Biology Group . 2022 October 19;3(10):1224-1228. doi: 10.37871/jbres1581.
Zinc Concentrations in Different Parts of the Gastropod, Faunus ater, Collected from Intertidal Areas of Peninsular Malaysia
Chee Kong Yap1*, Krishnan Kumar2, Hisyam MND1, Wan Hee Cheng2, Wan Mohd Syazwan1, Noor Azrizal-Wahid1, Rosimah Nulit1, Mohd. Hafiz Ibrahim1, Muskhazli Mustafa1, Hideo Okamura3, Yoshifumi Horie3, Moslem Sharifinia4, Mehrzad Keshavarzifard4, Geetha Subramaniam2, Meng Chuan Ong5,6, Mohamad Saupi Ismail7, and Franklin Berandah Edward8
2Faculty of Health and Life Sciences, Inti International University, Persiaran Perdana BBN, 71800 Nilai, Negeri Sembilan, Malaysia
3Graduate School of Maritime Sciences, Faculty of Maritime Sciences, Kobe University, Kobe 658-0022, Japan
4Shrimp Research Center, Iranian Fisheries Science Research Institute, Agricultural Research, Education and Extension Organization (AREEO), Bushehr 7516989177, Iran
5Faculty of Science and Marine Environment, Universiti Malaysia Terengganu, 21030 Kuala Nerus, Terengganu, Malaysia
6Ocean Pollution and Ecotoxicology (OPEC) Research Group, Universiti Malaysia Terengganu, 21030 Kuala Nerus, Terengganu
7Fisheries Research Institute, Batu Maung, Pulau Pinang 11960, Malaysia
8Natural Resources and Environment Board, Petra Jaya, Kuching 93050, Sarawak, Malaysia
- Zn
- Different tissues
- Faunus ater
Abstract
Intertidal gastropods, Faunus ater (F. ater), were collected from four sampling sites in Peninsular Malaysia. It was found that the concentrations of Zinc (Zn) (mg/kg dry weight) ranged from 347.23 to 356.21 for Digestive Caecum (DC), 95.00 to 100.91 for foot, 102.73 to 109.00 for muscle, 42.73 to 177.72 for operculum, 87.57 to 161.76 for remainder, and 24.42 to 53.96 for shell of F. ater. Current results indicate that the intertidal waters of Pantai Sri Tujoh has a higher bioavailability of Zn and require further research. According to the current findings, F. ater may be employed as a biomonitor of Zn pollution and bioavailability.
Introduction
It's critical to comprehend the intertidal mollusks' biological ranges and background populations before sustainable resource exploitation from coastal settings [1-4]. As a biomonitor of heavy metal contamination in Peninsular Malaysia, intertidal gastropods other than the well-known green-lipped mussel Perna viridis should be considered [5-8]. This is due to the possibility that more intertidal biomonitors may provide more accurate data on metal contamination and bioavailability [9].
Numerous studies have demonstrated the efficacy of gastropods as biomonitors of heavy metal contaminations. The soft tissues of other mollusk species have also been reported in the literature to contain potentially hazardous metal. The gastropods include Lymnaea stagnalis, Contectiana listeria, and Bithynia tentaculata from the South Ural lakes in Russia, Telescopium telescopium from the Blanakan silvofishery ponds in Indonesia, 7 bivalves, and 13 gastropods from Palk Bay in India, Filopaludina javanica from some lakes in Bogor, Indonesia, and mud snails from the Southwest Crimea, Black Sea [10].
Aquatic mollusks have a tendency to accumulate heavy metals to larger levels in their various components, reaching concentrations that are noticeably higher than those of the ambient water concentrations [11,12]. Furthermore, Rainbow [13] asserts that the use of particular organisms as biomonitors of the bioavailability of heavy metals in coastal waters enables comparisons to be made across time and space because biomonitors offer integrated measurements of the ambient metal concentrations that are ecotoxicologically significant in those waters. On the other hand, according to Rainbow PS, et al. [9], a biomonitor can offer details on certain metal bioavailabilities for that biomonitor. However, it is generally accepted as acceptable to extrapolate about the site's total metal bioavailabilities from that biomonitor. F. ater may be helpful as a potential biomonitor of Zn bioavailabilities in the tested settings.
The concentrations of Cd, Cu, Ni, and Pb in the shell and soft tissue parts of F. ater from four sampling sites in Peninsular Malaysia were previously reported by Yap CK, et al. [1]. The Zn concentrations in the sediment and aquatic organisms are typically higher in the vicinity of point sources of Zn and decrease in the absence of those sources. The objective of the current study, which is a continuation of the research to disclose previously unreported data on Zn distributions in the shell and different parts of soft tissues of F. ater (Potamididae) by Yap CK, et al. [1] at four sampling sites, Pantai Sri Tujoh, Pantai Bisikan Bayu, Kg. Telaga Nenas, Kesang Laut in the East and West Coast of Peninsular Malaysia. The gastropod is a brackish-water snail that dwells in mangrove swamps. Thailand has reported on the distribution of this species [2,3].
Materials and Methods
Samples were collected from Peninsular Malaysia during June to September 2007 in the west coast; Telaga Nenas (KTN) and Kesang Laut (KL), as well as in the east coast; Pantai Seri Tujoh (PST) and Pantai Bisikan Bayu (PBB) (Figure 1). The family, genus, and species of F. ater were characterized by using the identification keys created by Uptham ES, et al. [14], Brandt RAM [15], and Van Benthem Jutting [16].
About 30 snails with shell lengths ranging from 3.82 - 6.79 cm were collected from each sampling site. The snails were transferred to the lab and stored at -10°C until analysis. The maximum shell lengths (from tip to aperture) for PST, PBB, KTN, and KL were respectively 4.83-6.79 cm, 3.82-5.20 cm, 3.98-6.78 cm, and 4.33-6.71 cm.
The F. ater soft tissues were painstakingly separated into five sections (Digestive Caecum (DC), the foot, the operculum, and the remaining tissues) before the analyses. The shell and different parts of soft tissues were then dried for a further 72 hours at 105°C to achieve constant dry weights [17].
The dried samples were weighed and placed in acid-washed digesting tubes. They were digested with 10 ml of concentrated nitric acid (BHD 69%, AnalaR grade). After being at a low temperature (40°C) for an hour, the samples were placed in a digestion block and heated to a high temperature (140°C) for 3 hours [8]. Dilute the cooled samples to 40ml, double deionized water was employed. The digested samples were then filtered using Whatman No. 1 filter paper and placed into pill boxes that had been acid-washed (filter speed: medium). Zinc in samples was determined using an air-acetylene flame Atomic Absorption Spectrophotometer (AAS) Perkim-Elmer™ Model 800. The standard solutions of Zn (1000 ppm) were prepared from MERCK Titrisol.
The recoveries for the Zn standard solutions during running of the instrumental analysis were between 80 and 110%, which was satisfactory. The Certified Reference Material (CRM) for dogfish liver was used to verify the analytical techniques for the gastropod (DOLT-3, National Research Council Canada). All of the metals' recoveries, specifically (certified CRM: 86.6 2.40 mg/kg dry weight; measured CRM: 80.9 1.94; percentage of recovery: 93.4 2.24%), were satisfactory.
Statistical Analysis
Using the statistical program SPSS version 12, a one-way post hoc ANOVA-Student Newman-Keuls (SNK) analysis was used to find significant differences between the mean values.
Results and Discussion
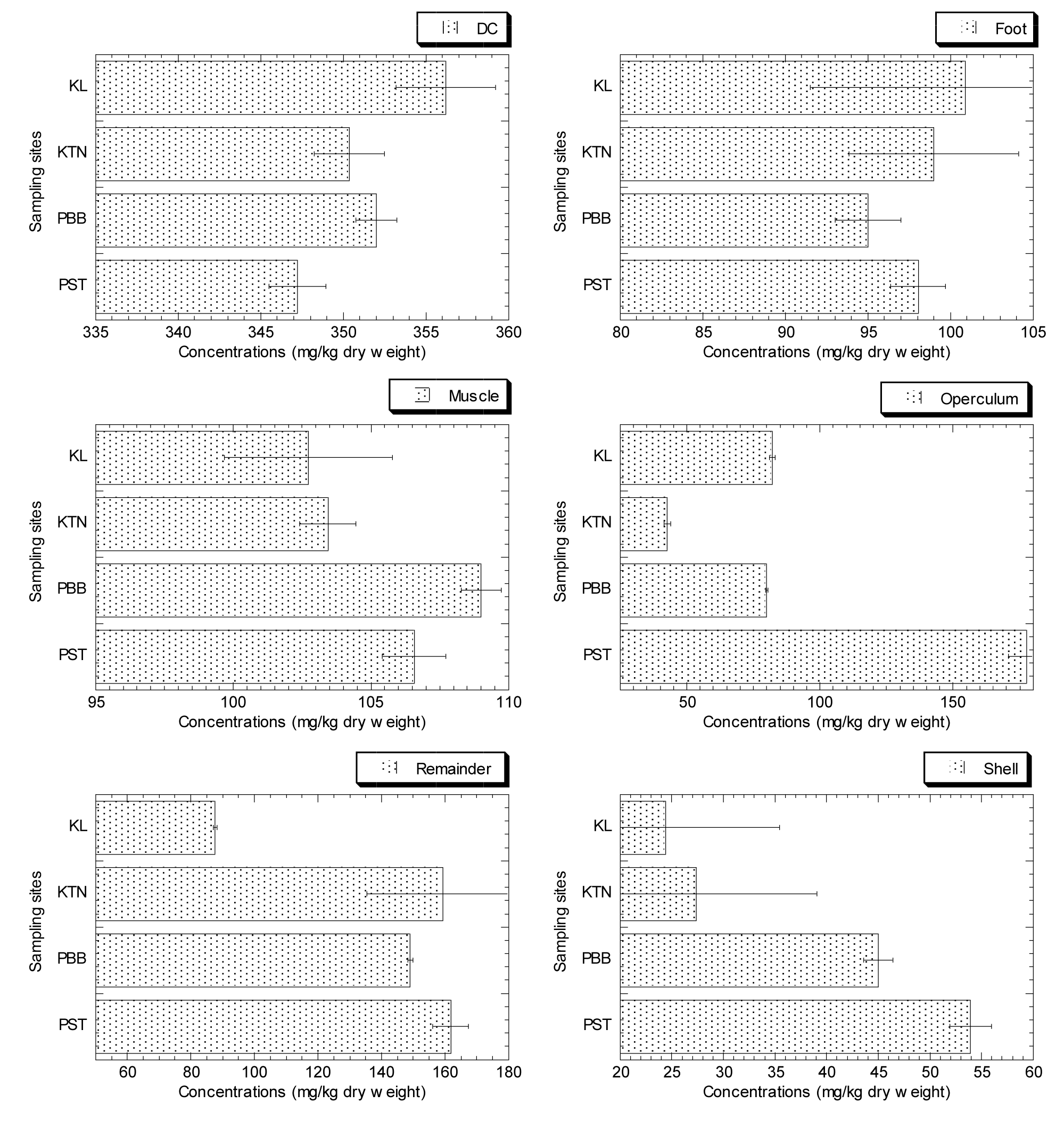
The Zn concentrations in the shell, DC and remaining parts in soft tissue of F. ater from the four sampling sites are displayed in table 1. It was found that Zn concentrations (mg/kg dry weight) ranged from 347.23 to 356.21 for DC, 95.00 to 100.91 for foot, 102.73 to 109.00 for muscle, 42.73 to 177.72 for operculum, 87.57 to 161.76 for remainder, and 24.42 to 53.96 for shell of F. ater. The overall order of Zn levels follows: DC > remainder > muscle ≥ foot ≥ operculum > shell. It was found that the DC and the other remainder of soft tissue of F. ater accumulated high concentrations of Zn, while, the lowest Zn concentrations were found in the shell of F. ater.
According to findings from other research, the variation in metal concentrations in gastropods may be due to variations in the affinities of the metals to the binding sites of the metallothioneins in the various tissues [18,19]. Additionally, metal accumulations in different tissues may be linked to the function of a particular organ in mollusks.
From the perspective of bioaccumulation, the high Zn concentrations in DC have proven to be significant. It is remarkable that the DC, a part of the digestive glands, has a higher-than-normal Zn concentration. This discovery is in line with earlier investigations on the digestive glands of marine gastropods, specifically Patella caerulea [20] and marine gastropods in general [21]. In the past, large concentrations of Pb and Ni, were found in the DC of Telescopium telescopium by Yap and Noorhaidah [22] and Yap CK, et al. [23], respectively. Due to the high Zn concentration, it was hypothesised that this DC acts as a repository for Zn, maybe in the form of metallothionein or granules as a Zn detoxification mechanism [23,24].
Figure 2 shows that KL has the largest concentrations of Zn in the DC and foot, PBB in the muscle, and PST in the operculum, shells, and remaining tissues. The Zn bioavailability of the sampling sites was calculated using the accumulation of Zn in the various parts in soft tissue of F. ater. The highest Zn levels were found in PST, which led researchers to conclude that PST has a high Zn bioavailability [25,26].
The current results also showed that Zn bioaccumulation varied among the different tissues of F. ater. However, it is still somewhat difficult to determine which sampling site had the highest bioavailability of Zn in coastal intertidal waters.
The cumulated concentrations in a biomonitor, are a good indicator of the integrated bioavailability and contamination of the sample sites [9]. This is because the bioaccumulate metals in tissues of biomonitor are proportion to the degree of environmental contamination. Such accumulated levels in a biomonitor are compared between sites in order to measure the bioavailabilities and contaminations of heavy metals at the sample locations [27].
The characteristics (anthropogenic activities) of the sampling locations may also have an impact on the pollution and metal bioavailability in these areas. Table 1 show that all four locations are well-known as fishing communities and aquacultural regions. It is believed that in addition to natural resources, anthropogenic activities that may be seen in these places may also affect the metal bioavailabilities of these four sampling locations [5]. The organic wastes discharged from fish/or mussel farms allegedly had an impact on the water quality surrounding fish culture zones, which may have contaminated the water and increased Zn bioavailability, according to Wu RSS, et al. [28] and Yap CK, et al. [6].
| Table 1: Overall statistics of Zn concentrations (mg/kg dry weight) in Faunus ater collected from four sampling sites in Peninsular Malaysia. | ||||||
| DC | Foot | Muscle | Operculum | Remainder | Shell | |
| Minimum | 347.23 | 95.00 | 102.73 | 42.73 | 87.57 | 24.42 |
| Maximum | 356.21 | 100.91 | 109.00 | 177.72 | 161.76 | 53.96 |
| Mean | 351.45 | 98.24 | 105.44 | 95.62 | 139.40 | 37.69 |
| Median | 351.18 | 98.52 | 105.01 | 81.01 | 154.13 | 36.19 |
| SD | 3.74 | 2.46 | 2.90 | 57.64 | 34.99 | 14.15 |
| SE | 1.87 | 1.23 | 1.45 | 28.82 | 17.49 | 7.07 |
| Skewness | 0.23 | -0.37 | 0.30 | 0.80 | -1.07 | 0.17 |
| Kurtosis | -1.11 | -1.06 | -1.55 | -0.85 | -0.74 | -1.72 |
| Note: DC: Digestive Caecum; SD: Standard Deviation; SE: Standard Error | ||||||
Conclusion
From the present findings, it was concluded that the DC of F. ater accumulated high concentrations of Zn. On the other hand, PST intertidal coastal waters may have a higher bioavailability of Zn. Current results indicate that the tidal waters of PST has a higher bioavailability of Zn and require further research. The results gathered in the present study suggested that F.ater could be used as a potential biomonitor of heavy metal contamination in the intertidal area of Peninsular Malaysia and as an alternative biomonitor mollusc to the well-established green-lipped mussel R. viridis.
Acknowledgment
The authors would like to express their gratitude for the financial assistance received through the Research University Grant Scheme (RUGS), [Vote No.: 91229], by Universiti Putra Malaysia, and the e-Science Fund, [Vote No.: 5450338], by the Ministry of Science, Technology and Innovation, Malaysia.
References
- Yap CK, Hisyam MND, Edward FB, Tan SG. Concentrations of heavy metal in the different parts of gastropod, Faunus ater collected from the intertidal area of Peninsular Malaysia. Pertanika Journal of Tropical Agricultural Science. 2010;33(1):45-60.
- Sri-aroon P, Lohachit C, Harada M. Brackish-water mollusks of Surat Thani Province, southern Thailand. Southeast Asian J Trop Med Public Health. 2005;36 Suppl 4:180-8. PMID: 16438206.
- Sri-Aroon P, Lohachit C, Harada M, Chusongsang P, Chusongsang Y. Malacological survey in Phang-Nga Province, southern Thailand, pre- and post-Indian Ocean tsunami. Southeast Asian J Trop Med Public Health. 2006;37 Suppl 3:104-9. PMID: 17547062.
- Tam NFY, Yao, MWY. Normalisation and heavy metal contamination in mangrove sediments. Science of Total Environment. 1998;216:33-39.
- Yap CK, Ismail A, Tan SG, Omar H. Concentrations of Cu and Pb in the offshore and intertidal sediments of the west coast of Peninsular Malaysia. Environ Int. 2002 Dec;28(6):467-79. doi: 10.1016/s0160-4120(02)00073-9. PMID: 12503912.
- Yap CK, Ismail A, Tan SG. Background concentrations of Cd, Cu, Pb and Zn in the green-lipped mussel Perna viridis (Linnaeus) from Peninsular Malaysia. Marine Pollution Bulletin. 2003;46:1035-1048.
- Yap CK, Ismail A, Tan SG, Rahim Ismail A. Assessment of different soft tissues of the green-lipped mussel Perna viridis (Linnaeus) as biomonitoring agent of Pb: Field and laboratory studies. Water, Air and Soil Pollution. 2004;153:253-268.
- Yap CK, Ismail A, Cheng WH, Tan SG. Crystalline style and tissue redistribution in Perna viridis as indicators of Cu and Pb bioavailabilities and contamination in coastal waters. Ecotoxicol Environ Saf. 2006 Mar;63(3):413-23. doi: 10.1016/j.ecoenv.2005.02.005. PMID: 16406592.
- Rainbow PS, Smith BD, Lau SSS. Biomonitoring of trace metal availabilities in the Thames estuary using a suite of littoral biomonitors. Journal of the Marine Biological Association of United Kingdom. 2002;82:793-799. doi: 10.1017/S002531540200615X.
- Ryabushko VI, Toichkin AM, Kapranov SV. Heavy Metals and Arsenic in Soft Tissues of the Gastropod Rapana venosa (Valenciennes, 1846) Collected on a Mollusk Farm Off Sevastopol (Southwestern Crimea, Black Sea): Assessing Human Health Risk and Locating Regional Contamination Areas. Bull Environ Contam Toxicol. 2022 Jun;108(6):1039-1045. doi: 10.1007/s00128-021-03451-w. Epub 2022 Jan 15. PMID: 35032177.
- Saha M, Sarkar SK, Bhattacharya B. Interspecific variation in heavy metal body concentrations in biota of Sunderban mangrove wetland, northeast India. Environ Int. 2006 Feb;32(2):203-7. doi: 10.1016/j.envint.2005.08.012. Epub 2005 Oct 5. PMID: 16213017.
- Kumar K, Elias S, Yap CK, Prakash B, Cheng WH, Chong MY. Distribution of heavy metals in sediments and soft tissues of the Cerithidea obtusa from Sepang River, Malaysia. Indones J Chem. 2022;22(4):1070-1080. doi: 10.22146/ijc.72991.
- Rainbow PS. Biomonitoring of heavy metal availability in the marine environment. Marine Pollution Bulletin. 1995;31:183-192. doi: 10.1016/0025-326X(95)00116-5.
- Upatham ES, Sornmani S, Kittikoon V, Lohachit C, Burch JB. Identification key for fresh and brackish-water snails of Thailand. Malacological Revision. 1983;16:107-132.
- Brandt RAM. The non-marine aquatic Mollusca of Thailand. Archives of Molluskenkund. 1974;105:1-423.
- Jutting B, Van WSS. Syatematic studies on the non-marine mollusca of the Indo-Australian Archipelaco. Treubia. 1956;23:259-477.
- Mo C, Neilson B. Standardization of oyster soft dry weight measurements. Water Research. 1994;28:243-246. doi: 10.1016/0043-1354(94)90140-6.
- Roesijadi G. The significance of low molecular weight, metallothionein-like protein in marine invertebrates: Current status. Marine Environment Research. 1980;4:167-179. doi: 10.1016/0141-1136(81)90032-5.
- Viarengo A , Palmero S, Zanicchi G, Capelli R, Vaissiere R, Orunesu M. Role of metallothioneins in Cu and Cd accumulation and elimination in the gill and digestive gland cells of Mytilus galloprovincialis (Lam.). Marine Environmental Research. 1984;16:23-36. doi: 10.1016/0141-1136(85)90018-2
- Yüzereroğlu TA, Gök G, Coğun HY, Firat O, Aslanyavrusu S, Maruldali O, Kargin F. Heavy metals in Patella caerulea (Mollusca, Gastropoda) in polluted and non-polluted areas from the Iskenderun Gulf (Mediterranean Turkey). Environ Monit Assess. 2010 Aug;167(1-4):257-64. doi: 10.1007/s10661-009-1047-x. Epub 2009 Jun 20. PMID: 19543988.
- Nott JA, Nicolaidou A. Metals in gastropods-metabolism and bioreduction. Marine Environmental Research. 1989;28(1-4):201-205. doi: 10.1016/0141-1136(89)90225-0.
- Yap CK, Noorhaidah A. Gill and digestive caecum of Telescopium telescopium are biomonitors of Pb bioavailability and contamination by Pb in the tropical intertidal area. Sains Malaysiana. 2011;40(10):1075-1085.
- Yap CK, Noorhaidah A, Tan SG. Digestive caecum and tissue redistribution in gill of Telescopium telescopium as indicators of Ni bioavailabilities and contamination in the tropical intertidal area. Water, Air and Soil Pollution. 2012;223:2891-2905. doi: 10.1007/s11270-012-1073-0.
- Lobel PB, Mogie P, Wright DA, Wu BL. Metal accumulation in four molluscs. Marine Pollution Bulletin. 1982;13(5):170-174. doi: 10.1016/0025-326X(82)90089-3.
- Yap CK, Aziran Y, Cheng WH. Distribution of heavy metal concentrations in the different soft tissues and shells of the bivalve Psammotaea elongata and gastropod Faunus ater collected from Pantai Sri Tujuh, Kelantan. Journal of Sustainability Science and Management. 2009;4(1):66-74.
- Yap CK, Mohd Ruszaidi S, Cheng WH, Tan SG. Heavy metal concentrations in the mangrove snail, Nerita lineata and surface sediments collected from Klang River Estuary, Selangor, Malaysia J Sustainability Sci Manage. 2010;5(1):1-12.
- Phillips DJH, Rainbow PS. Biomonitoring of trace aquatic contaminants. 2nd ed. London: Chapman and Hall; 1994.
- Wu RSS, Mackay DW. Lau TC, Yam V. Impact of marine fish farming on water quality and bottom sediment: A case study in the sub-tropical environment. Marine Environmental Research. 1994;38:115-145. doi: 10.1016/0141-1136(94)90004-3.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.