A Longitudinal Observational Cohort Study. Arthritis Rheumatol. 2019 Jun;71(6):1000-10. doi: 10.1002/art.40827. PMID: 30592383.
- Cao Y, Shen J, Ran ZH. Association between Faecalibacterium prausnitzii Reduction and Inflammatory Bowel Disease: A Meta-Analysis and Systematic Review of the Literature. Gastroenterol Res Pract. 2014 2014:872725. doi: 10.1155/2014/872725. PMID: 24799893.
- Pan H, Guo R, Ju Y, Wang Q, Zhu J, Xie Y, Zheng Y, Li T, Liu Z, Lu L, Li F, Tong B, Xiao L, Xu X, Leung EL, Li R, Yang H, Wang J, Zhou H, Jia H, Liu L. A single bacterium restores the microbiome dysbiosis to protect bones from destruction in a rat model of rheumatoid arthritis. Microbiome. 2019 Jul 17;7(1):107. doi: 10.1186/s40168-019-0719-1. PMID: 31315667.
- Marietta EV, Murray JA, Luckey DH, Jeraldo PR, Lamba A, Patel R, Luthra HS, Mangalam A, Taneja V. Suppression of Inflammatory Arthritis by Human Gut-Derived Prevotella histicola in Humanized Mice. Arthritis Rheumatol. 2016 Dec;68(12):2878-88. doi: 10.1002/art.39785. PMID: 27337150.
- Mu Q, Zhang H, Liao X, Lin K, Liu H, Edwards MR, Ahmed SA, Yuan R, Li L, Cecere TE, Branson DB, Kirby JL, Goswami P, Leeth CM, Read KA, Oestreich KJ, Vieson MD, Reilly CM, Luo XM. Control of lupus nephritis by changes of gut microbiota. Microbiome. 2017 Jul 11;5(1):73. doi: 10.1186/s40168-017-0300-8. PMID: 28697806.
- Jenks K, Stebbings S, Burton J, Schultz M, Herbison P, Highton J. Probiotic therapy for the treatment of spondyloarthritis: a randomized controlled trial. J Rheumatol. 2010 Oct;37(10):2118-25. doi: 10.3899/jrheum.100193. PMID: 20716665.
- Kloppenburg M, Breedveld FC, Terwiel JP, Mallee C, Dijkmans BA. Minocycline in active rheumatoid arthritis. A double-blind, placebo-controlled trial. Arthritis Rheum. 1994 May;37(5):629-36. doi: 10.1002/art.1780370505. PMID: 8185689.
- Tilley BC, Alarcón GS, Heyse SP, Trentham DE, Neuner R, Kaplan DA, Clegg DO, Leisen JC, Buckley L, Cooper SM, Duncan H, Pillemer SR, Tuttleman M, Fowler SE. Minocycline in rheumatoid arthritis. A 48-week, double-blind, placebo-controlled trial. MIRA Trial Group. Ann Intern Med. 1995 Jan 15;122(2):81-9. doi: 10.7326/0003-4819-122-2-199501150-00001. PMID: 7993000.
- O'Dell JR, Haire CE, Palmer W, Drymalski W, Wees S, Blakely K, Churchill M, Eckhoff PJ, Weaver A, Doud D, Erikson N, Dietz F, Olson R, Maloley P, Klassen LW, Moore GF. Treatment of early rheumatoid arthritis with minocycline or placebo: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 1997 May;40(5):842-8. doi: 10.1002/art.1780400510. PMID: 9153544.
- Mu Q, Tavella VJ, Kirby JL, Cecere TE, Chung M, Lee J, Li S, Ahmed SA, Eden K, Allen IC, Reilly CM, Luo XM. Antibiotics ameliorate lupus-like symptoms in mice. Sci Rep. 2017 Oct 20;7(1):13675. doi: 10.1038/s41598-017-14223-0. PMID: 29057975.
- Schäfer AL, Eichhorst A, Hentze C, Kraemer AN, Amend A, Sprenger DTL, Fluhr C, Finzel S, Daniel C, Salzer U, Rizzi M, Voll RE, Chevalier N. Low Dietary Fiber Intake Links Development of Obesity and Lupus Pathogenesis. Front Immunol. 2021 12:696810. doi: 10.3389/fimmu.2021.696810. PMID: 34335609.
- Zhang Y, Liu Q, Yu Y, Wang M, Wen C, He Z. Early and Short-Term Interventions in the Gut Microbiota Affects Lupus Severity, Progression, and Treatment in MRL/lpr Mice. Front Microbiol. 2020 11:628. doi: 10.3389/fmicb.2020.00628. PMID: 32346376.
- Ogrendik M. Treatment of ankylosing spondylitis with moxifloxacin. South Med J. 2007 Apr;100(4):366-70. doi: 10.1097/SMJ.0b013e31802fa2a8. PMID: 17458395.
- Yang L, Liu B, Zheng J, Huang J, Zhao Q, Liu J, Su Z, Wang M, Cui Z, Wang T, Zhang W, Li Q, Lu H. Rifaximin Alters Intestinal Microbiota and Prevents Progression of Ankylosing Spondylitis in Mice. Front Cell Infect Microbiol. 2019 9:44. doi: 10.3389/fcimb.2019.00044. PMID: 30886835.
- Antushevich H. Fecal microbiota transplantation in disease therapy. Clin Chim Acta. 2020 Apr;503:90-8. doi: 10.1016/j.cca.2019.12.010. PMID: 31968211.
- de Groot P, Nikolic T, Pellegrini S, Sordi V, Imangaliyev S, Rampanelli E, Hanssen N, Attaye I, Bakker G, Duinkerken G, Joosten A, Prodan A, Levin E, Levels H, Potter van Loon B, van Bon A, Brouwer C, van Dam S, Simsek S, van Raalte D, Stam F, Gerdes V, Hoogma R, Diekman M, Gerding M, Rustemeijer C, de Bakker B, Hoekstra J, Zwinderman A, Bergman J, Holleman F, Piemonti L, De Vos W, Roep B, Nieuwdorp M. Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomised controlled trial. Gut. 2021 Jan;70(1):92-105. doi: 10.1136/gutjnl-2020-322630. PMID: 33106354.
- Zeng J, Peng L, Zheng W, Huang F, Zhang N, Wu D, Yang Y. Fecal microbiota transplantation for rheumatoid arthritis: A case report. Clin Case Rep. 2021 Feb;9(2):906-9. doi: 10.1002/ccr3.3677. PMID: 33598269.
- Huang C, Yi P, Zhu M, Zhou W, Zhang B, Yi X, Long H, Zhang G, Wu H, Tsokos GC, Zhao M, Lu Q. Safety and efficacy of fecal microbiota transplantation for treatment of systemic lupus erythematosus: An EXPLORER trial. J Autoimmun. 2022 Jun;130:102844. doi: 10.1016/j.jaut.2022.102844. PMID: 35690527.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.