Biology Group . 2022 December 05;3(12):1451-1459. doi: 10.37871/jbres1619.
Molecular Detection of Anaplasma phagocytophilum, Babesia odocoilei, Babesia species and Borrelia burgdorferi Sensu Lato in Songbirds
John D Scott1*, Elena McGoey2, Ana Morales3 and Risa R Pesapane2,4
2School of Environmental and Natural Resources, College of Food, Agricultural, and Environmental Sciences, The Ohio State University, Columbus, OH 43210, USA
3McGill Bird Observatory, Ste Anne de Bellevue, QC, Canada H9X 0A6
4Department of Veterinary Preventive Medicine, College of Veterinary Medicine, The Ohio State University, 1920 Coffey Rd., Columbus, OH 43210, USA
- Bird blood
- Tick-borne zoonotic pathogens
- Anaplasma phagocytophilum
- Babesia odocoilei
- Borrelia burgdorferi sensu lato
- Blacklegged tick
- Ixodes scapularis
- Zoonosis
- Ground-foraging songbirds
- Passerine birds
- Infectious diseases
- Molecular biology
Abstract
The blacklegged tick, Ixodes scapularis, is known to carry various tick-borne zoonotic pathogens with the potential to cause debilitating human and animal diseases. Juvenile I. scapularis parasitize songbirds and, perhaps, these avifauna are competent hosts of common microbial pathogens. We extracted brachial venous blood from 18 ground-foraging passerine birds that were parasitized by I. scapularis larvae and nymphs. Using molecular identification, namely PCR, DNA sequencing, and Basic Local Alignment Search Tool (BLAST), we targeted Anaplasma phagocytophilum, Babesia spp. and Borrelia burgdorferi sensu lato. Overall, 15 (83%) of 18 passerine birds were positive for 3 microbial zoonotic pathogens that comprised of A. phagocytophilum (n = 8), Babesia odocoilei (n = 6), Babesia spp. 20-5A74 (n = 1), and B. burgdorferi sensu lato (n = 9). The pathogen load consisted of 8 singles, 5 doubles, and 2 triples. One novel Babesia sp. (Babesia spp. 20-5A74) was found, and the remaining Babesia infections were B. odocoilei. Our findings reveal that ground-foraging, passerine birds are avian hosts of zoonotic pathogens. We provide the first-ever documentation that songbirds are hosts of B. odocoilei. Based on our data, B. odocoilei outnumbered other Babesia spp., and elucidated the authentic fact that B. odocoilei is the predominant Babesia sp. in North America. As avian hosts, passerine birds play a significant role in the enzootic transmission cycle of B. burgdorferi sensu lato, A. phagocytophilum, and Babesia species.
Introduction
Principal vectors of zoonotic pathogens in North America are the blacklegged tick, Ixodes scapularis (Acari: Ixodidae), and the western blacklegged tick, Ixodes pacificus [1]. Both larvae and nymphs of these ixodid ticks parasitize songbirds (Order: Passeriformes). Epidemiologically, I. scapularis carries and transmits at least six tick-borne, zoonotic pathogens that include several genospecies within the Borrelia burgdorferi sensu lato (Bbsl) complex [2,3], Borrelia miyamotoi [4,5], Babesia spp. (Bspp) [6-8], Anaplasma phagocytophilum (Aph) [9,10], Ehrlichia muris eauclairensis [11], and the virus of Powassan Virus Disease [12-14]. Ecologically, I. scapularis parasitizes at least 82 bird species [15] and 55 mammalian hosts, including humans [7,16-21]. Polymicrobial infections have been reported in patients [22], and multiple pathogens (i.e., Bbsl, B. miyamotoi, A. phagocytophilum, B. microti) have been detected in a single I. scapularis [23].
The Lyme disease bacterium was first isolated from the blood of Common Yellowthroat (Geothlypis trichas), American Robin (Turdus migratorius), and Gray Catbird (Dumetella carolinensis) collected in Connecticut [16]. As well, Bbsl was cultured from a Song Sparrow (Melospiza melodia) collected in Wisconsin [24] and, likewise, in Connecticut [16-18]. In Canada, Bbsl-positive I. scapularis larvae have been collected from Song Sparrows [25,26]. Researchers put xenodiagnostic larvae on American Robins, and determined that this avian host is a competent host of Bbsl [27]. Anaplasma phagocytophilum has been detected in the blood of birds collected in California [28]. Specifically, blood samples from a Golden-crowned Sparrow (Zonotrichia atricapilla) and European Starling (Sturnus vulgaris) were positive for A. phagocytophilum.
Worldwide, several different Babesia spp. (Apicomplexa: Piroplasmida: Babesiidae) cause human babesiosis, and they include B. crassa-like [29], B. divergens [30], Babesia divergens-like MO-1 [31], B. duncani [32], B. microti [33], B. motasi [34], B. odocoilei [7], Babesia spp. XXB/HangZhou [35], Babesia sp. TW1 [36], Babesia spp. CA1, CA3, and CA4 [37], and B. venatorum [38]. Also, at least 111 valid Babesia species are present in environmental habitats around the globe [39]. Using molecular-based characterization, molecular biologists and veterinary researchers recently discovered Babesia odocoilei (Bod) in red deer, Cervus elaphus, in the United Kingdom [40].
Migratory songbirds widely disperse I. scapularis larvae and nymphs infected with tick-borne, zoonotic pathogens, including B. odocoilei. Combining datasets, B. odocoilei is common in North America. In the USA, tick researchers have reported B. odocoilei in Indiana [41-43], Michigan [44] Maine [42,43], Massachusetts [41-43], New York [45], Oklahoma [46,47], Pennsylvania [48,49] Texas [50,51], Virginia [52], and Wisconsin [42,43]. As well, B. odocoilei has been detected in I. pacificus in California [53]. In Canada, B. odocoilei has been detected in Saskatchewan [54], Ontario [7,15,55-59], and Quebec [55,57,58]. And yet, acarologists and ecologists have not reported B. microti in these three provinces [7,15,21,55-59].
Babesia odocoilei, which is a sequestering Babesia sp., can be recalcitrant to treat in human patients [7]. In contrast, B. microti is a non-sequestering species, and is relatively easy to treat. Biogeographically, there is paucity of B. microti continentwide [55].
The purpose of this tick-host-pathogen study was to take blood samples from ground-foraging songbirds that were parasitized by blacklegged ticks, I. scapularis, and determine whether passerine birds are hosts A. phagocytophilum, Babesia spp., and B. burgdorferi sensu lato.
Materials and Methods
Bird blood collection
Songbirds were captured using standard mist nets in two different locations: an arboreal area near Montée Biggar, Quebec, Canada; 45.09 N, 74.22 W and, also, McGill Bird Observatory, Ste-Anne-de-Bellevue, Quebec; 45.43 N, 73.94 W.
Blood (~50 µL) was collected in non-heparinized microhematocrit capillary tubes (FisherbrandTM, Saint-Laurent, Quebec, Canada) from the brachial vein of each bird, by trained individuals, between 16 June 2021 to 25 August 2021. The blood was then transferred to 1.5 mL cryogenic tubes (Thermo Fisher ScientificTM, Mississauga, Canada; NalgeneTM, Singapore, Southeast Asia) filled with 0.5 mL Queen’s lysis buffer solution [60]. Samples were stored inside a fridge for up to 6 months at 4oC until they were shipped to the lab for subsequent analysis. Banding and sampling of songbirds were granted under animal use protocol 2007-5446 for McGill University, and federal banding permits were issued by the Canadian Wildlife Service.
DNA isolation and pathogen detection
Genomic DNA was extracted from songbird blood using the PureLink Genomic DNA Mini Kit (Invitrogen, Waltham, MA, USA) according to manufacture’s instructions. Detection of B. burgdorferi s.l. and A. phagocytophilum was performed using 20 µL real-time PCR reaction of Taqman Fast Advanced Master Mix (Applied Biosystems, Waltham, MA. USA) and previously established primers targeting the 16S rDNA and msp2 genes, respectively [61,62]. A cycle threshold ≤ 40 with a characteristic curve was considered positive, and all positives were run in duplicate. Detection of Babesia spp. was achieved using primers targeting the 18S gene in conventional PCR followed by sequencing as previously described [15,58]. Molecular biology grade water and synthetic gBlock gene fragments (Integrated DNA Technologies, Coralville, IA, USA) of B. burgdorferi (MH781147), A. phagocytophilum (AY151054.1) and B. microti (MT974173.1) were included in all PCR reactions as controls.
Results
Tick collection
In total, brachial venous bloods from 18 ground-forging passerine birds (6 Song Sparrows, Melospiza melodia Wilson; 4 Common Yellowthroats, Geothlypis trichas L.; 4 Veeries, Catharus fuscescens Stephens; 1 Ovenbird, Seiurus aurocapillus L.; 1 Brown-headed Cowbird, Molothrus ater Boddaert; 1 Swainson’s Thrush, Catharus ustulatus Nuttall; and 1 American Robin, Turdus migratorius L.) were selected for testing and analysis. Qualified bird banders identified passerine birds to bird species. From these tick-infested passerines, 30 I. scapularis (9 larvae, 21 nymphs) were also collected.
Molecular detection of pathogens
Overall, 24 infections were detected, namely 9 Bbsl (38%), 8 Aph (33%), 6 B. odocoilei (25%), and one Babesia spp. 20-5A74 (4%), in 15 (83%) of 18 songbirds. These infections comprised of 8 single infections, 5 co-infections (n = 10), and 2 polymicrobial infections (n = 6) (Table 1).
Nine birds (50%) were infected with Bbsl, six birds (33%) with B. odocoilei, and eight birds (44%) with Aph. A single bird (Common Yellowthroat) was infected with a Babesia spp. 20-5A74 strain (Table 1).
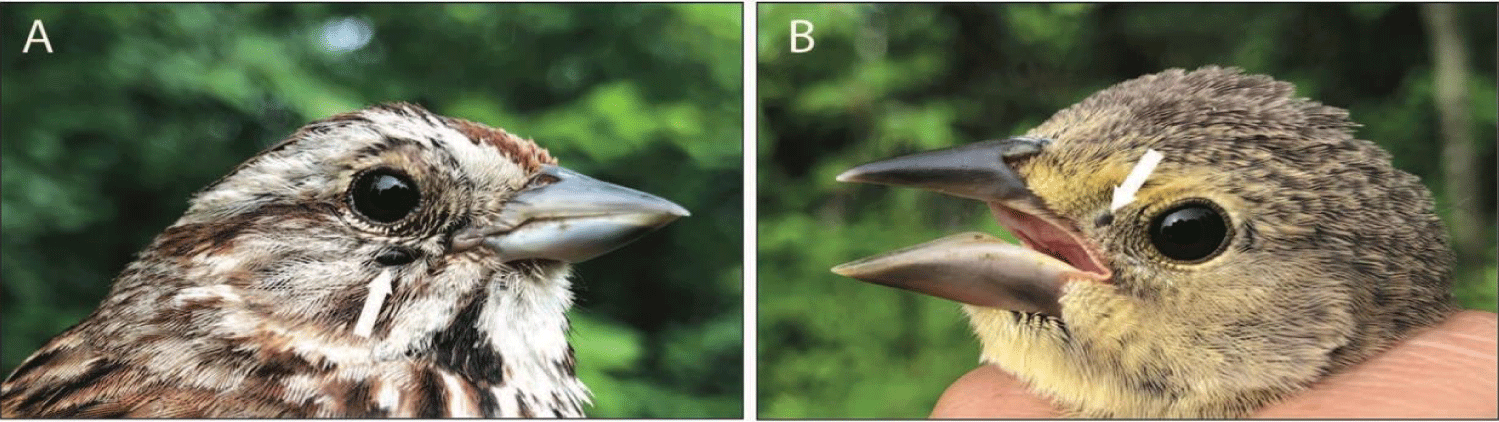
Blood from four different Song Sparrows were positive for Bbsl which also suggests that Song Sparrows are avian hosts of Bbsl. One (SOSP*0976) of these four Song Sparrows had a triple infection (i.e., Bbsl, Aph, Bod) (Figure 1A). Likewise, a juvenile Brown-headed Cowbird (BHCO*1471) had three endogenous pathogens (i.e., Bbsl, Aph, Bod) simultaneously as a polymicrobial infection (Figure 1B). Three individual songbirds (1 Common Yellowthroat, 1 Veery, and 1 Song Sparrow) were not infected with target pathogens.
Since the seven Babesia sequences for B. odocoilei were all almost 99.7% or more identical to sequences in GenBank, we did not deposit them to this publically available databank because they would not contribute to known diversity.
Discussion
The tick I. scapularis carries various pathogens with the potential of producing serious human and animal diseases. Babesia odocoilei-infected I. scapularis ticks have previously been collected from songbirds, but we unveil this Babesial parasite for the first time in bird blood. After numerous B. odocoilei collections in Ontario and Quebec, B. microti has not been found in blacklegged ticks or songbirds. Not only do ground-frequenting songbirds transport ticks, they may also be hosts for tick-borne, zoonotic pathogens. Migratory songbirds widely disperse zoonotic pathogens across North America and, therefore, one does not have to frequent or live in an endemic area to contract human babesiosis caused by B. odocoilei.
Tick-Host-Pathogen enzootic transmission cycle
Each microbial pathogen in the present study has its own tick-host pathways for sustaining viability. For polymicrobial infections, each one can have a different source and enzootic route. Tick-borne, zoonotic pathogens can be sourced from ixodid ticks, and transmitted during bird parasitism [15,55-59]. Since I. scapularis ticks are typically infected, we sampled ground-foraging songbirds with attached ticks. Although we selected these parameters, there was no assurance of infectivity. Depending on the time of year, sources and pathways of pathogens can vary greatly. Moreover, the combination of pathogens, can vary in the enzootic transmission cycle.
Even though I. scapularis females do not parasitize songbirds, they can transmit B. odocoilei to the next generation via transovarial transmission (gravid female to eggs to larvae) [1,46,63]. When larvae acquire B. odocoilei, they can transmit B. odocoilei to the next life stages via transstadial passage (larva to nymph and/or nymph to adult). Notably, neither larvae nor nymphs require a B. odocoilei-infected host to acquire infection. If the mobile life stages (i.e., larvae, nymphs, and females) of I. scapularis do not imbibe a replete blood meal, transmission of B. odocoilei is discontinued.
Alternatively, I. scapularis must acquire B. burgdorferi s.l. and A. phagocytophilum from infected hosts. Both bacterial infections typically stop when I. scapularis adults die. However, ground-foraging songbirds often feed on spent females after she lays her eggs, and may become infected orally. Hosts are paramount in sustaining these bacterial infections [63].
Although inter-generational studies have not been done with passerines, they can hold microbial infections as competent hosts [27]. Four (80%) of five Song Sparrows were infected with B. burgdorferi s.l. which suggest this bird species is a competent host. As well, three (75%) of four Common Yellowthroats were infected with A. phagocytophilum suggesting they are competent hosts. Similarly, three (75%) of the four Common Yellowthroats were infected with Babesia spp. Furthermore, two (50%) of four Veeries were infected with B. odocoilei which, again, suggests that Veeries are competent hosts of this apicomplexan parasite.
Polymicrobial infections occur in I. scapularis and their hosts. In the present study, an adult Song Sparrow (SOSP*0976) was infected with A. phagocytophilum, B. odocoilei, and B. burgdorferi s.l.; however, the single I. scapularis nymph was negative. Likewise, a juvenile Brown-headed Cowbird (BHCO*1471) was infected simultaneously with these three tick-borne zoonotic pathogens; however, the engorged larvae, which molted via transstadial passage to nymphs, were negative. These enzootic findings suggest vertical transmission (mother songbird to filial offspring) or previous infections via bird parasitism by ticks. When juvenile I. scapularis are negative, and the hosts’ bloods are positive, this enzootic situation suggests that these avian hosts are retaining tick-borne zoonotic pathogens in their bodies, and are competent hosts.
With respect to B. burgdorferi s.l., I. scapularis females do not facilitate transovarial transmission [62]. Therefore, when an I. scapularis larva parasitizes a songbird, the only avenue for it to become infected with B. burgdorferi s.l. is an infected host (Figure 2). Birds are known to harbor Bbsl in their bodies for extended periods of time [27,64,65]. Once acquired from infected hosts, many tick-borne pathogens are confined within the tick gut lumen, and are surrounded by tick-microbe interactions and discrete midgut barriers [66]. There are many barriers that pathogens encounter when passing from host to tick. These biological hindrances include host blood chemistry, tick defenses, competition from other pathogens/microbes within the tick, and sequence of infection (e.g., some pathogens may exclude others depending on the order of exposure) [66−69]. A tick can initially test positive for a microorganism but not survive due to tick characteristics and microbial interactions in the midgut lumen.
Overall, B. microti is sparse in North America. After numerous tick-host-pathogen studies, researchers have not found B. microti in I. scapularis and songbirds collected in Ontario and Quebec [7,8,15,21,55-59]. Similarly, a tick-pathogen survey of 299 questing I. scapularis adults in Pennsylvania found B. odocoilei in 15.4%, whereas B. microti accounted for only 0.7% [49]. In Maryland, none of the 348 I. scapularis nymphs collected was positive for B. microti [70]. In a recent tick-host-pathogen study, the ratio of B. odocoilei to B. microti was 41 to 1 [55]. By far, B. odocoilei was the predominant Babesia sp. With the exception of one Babesia strain (i.e., Babesia spp. 21-5A74), all Babesia spp. in the present study were B. odocoilei (Table 1). Moreover, since most commercial laboratories do not test humans for B. odocoilei, and the fact that B. odocoilei cross-reacts with B. duncani [7,8], B. odocoilei has often been misrepresented or discounted as B. duncani [7,8].
| Table 1. DNA sequence analysis of the 18S gene of Babesia spp. in blood of songbirds captured at Montée Biggar, Quebec, 2021. | ||||
| Bird Species | Bird Code | Date Collected | Babesia species | BLAST Match |
| Common Yellowthroat | COYE*2978 | 12-Jul | B. odocoilei | 99.8/99 |
| Common Yellowthroat | COYE*7109 | 16-Jun | B. spp. 20-5A74 | 94/96 |
| Common Yellowthroat | COYE*7128 | 23-Jun | B. odocoilei | 98.8/100 |
| Veery | VEER*1549 | 12-Jul | B. odocoilei | 100/100 |
| Veery | VEER*3257 | 16-Jun | B. odocoilei | 99.2/100 |
| Song Sparrow | SOSP*0976 | 4-Jul | B. odocoilei | 99/100 |
| Brown-headed Cowbird | BHCO*1471 | 4-Jul | B. odocoilei | 98.9/100 |
Survival of Babesia odocoilei in nature
Over millions of years, B. odocoilei has honed itself to parasitize a wide range of vertebrate hosts [38,63]. This sequestering piroplasmid can dwell in all four developmental life stages (eggs, larvae, nymphs, adults) of I. scapularis ticks [1,59]. Babesia odocoilei-infected, gravid females can pass this infection to offspring via transovarial transmission [1,46,63]. From the midgut epithelium, kinetes (infective Babesial spores) move through the tick body fluid (haemolymph) to peripheral tissues, including the ovaries. After mating, the eggs become infected with B. odocoilei kinetes, and are ready to be deposited on the forest floor. After 5-6 wk, the clutch of eggs hatch to I. scapularis larvae, and are ready to transmit B. odocoilei to suitable hosts. As well, larvae can transmit B. odocoilei infection to hosts by transstadial passage [1,46,63]. In fact, a B. odocoilei-infected I. scapularis female can transmit B. odocoilei infection from one tick generation to the next generation without parasitizing infected hosts. In nature, this generational sequel completes an enzootic transmission cycle of B. odocoilei. Because of their minute size (0.75 mm), and capability to transmit B. odocoilei, I. scapularis larvae pose a substantial threat to the local human population, especially after mid-July when a clutch of eggs hatch to larvae. Protective acaricide-treated clothing is important, especially when the temperatures are above freezing and there is no snow cover. Not only are white-tailed deer, Odocoileus virginianus, hosts of all three host-seeking life stages (larvae, nymphs, adults) of I. scapularis, these cervids are reservoirs of B. odocoilei [46,71]. Based on our findings, songbirds not only transport larval and nymphal I. scapularis, they are competent hosts of at least three tick-borne zoonotic microorganisms.
The blood sample of a Common Yellowthroat (COYE*7109) was confirmed positive for the apicomplexan species, Babesia spp. 20-5A74. This novel strain was likewise detected in an I. scapularis female parasitizing a domestic cat residing in the western part of eastern Ontario [15]. Ecologically our findings show that passerine birds can harbor polymicrobial infections including A. phagocytophilum, B. odocoilei, Babesia spp. 20-5A74 and B. burgdorferi s.l.
Differences in Babesia species
Babesia odocoilei has special adaptations to live in ixodid ticks and certain avian and mammalian hosts, including humans. Pathologically, B. odocoilei causes human babesiosis [7,8], and ecologically, all four life stages of I. scapularis can harbor this zoonotic infection [1,63]. In mammalian hosts, sequestering Babesia spp., such B. odocoilei maintain infection by using cytoadherence (adheres to endothelium cells and lining) [72]. Additionally, B. odocoilei exhibits sequestration (intravascular Babesia entanglements consisting of uninfected- and infected-erythrocytes bonded by fibrin strands), and occlude and block capillaries and post-capillary venules [73,74]. Sequestering Babesia spp. can complete their life cycle within self-perpetuating entanglements (local proliferation), and cause febrile symptoms. Thus, they remain isolated from the circulating immune system and spleen [74]. Compared to non-sequestering Babesia species (i.e., B. microti), patients that are infected with B. odocoilei are typically recalcitrant to treat with standard anti-Babesia regimens [7,8]. Patients may become life-long carriers and, occasionally, death results [31,75]. Sequestration has been documented in other Babesia spp. including Babesia bovis in cattle [72], Babesia canis in dogs [76], Babesia lengau in domestic cats [77]. In contrast, other Babesia spp., which are non-sequestering, include B. bigemina, B. divergens, and B. microti [78]. Since there at least 111 valid Babesia species, more yet-to-be-recognized, sequestering Babesia species will be flourishing in indigenous areas around the globe.
Human babesiosis caused by Babesia odocoilei
Human babesiosis caused by B. odocoilei is now being diagnosed and treated clinically [7,8]. Presentation in human patients varies from asymptomatic to debilitating with variable circulatory, gastrointestinal, rheumatological and neurological manifestations. Symptoms typically include fatigue, exertional intolerance, inflammation, ischemia, impaired cognition, cold intolerance, digital numbness, sweats (especially at night), insomnia, tissue/organ dysfunction, muscle aches (especially legs), and loss of balance [7,8]. Since B. odocoilei sequesters in the capillaries of the brain, this intraerythrocytic infection can produce cerebral pathophysiology, coma-like symptomology, and brain fog. Self-perpetuating, fibrin-bonded entanglements cause occlusions in capillaries, and hinder blood circulation. These entanglements induce capillary blockage and, thus, impede the transfer of oxygen and nutrients. Consequently, mitochondria are forced to produce ATP anaerobically and, therefore, these minute organelles operate inefficiently producing excess lactic acid. Diminutive ATP exists for normal cellular functions, including the Na+/K+ pump [79]. Mitochondria dysfunction and occlusion of capillaries associated with sequestering Babesia spp. help to explain the symptoms of human babesiosis caused by B. odocoilei. Humans with a sequestering Babesial infection can have cognitive and mood disorders ranging from minimal to severe [80]. Babesia odocoilei infections are typically persistent because this piroplasmid sequesters in capillaries and venules. Once this infection develops to the advanced stage, this deep-seated, stealth infection is recalcitrant to treat with current anti-Babesia regimes and, thus, this Babesial infection is chronic [7,8]. Since there has not been a valid commercial test for B. odocoilei aimed at human subjects, this particular human babesiosis has been a longstanding issue (i.e., cross-reactions) that has been misrepresented by invalid serology tests and unsubstantiated medical evaluations [1,55]. Tick bite is the normal mode of transmission, but blood transfusion is also apparent. Case reports of congenital babesiosis have been documented [81-83]. Infants can acquire babesiosis from tick bites, blood transfusions, or congenitally via vertical transmission (mother to filial offspring). Babesiosis in neonates can present with febrile thrombocytopenia, fevers, and parasitemia.
Conclusion
We provide the first documentation of songbirds as hosts of B. odocoilei, namely Song Sparrows, Common Yellowthroats, and Veeries. Since transovarial transmission does not apply to B. burgdorferi s.l. and A. phagocytophilum, we provide substantive evidence that Song Sparrow and Common Yellowthroats are competent hosts of these two zoonotic pathogens. Because B. odocoilei exhibits transovarial transmission, we also show supportive evidence that Common Yellowthroats are competent hosts of B. odocoilei. Based on our data, B. odocoilei outnumbers other Babesia species, and elucidates its predominance within the Temperate Zone of North America. In addition, we show that songbirds can harbor at least three different pathogens concurrently. Molecular analysis yielded A. phagocytophilum, B. odocoilei, Babesia spp. 20-5A74, and Borrelia burgdorferi s.l. in the blood of songbirds captured in southern Canada. As avian hosts, passerines play a noteworthy role in the enzootic transmission cycle of B. burgdorferi s.l., A. phagocytophilum and Babesia spp. Unequivocally, B. odocoilei is the predominant Babesia spp. in North America. Because songbirds transport pathogen-laden I. scapularis throughout North America, outdoors people do not have to live in or visit an endemic area to contract tick-borne, zoonotic pathogens. Healthcare practitioners must be cognizant that patients with a tick bite may have acquired polymicrobial infections.
Acknowledgments
Ethical consideration
Ethical approval for banding and sampling of songbirds were granted under animal use protocol 2007-5446 for McGill University, and federal banding permits issued by the Canadian Wildlife Service.
Authors’ contributions
Conceptualization and design: JDS and RRP. Collection and methodology: JDS, AM, EM and RRP. Formal analysis: JDS, EM, and RRP. Drafting of manuscript: JDS, EM, and RRP. Accuracy of data analysis: JDS, RRP. All authors read and approved the final version of the manuscript.
Competing financial and investment interests
The authors declare that they have no competing financial and investment interests related to these tick-borne zoonotic studies.
Funding
This research was supported in part by the Mary Alice Holmes Memorial Foundation, Lyme Ontario, and a philanthropic donor Diane Kindree. Research reported in this publication was supported through Shared Resources of The Ohio State University Comprehensive Cancer Center and the National Institutes of Health under grant number P30 CAO16058.
Recognition
We thank bird banders who collected field samples and took photographs. We are grateful to Amanda Green for computer graphics and Catherine Scott for logistical support.
References
- Nicholson WA, Sonenshine DE, Noden BH. Ticks (Ixodida). In Medical and Veterinary Entomology, 3rd ed. In: Mullen GR, Durden LA, Editors. Academic Press/Elsevier: London, UK; 2019. p. 603–672. ISBN 978-0-12-814043-7.
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP: Lyme disease―a tick-borne spirochetosis? Science. 1982;216:1317−1319.
- Wilske B, Preac-Mursic V, Göbel UB, Graf B, Jauris S, Soutschek E, Schwab E, Zumstain G. An OspA serotyping system for Borrelia burgdorferi based on reactivity of monoclonal antibodies and OspA sequence analysis. J Clin Microbiol. 1993;31:340−350.
- Jobe DA, Lovrich SD, Oldenburg, DG, Kowalski TJ, Callister SM. Borrelia miyamotoi infection in patients from upper midwestern United States, 2014-2015. Emerg Infect Dis. 2016;22:1471−1473.
- Wagemakers A, Oei A, Fikrig MM, Miellet WR, Hovius JW. The relapsing fever spirochete Borrelia miyamotoi is cultivable in a modified Kelly-Petterkofer medium, and is resistant to human complement. Parasit Vectors. 2014;7:418.
- Narurkar R, Mamonska-Dyga A, Nelson JC, Liu D. Autoimmune hemolytic anemic associated with babesiosis. Biomark Res. 2017;5:14.
- Scott JD, Sajid MS, Pascoe EL, Foley JE. Detection of Babesia odocoilei in humans with babesiosis symptoms. Diagnostics.2021;11:947.
- Michaud, S. Lyme, Anaplasma phagocytophilum, Bartonella henselae and possible Babesia odocoilei acute infections among 101 patients in a small community hospital in Quebec in 2021: A retrospective chart review. Proceedings of International Lyme and Associated Diseases Society, Orlando, Florida, USA, 2022.
- Dumler JS, Choi KS, Garcia-Garcia JC, Barat NS, Scorpio DG, Garyu JW, Grab DJ, Bakken JS. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis. 2005;11:1828−1834.
- Kandhi S, Ghazanfar H, Qureshi ZA, Kalangi H, Jyala A, Perez, ESA. An atypical presentation of a severe case of Anaplasma phagocytophilum. Cureus. 2022;14: e23224.
- Pritt BS, Allerdice MEJ, Sloan LM, Paddock CD, Munderdoh UG, Rikihisa Y, Tajima T, Paskewitz SM, Neitzel DF, Johnson DKH, et al. Proposal to reclassify Ehrlichia muris as Ehrlichia muris subsp. muris subsp. nov. and description of Ehrlichia muris subsp. eauclairensis subsp. nov., a newly recognized tick-borne pathogen of humans. Int J Syst Evol Microbiol. 2017;67: 2121−2126.
- Campagnolo ER, Tewari D, Farone TS, Livengood JL, Mason KL: Evidence of Powassan/deer tick virus in adult blacklegged ticks (Ixodes scapularis) recovered for hunter-harvested white-tailed deer (Odocoileus virginianus) in Pennsylvania: A public health perspective. Zoonoses Public Health. 2018;65:e589−594.
- Knox KK, Thomm AM, Harrington YA, Ketter E, Patitucci JM, Carrigan DR. Powassan/deer tick virus and Borrelia burgdorferi infection in Wisconsin tick populations. Vector Borne Zoonotic Dis. 2017;17:463−466.
- Anderson JF, Armstrong PM. Prevalence and genetic characterization of Powassan virus strains infecting Ixodes scapularis in Connecticut. An J Trop Med Hyg. 2012;87:754−759.
- Scott JD, Pesapane RR. Detection of Anaplasma phagocytophilum, Babesia odocoilei, Babesia sp., Borrelia burgdorferi sensu lato, and Hepatozoon canis in Ixodes scapularis ticks collected in eastern Canada.Pathogens. 2021;10:1265.
- Anderson JF, Magnarelli LA. Avian and mammalian hosts for spirochete-infected ticks and insects in a Lyme disease focus in Connecticut. Yale J Biol Med. 1984;57:627641.
- Anderson JF, Johnson RC, Magnarelli LA, Hyde FW. Involvement of birds in the epidemiology of Lyme disease agent Borrelia burgdorferi. Infect Immun. 1986;51:394-396.
- Anderson JF, Magnarelli LA: Enzootiology of Borrelia burgdorferi in the northeastern and northcentral United States. In: Biology of Ixodes ricinus Complex Ticks and Lyme Disease. Acarology IX Symposia;Ohio Biological Survey: Columbus, OH, USA, 1999;2:385−389.
- Scott JD, Clark KL, Foley JE, Anderson JF, Bierman BC, Durden LA. Extensive distribution of the Lyme disease bacterium, Borrelia burgdorferi sensu lato, in multiple tick species parasitizing avian and mammalian hosts across Canada. Healthcare. 2018;6:131.
- Scott JD. First report of Ixodes scapularis ticks parasitizing a North American porcupine in Canada. Parasitologia. 2021;1:45−49.
- Scott JD, Clark KL, Durden LA. Presence of Babesia odocoilei and Borrelia burgdorferi sensu lato in a tick and dual parasitism of Amblyomma inornatum and Ixodes scapularis on a bird in Canada.Healthcare. 2019;7:46.
- Stricker RB. Lyme disease: a potential polymicrobial infection. ASM News.2003;69:265.
- Sanchez-Vicente S, Tagliafierro T, Coleman JL, Benach JL, Tokarz R. Polymicrobial nature of tick-borne diseases. mBio. 2019;10:e02055-19.
- McLean RG, Ubico SR, Hughes CAN, Engstrom SM, Johnson RC. Isolation and characterization of Borrelia burgdorferi from blood of a bird captured in the Saint Croix River Valley. J Clin Microbiol. 1993;31:2038−2043.
- Scott JD, Lee MK, Fernando K, Durden LA, Jorgensen DR, Mak S, Morshed MG. Detection of Lyme disease spirochete, Borrelia burgdorferi sensu lato including three novel genotypes in ticks (Acari: Ixodidae) collected from songbirds (Passeriformes) across Canada. J Vect Ecol. 2010;35:124−139.
- Scott, JD, Anderson JF, Durden LA. Widespread dispersal of Borrelia burgdorferi-infected ticks collected from songbirds across Canada.J Parasitol. 2012;98:49−59.
- Richter D, Spielman A, Komar N, Matuschka FR. Competence of American Robins as reservoir hosts for Lyme disease spirochetes. Emerg Infect Dis. 2000;6:133−138.
- Dingler RJ, Wright, SA, Donohue AM, Macedo PA, Foley JE. Surveillance for Ixodes pacificus and the tick-borne pathogens Anaplasma phagocytophilum and Borrelia burgdorferi in birds from California’s inner coast range. Ticks Tick Borne Dis. 2014;5:436−445.
- Jia N, Zheng YC, Jiang JF, Jiang RR, Jiang BG, Wei R, Liu HB, Huo QB, Sun Y, Chu YL, et al. Human babesiosis caused by a Babesia crassa-like pathogen: A case series. Clin Infect Dis.2018;67:1110−1119.
- Zintl A, Mulcahy G, Skerrett, HE, Taylor SM, Gray JS. Babesia divergens, a bovine blood parasite of veterinary and zoonotic importance. Clin Microbiol Rev. 2003;16:622−636.
- Herwaldt BL, Persing DH, Precigout EA, Goff WL, Mathiesen DA, Taylor PW, Eberhard ML, Gorenflot AF. A fatal case of babesiosis in Missouri: Identification of another piroplasm that infects humans. Ann Intern Med. 1996;124:643−650.
- Conrad PA, Kjemtrup AM, Carreno RA, Thomford J, Wainwright K, Eberhard M, Quick R, Telford SR III, Herwaldt BL. Description of Babesia duncani n. sp. (Apicomplexa: Babesiidae) from humans and its differentiation from other piroplasms. Int J Parasitol. 2006;36:779−789.
- Gray JS, Weiss LM: Babesia microti. InEmerging Protozoan Pathogens; Chan N, editor. Abingdon, UK: Taylor and Francis, 2008. p. 303−349.
- Kim JY, Cho SH, Joo HN, Tsuji M, Cho SR, Park IJ, Chung GT, Ju JW, Cheun HI, Lee, HW, et al. First case of human babesiosis in Korea: Detection and characterization of a novel type of Babesia sp. (K01) similar to ovine Babesia. J Clin Microbiol. 2007;45:2084−2087.
- Man SQ, Qiao K, Cui J, Feng M, Fu YF, Cheng XJ. A case of human infection with a novel Babesia species in China. Infect Dis Poverty. 2016;5:28.
- Shih CM, Liu LP, Chung WC, Ong SJ, Wang CC. Human babesiosis in Taiwan: Asymptomatic infection with a Babesia microti-like organism in a Taiwanese woman. J Clin Microbiol. 1997;35:450−454.
- Kjemtrup AM, Conrad PA. Human babesiosis: An emerging tick-borne disease. Int J Parasitol. 2000;30:1323−1337.
- Herwaldt BL, Cacciò S, Gherlinzoni F, Aspöck H, Slemenda SB, Piccaluga P, Martinelli G, Edelhofer R, Hollenstein U, Poletti G, et al. Molecular characterization of a non-Babesia divergens organism causing zoonotic babesiosis in Europe. Emerg Infect Dis. 2003;9:943−948.
- Schnittger L, Rodriguez AE, Florin-Christensen M, Morrison DA. Babesia: A world emerging. Infect Genet Evol. 2012;8:1788−1809.
- Gray A, Capewell P, Zadoks R, Taggart MA, French AS, Katzer F, Shiels BR, Weir W. Wild deer in the United Kingdom are a potential reservoir for the livestock parasite Babesia divergens. Cur Res Parasitol Vector Borne Dis. 2021;1:100019.
- Armstrong PMP, Katavolos P, Caporale DA, Smith RP. Spielman A, Telford SR III. Diversity of Babesia infecting deer ticks (Ixodes dammini). Am J Trop Med Hyg. 1998;58:739−742.
- Steiner FE, Pinger RR, Vann CN, Abley MJ, Sullivan B, Grindle N, Clay K, Fuqua C: Detection of Anaplasma phagocytophilum and Babesia odocoilei DNA in Ixodes scapularis (Acari: Ixodidae) collected in Indiana. J Med Entomol.2006;43:437−442.
- Steiner FE, Pinger RR, Vann CN, Grindle N, Civitello D, Clay K, Fuqua C. Infection and co-infection rates of Anaplasma phagocytophilum variants, Babesia spp., Borrelia burgdorferi, and the rickettsial endosymbiont in Ixodes scapularis (Acari: Ixodidae) from sites in Indiana, Maine, Pennsylvania and Wisconsin. J Med Entomol. 2008;45:289−297.
- Hamer SA, Roy PL, Hickling GJ, Walker ED, Foster ES, Barber CC, Tsao JI. Zoonotic pathogens in Ixodes scapularis, Michigan. Emerg Infect Dis. 2007;13:1131−1133.
- Schoelkopf L, Hutchinson CE, Bendele KG, Goff WL, Willette M, Rasmussen JM, Holman PJ. New ruminant hosts and wider geographic range identified for Babesia odocoilei (Emerson and Wright 1970). J Wildl Dis. 2005;41:683−690.
- Holman PJ, Madeley J, Craig TM, Allsopp BA, Allsopp MT, Petrini KR, Waghela SD, Wagner GG. Antigenic, phenotypic and molecular characterization confirms Babesia odocoilei isolated from three cervids. J Wildl Dis. 2000;36:518−530.
- Waldrup KA, Kocan AA, Qureshi T, Davis DS, Baggett D, Wagner GG. Serological prevalence and isolation of Babesia odocoilei among white-tailed deer (Odocoileus virginianus) in Texas and Oklahoma. J Wildl Dis. 1989;25:194−201.
- Shock BC, Moncayo A, Cohen S, Mitchell EA, Williamson PC, Lopez G, Garrison LE, Yabsley MJ. Diversity of piroplasms detected in blood-fed and questing ticks from several states in the United States. Ticks Tick Borne Dis. 2014;5:373−380.
- Livengood J, Hutchinson ML, Thirumalapura N, Tewari D. Detection of Babesia, Borrelia, Anaplasma, and Rickettsia spp. in adult black-legged ticks (Ixodes scapularis) from Pennsylvania, United States, with a Luminex multiplex bead assay. Vector Borne Zoonotic Dis. 2020;20:406−411.
- Emerson HR and Wright WT: The isolation of a Babesia in white-tailed deer. Bull Wild Dis Assoc.1968;4:142−143.
- Emerson HR, Wright WT: Correction. J Wildl Dis. 1970;6:519.
- Perry BD, Nichols DK, Cullom ES. Babesia odocoilei Emerson and Wright, 1970 in white-tailed deer, Odocoileus virginianus (Zimmermann), in Virginia. J Wildl Dis. 1985;21:149−152.
- Eshoo MW, Carolan HE, Massire C, Chou DM, Crowder CD, Rounds MA, Phillipson CA, Schutzer SE, Ecker DJ. Survey of Ixodes pacificus ticks in California reveals a diversity of microorganisms and a novel and widespread Anaplasmataceae species. PLoS ONE. 2015;10:e0135828.
- Pattullo KM, Wobeser G, Lockerbie BP, Burgess HJ. Babesia odocoilei infection in a Saskatchewan elk (Cervus elaphus canadensis) herd. J Veter Diagn Invest. 2013;25:535−540.
- Scott JD, McGoey E, Pesapane RR. Tick-Borne pathogens Anaplasma phagocytophilum, Babesia odocoilei, and Borrelia burgdorferi sensu latoin blacklegged ticks widespread across eastern Canada. J Biomed Res Environ Sci. 2022;3:1249−1256.
- Scott JD, Clark KL, Coble NM, Ballantyne, TR. Detection and transstadial passage of Babesia species and Borrelia burgdorferi sensu lato in ticks collected from avian and mammalian hosts in Canada. Healhcare. 2019;7:155.
- Scott JD, Pascoe EL, Sajid MS, Foley JE. Detection of Babesia odocoilei in Ixodes scapularis ticks collected from songbirds in Ontario and Quebec, Canada. Pathogens. 2020;9:781.
- Scott JD, Pascoe E, Sajid MS, Foley J. Monitoring of nesting songbirds detects established population of blacklegged ticks and associated Lyme disease endemic area in Canada. Healthcare. 2020;8:59.
- Scott JD, Pascoe EL, Sajid MS, Foley JE. Detection of Babesia odocoilei in Ixodes scapularis ticks collected in southern Ontario, Canada. Pathogens.2021;10:327.
- Seutin G, White BN, Boag PT: Preservation of avian blood and tissue samples for DNA analysis. Can J Zool. 1991;69:82-90.
- Barbour AG, Bunikis J, Travinsky B, Hoen AG, Diuk-Wasser MA, Fish D, Tsao JI. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am J Trop Med Hyg. 2009;81:1120-1131.
- Drazenovich N, Foley J, Brown RN. Use of real-time quantitative PCR targeting the msp2 protein gene to identify cryptic Anaplasma phagocytophilum infections in wildlife and domestic animals. Vector Borne Zoonotic Dis. 2006;6:83-90.
- Jalovecka M, Sojka D, Ascencio M, Schnittger L. Babesia life cycle―when phylogeny meets biology. Trends Parasitol. 2019;35:356-368.
- Burgess EC: Experimental inoculation of mallard ducks (Anas platyrhynchos platyrhynchos) with Borrelia burgdorferi. J Wildl Dis. 1989;25:99-102.
- Haninová K, Taragelová V, Koci J, Schäfer SM, Hails R, Ullmann AJ, Piesman J, Lubuda M, Kurtenbach K. Association of Borrelia garinii and B. valaisiana with songbirds in Slovakia. Appl Environ Microbiol. 2003;69:2825-2830.
- Kitsou C, Foor SD, Dutta S, Bista S, Pa U. Tick gut barriers impacting tick-microbe interactions and pathogen persistence. Mol Microbiol. 2021;116:1241-1248.
- Bonnet SI, Pollet T. Update on the intricate tango between tick microbiomes and tick-borne pathogens. Parasite Immunol. 2021;43:e12813.
- Aivelo T, Norberg A, Tschirren B. Bacterial microbiota composition of Ixodes ricinus ticks: The role of environmental variation, tick characteristics and microbial interactions. PeerJ. 2019;7:e8217.
- Durand J, Herrmann C, Genné D, Sarr A, Gern L, Voordouw MJ: Multistrain infections with Lyme borreliosis pathogens in the tick vector. Appl Environ Microbiol. 2017;83:e02552-16.
- Swanson KI, Norris DE. Co-circulation of microorganisms in questing Ixodes scapularis nymphs in Maryland. J Vector Ecol. 2007;32:243-251.
- Waldrup KA, Kocan AA, Barker RW, Wagner GG. Transmission of Babesia odocoilei in white-tailed deer (Odocoileus virginianus) by Ixodes scapularis (Acari: Ixodidae). J Wildl Dis. 1990;26:390-391.
- O’Connor RM, Long JA, Allred DR. Cytoadherence of Babesia bovis-infected erythrocytes to bovine brain capillary endothelial cells provides an in vitro model for sequestration. Infect Immun. 1999;67:3921-3928.
- Schetters TP, Kleuskens J, Scholtes N, Gorenflot A. Parasite localization and dissemination in the Babesia-infected host. Ann Trop Med Parasitol. 1998;92:513-519.
- Allred DR, Al-Khedery B. Antigenic variation and cytoadhesion in Babesia bovis and Plasmodium falciparum: Different logics achieve the same goal. Mol Biochem Parasitol. 2004;134:27-35.
- Selig T, IIyas S, Theroux C, Lee J. Fatal babesiosis in an immunocompetent patient. R I Med J. 2022;105:20-23.
- Schetters T: Mechanisms involved in the persistence of Babesia canis infection in dogs. Pathogens. 2019;8:94.
- Bosman AM, Oosthuizen MC, Venter EH, Steyl JC, Gous TA, Penzhorn BL. Babesia lengau associated with cerebral and haemolytic babesiosis in two domestic cats. Parasit Vectors. 2013;6:128.
- Mosqueda J, Olvera-Ramirez A, Aguilar-Tipacamu G, Canto GJ. Current advances in detection and treatment of babesiosis. Curr Med Chem. 2012;19:1504-1518.
- Pirahanchi Y, Jessu, R, Aeddula NR. Physiology, sodium potassium pump. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2022 Jan-. NCBI Bookshelf. National Library of Medicine, National Institutes of Health.
- Schwarz S, Mohr A, Knauth M, Wildemann B, Storch-Hagenlocher B. Acute disseminated encephalomyelitis: A follow-up study of 40 adult patients. Neurology. 2001;56:1313.
- Fox LM, Wingerter S, Ahmed A, Chou J, Phein L, Levy O. Neonatal babesiosis: case report and review of the literature. Pediatr Infect Dis J. 2006;25:169-73.
- Iyer S, Goodman K. Congenital babesiosis from maternal exposure: A case report. J Emerg Med. 2019;56:e39−e41.
- Walker S, Coray E, Ginsberg-Peltz J, Smith L. A five-week-old twin with profound anemia: A case report of asymmetric congenital babesiosis. Cureus. 2022;14;e22774.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.