General Science . 2022 August 30;3(8):994-999. doi: 10.37871/jbres1543.
The Role of “Smart-Flap” of Sternocleidomastoid Muscle in Cervical Spine Surgery: A Proposal of a New Surgical Technique
Fabrizio Cuzzocrea1, Matteo Ghiara2, Chiara Mossinelli2, Marco Benazzo2 and Vincenzo Pio Gagliardi1
2Department of Otorhinolaryngology, Fondazione IRCCS Policlinico San Matteo, Otorhinolaryngology, Pavia, Italy
- Cervical spine surgery
- Oesophageal fistula
- Oesophageal perforation
- Pedicled flap
- Sternocleidomastoid muscle flap
Abstract
In cervical spine surgery there are known risk of complications such as esophageal fistula and dural tear. Usually the gold standard in treatment is represented by surgery. The sternocleidomastoid flap is the most common used in the revision procedure. In this paper we propose a modified surgical technique of harvesting the SCM flap and we suggest its use as prevention in selected cases.
Introduction
The sternocleidomastoid flap is a pedicled flap that was first described by Jianu [1]. Since then, it has been used in various surgical fields: reconstruction procedure after oncological surgery of head and neck (mandible, floor of the mouth, oropharynx) [2], prevention or treatment of Frey Syndrome after parotidectomy [3], revision procedures in anterior cervical spine surgery [4].
As described in literature, the SCM flap in cervical spine surgery can be harvested either partial or complete. The use of a partial and split SCM flap was firstly described by Alvarez [5].
The principal implications of this flap in anterior cervical spine surgery are esophageal perforations and dural tears [6,7]. The muscle tissue has the objective of reinforcement and/or repairing these points of weakness. Furthermore it is suitable also in selected cases of cervical spondylodiscitis to support the healing process: the high blood supply of the muscle is supposed to deliver more antibiotics selectively at the infectious site [8].
The risk of esophageal perforation is relatively low, with a medium rate of 2% [6], although the incidence of this complication could be underestimated because of the delayed clinical presentation and diagnosis [9]. This clinical event represents a life-threating complication with high mortality rates (between 16% and 50%) [10].
Esophageal perforations can be classified in traumatic and iatrogenic. The latter one can be subdivided depending on the timing of presentation: intraoperative, early and delayed. In literature the difference between early and delayed is proposed with a cut-off ranging from 7 days to 30 days after surgery [4,10].
The most common etiology of esophageal perforation after cervical spine surgery is represented by “hardware failure” (41%), followed by chronic erosion by hardware (31%), intraoperative injury such as retraction and operative tools (19%) and graft extrusion and penetration (7%)4.
The clinical presentation may vary, depending on different factors: etiology, location of the perforation and timing of presentation. It can range from asymptomatic cases to dysphagia, neck pain, neck swelling and abscesses, subcutaneous emphysema, unexplained fever, pharyngo-esophago-cutaneous fistula, mediastinitis and septic shock [6,8,10].
The early diagnosis is very important in order to improve the prognosis [11]. The mortality varies between 20% in case of early treatment (within 24 hours) to 50% in case of delayed management [12-14]. The first diagnostic step is represented by contrast pharyngo-esophageal study (gastrografin, methylene blue and barium swallow study), followed by CT scan; additionally, endoscopy and MRI can also be used [4]. The sensitivity of all the mentioned methods is about 80% [15].
The treatment of the esophageal fistula after spine surgery could be conservative or surgical. The conservative management includes wide spectrum antibiotics [16], stopping oral intake and introducing enteral (nasogastric tube) or parenteral nutrition. This kind of management is indicated only in cases of asymptomatic and small defects (less than 1 cm). For all other situations and in case of intraoperative defect the gold standard is represented by surgery. The closure of the esophageal defect can be obtained with a primary suture with or without the additional use of a pedicled flap; the most commonly used flap is the sternocleidomastoid one, because of the well-known topography, proximity to the esophageal defect, fair plicability and robustness [17]. Another advantage of the use of SCM flap is the possibility to harvest it from the superior or inferior insertion, depending on the vascularization of the muscle [18]. In case of large defects (more than 1 cm) the use of the whole muscle flap is suggested, while in case of small defects (less than 1 cm) a split flap is indicated [5,8].
Incidental dural tear during anterior cervical spine surgery is reported around 6% [19]. The reported risk factors in literature are age, sex, smoking, pre-existing conditions (ossification of the posterior longitudinal ligament or ligamentum flavum, degenerative spondylolisthesis, synovial cyst, dural ectasia in neurofibromatosis type 1, Marfan’s disease, intradural disk herniation, compressive osteophytes), primary versus revision surgery, type of procedure performed, elevated surgical invasiveness and experience of the surgeon [20].
Dural tears that are not properly sealed, or those that are not recognized, lead to recurrent CSF leakage that induce debilitating orthostatic headache, nausea and vomiting. The subsequent possible complications are wound infections, meningitis, myelocutaneous fistula and pseudomeningocele [20].
Even if primary direct repair by a water-thigh suture is the preferred method of choice, durotomies in anterior cervical spine surgery are generally difficult to access due to the limited exposure. Many methods of durotomy coverage are described, such as application of tissue sealants, collagen matrix sponge onlay, blood patches and tissue grafting [21]. The use of muscle augment or muscle pedicle flap to repair dural defect is a well-known technique. It was firstly employed in lumbar surgery as well as in intracranial and skull base surgery, but rarely for cervical fistulas. In 2012 Lien, et al. [20] utilized a SCM flap to prevent CSF leaks following durotomies in two anterior cervical procedures7. Neither patient exhibited a persistent postoperative CSF leak that required revision surgery. In addition the literature reports the conservative management: postoperative bed rest in sitting position to decrease the fluid pressure and eventual lumbar subarachnoid drain.
Spondilodyscitis is the inflammation of the vertebral body and the intervertebral disk space. It has multifactorial etiology and requires a multidisciplinary approach [22]. The gold standard of treatment is medical with appropriate antibiotic therapy [23]. In this clinical condition surgery has additional different roles: identification of pathogens through biopsy; stabilization of the segment in cases of instability; decompression in cases of neurological compromise; eventual evacuation of abscesses [22]. The use of the SCM flap in order to increase the blood supply has already been described [8]; this could selectively increase the delivery of antibiotics in the site of infection.
The aim of this manuscript is to propose a modified surgical technique of SCM flap. This kind of surgery could be performed in primary procedures (prevention of pharyngoesophageal fistula in selected cases after anterior cervical spine surgery), in revision surgery after complications (pharyngoesophageal fistula and dural tear after cervical spine surgery) or in selected cases of spondylodiscitis in addition to medical therapy.
Vascular Supply and Surgical Technique
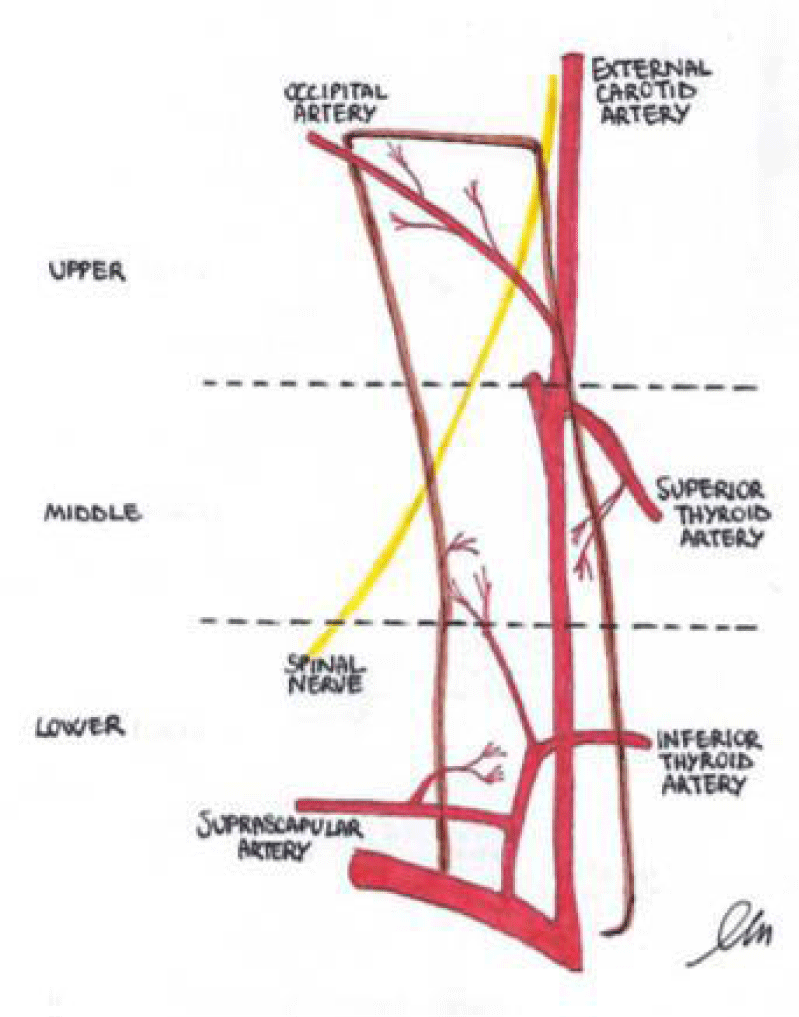
The vascular supply of the SCM muscle has been extensively described in previous studies [7,18,24]. The muscle receives supply from three main branches (Figure 1). The upper third of the muscle is constantly supplied by branches of the occipital artery. The middle third receives its supply from branches of the superior thyroid artery, whereas the inferior third is supplied by a branch of the thyrocervical trunk (suprascapular artery mostly).
The surgical technique was conceived as less invasive as possible in order to decrease the morbidity of the donor site and to prevent aesthetic damage as well as the so called “bulky” flap [25].
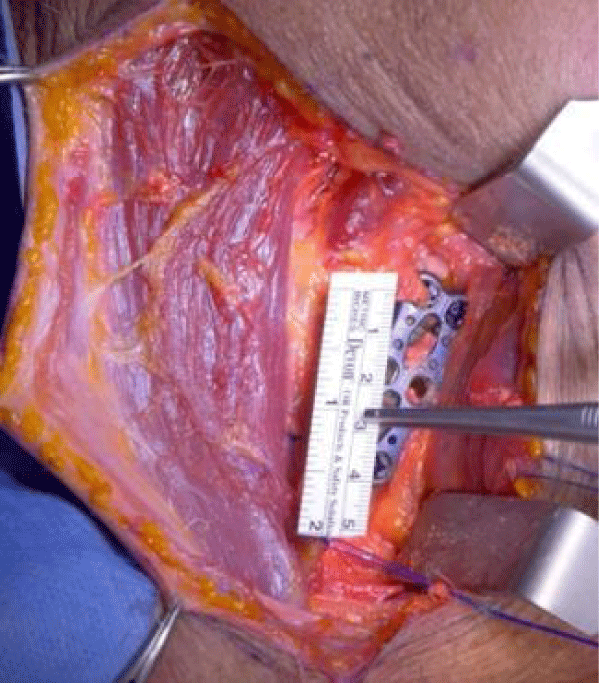
The procedure is designed to be performed with an enlarged antero-medial incision following the anterior border of the SCM muscle and by platysma muscle split along the line of its fibers. Depending on the distal extension of the incision the omohyoid muscle can be spared or cut. Once that the orthopedic procedure is over, the area to be covered must be measured and reported on the proximal or distal insertion of the SCM muscle (Figure 2).
The procedure will be described considering to be performed on the sternal portion: the muscle is split longitudinally to respect the fibers extending proximally as much as needed to cover the whole surface, but not exceeding the two thirds of the muscle belly in length [5].
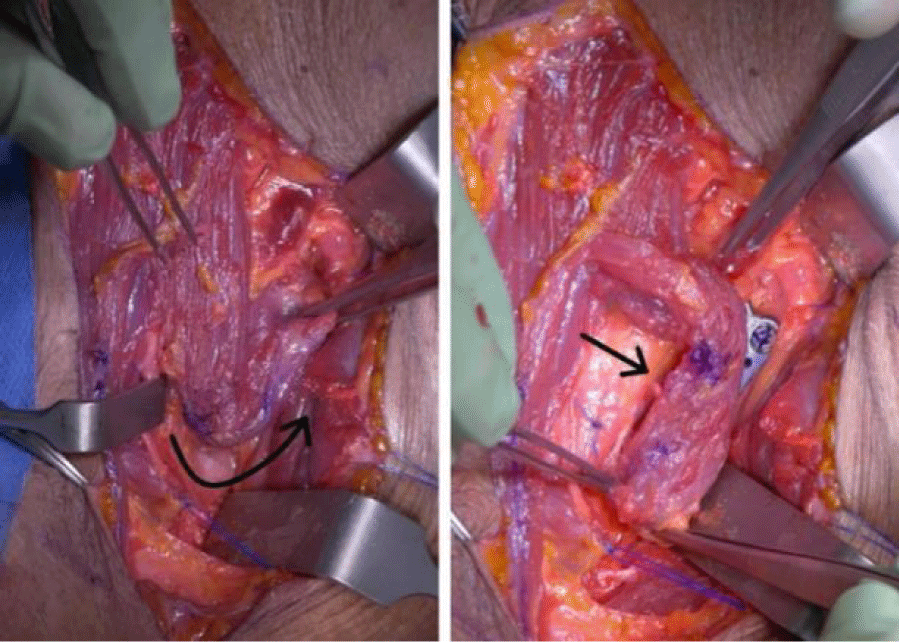
The distal end (sternal portion) is cut at the junction with the manubrium and transposed or rotated of 180° to cover the defect (Figure 3).
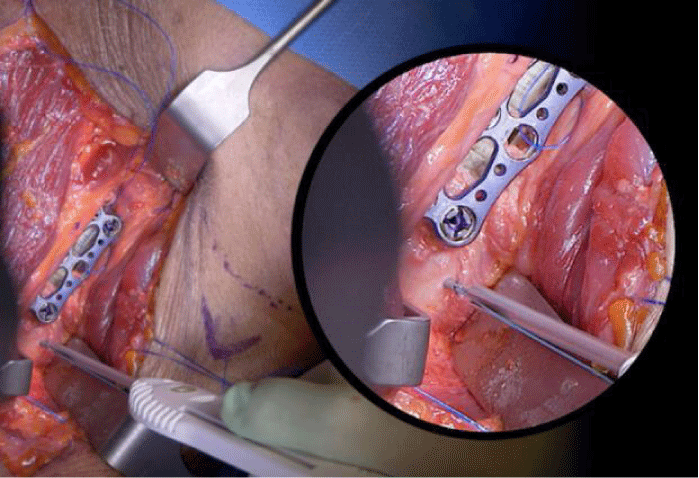
Once in place the proximal part is secured through a sliding notch to the proximal part of the plate and the caudal part is secured directly to the bony surface through a surgical anchor (whether absorbable or not) that has to be placed 1 or 2 cm below the disc level (Figure 4).
A second sliding notch allows the flap to adhere completely to the plate without excessive tension on the flap itself.
The edges of the flap are then sutured on the surrounding muscles below the deep vertebral fascia to guarantee the maximal adherence to the plate without excessive tension on the flap. The incision is then closed by layer. The use of a surgical drain is suggested to prevent hematoma formation.
Case Presentation
Case 1
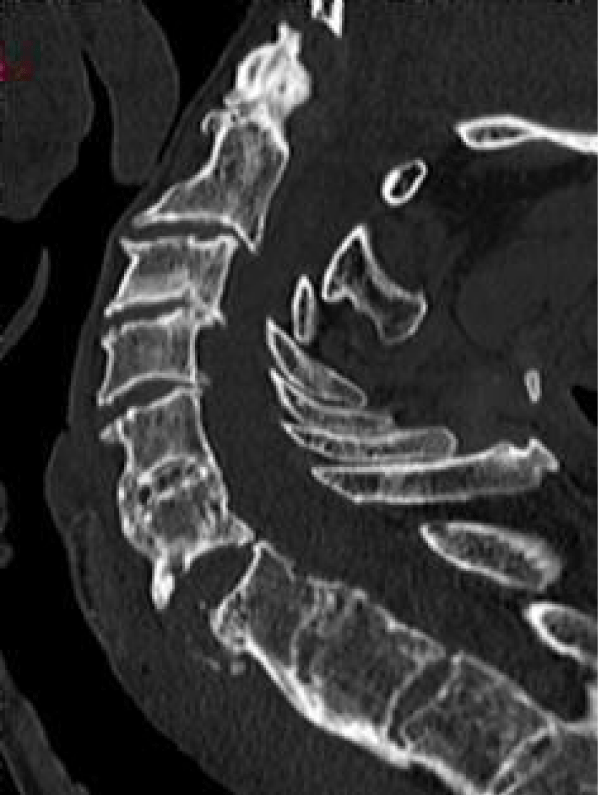
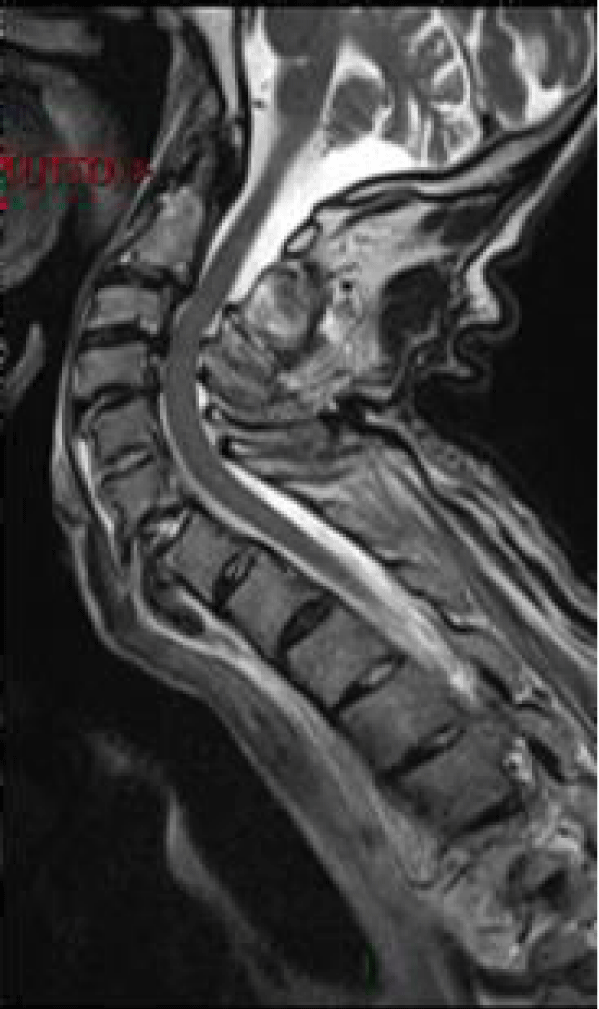
Man, 85 years old, chronic renal failure secondary to nephroangiosclerosis and arterial hypertension. He was admitted to the Department of Orthopedics after a car crash. The CT scan at the admission revealed multiple fractures: ribs, left ileo and ischiopubic rami and C6-C7 (B3 according to AO classification) (Figure 5). The patient underwent a cervical spine MRI (Figure 6) that confirmed the diagnosis without any sign of medullary trauma.
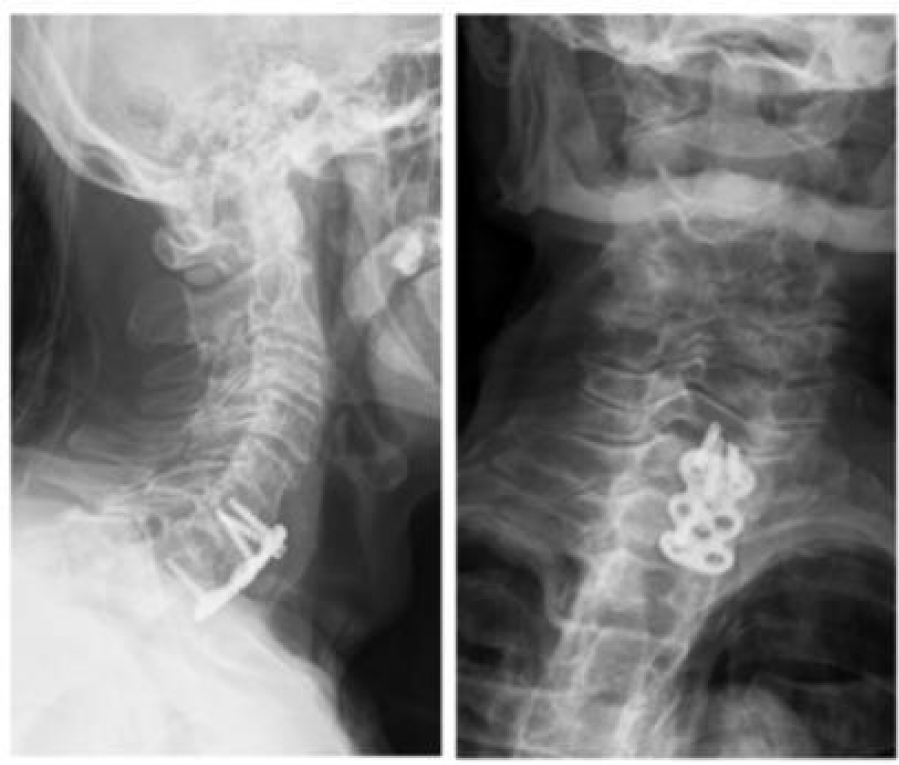
Once hemodynamically stable the patient was submitted to anterior cervical spine surgery: anterior C6-C7 arthrodesis with plate and screws (Figure 7).
After 3 days of normal course the patient started to complain dysphagia and fever. A chest x-ray turned out to be negative. An emergency CT-scan with barium meal showed positive blush of contrast in the neck and subcutaneous bubbles of air suggestive for esophageal perforation. Together with the ENT surgeons the patient underwent a second procedure: surgical toilette and closure of fistula reinforced by a smart SCM muscle flap. In 14th post-operative day the nasogastric tube was removed and the patient gradually went back to his normal eating habits. The patient was discharged in 20th post-operative day. At 3 months follow up he didn’t present any new complain and the implant maintains its stability without evidences of new esophageal fistula formation.
Case 2
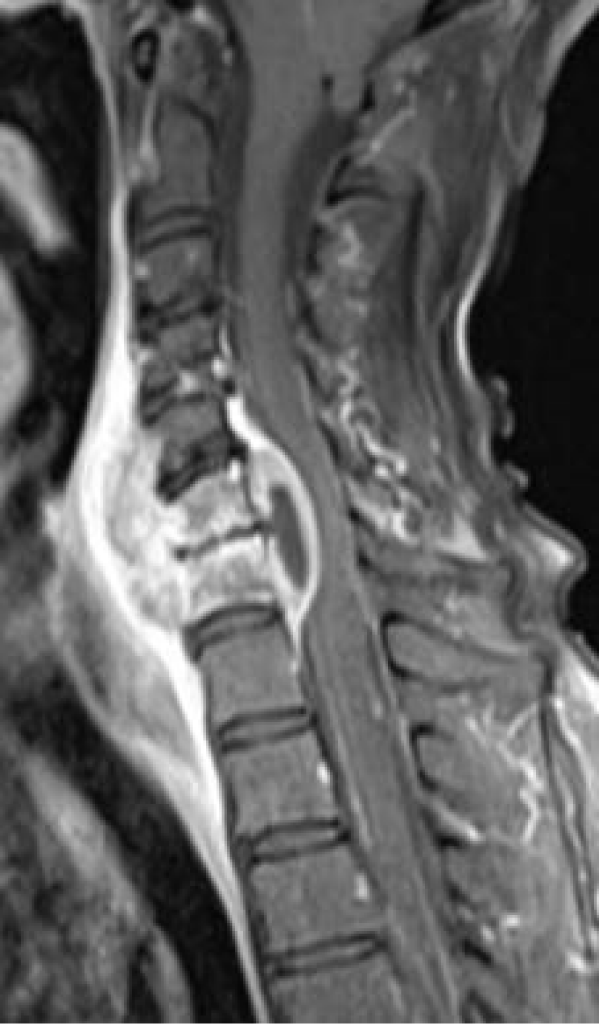
A woman, with 67 years old, had undergone right cervicotomy to remove a Schwannoma of the parapharyngeal space. After 45 days she complained worsening asthenia, dysphagia, bronchitis, lower limbs paraparesis and neurogenic bladder. She was admitted in another Hospital and treated as myasthenia gravis without any improvement. In the meantime, the clinical picture progressed to a complete tetraplegia. They performed an MRI (Figure 8) that showed a wide paravertebral and spinal abscess with medulla compression and signs of myelopathy.
At this point the patient came to our attention. We performed somatectomy of C6 and C7, anterior cervical arthrodesis C5-T1 with carbon fiber cage and plating and we covered it with a SCM smart flap. Posteriorly the procedure consisted in posterior arthrodesis C4-C5 to T1-T2. The neurologic condition improved immediately after the surgery. The intraoperative biopsies allowed the isolation of Streptococcus Oralis and Actinomyces Odontolyticus. After appropriate antibiotic therapy the patient was discharged with partial recovery of strength and sensibility to upper and lower limbs.
Discussion and Results
The SCM smart flap is a novel surgical technique to perform a split SCM muscle flap. The technique has been designed as easy as possible in order to be performed without a member of the ENT department. One of the advantages of this procedure is represented by the limited extension of the flap resulting in a lower morbidity to the donor site associated with a decreased operative time. This decreases the complications described with the use of the standard SCM muscle flap: functional loss, myonecrosis, bulky flap and cosmetic alterations.
This flap has different applications: we already described its use in cases of dural tears as well as in oesophageal fistula. It could be used also in selected cases to prevent delayed esophageal fistulas. In literature the chronic friction between the posterior wall of the oesophagus and plating system with formation of adhesions and traction-type diverticulum is described as the most common etiology in delayed perforations [26,27]. Considering the level of implant, the most vulnerable pharyngo-esophageal site is the Killian triangle, formed by the inferior constrictor pharyngeal and the cricopharyngeal muscles. In this region, corresponding to C5/C6, the posterior esophageal mucosa is unprotected by muscularis and is separated from the retroesophageal space only by the buccopharyngeal fascia [16]. Patient related risk factors can be considered old age, alcohol abuse, long term intubation, malnutrition and smoking. Implant related factors seem to be associated with implant profile: A lower implant decreases friction on the surrounding tissues [4]. Another independent intraoperative factor that has to be considered is the direct ischemic effect of excessive pressure exerted on the soft tissues by the retractors. Moreover, the tissue could be already damaged by an underlying pathological process (for example spondilodyscitis).
The surgeon should be aware that the most common timing of presentation for a delayed esophageal fistula is reported to be approximately two years whereas normal follow up for surgical uncomplicated patients is approximately one year. This could lead other physicians to difficult diagnosis in consideration of the more subtle and progressive symptoms that generally characterise the delayed lesions.
Furthermore, in literature removal of implants during these type of revisions is strongly suggested [10].
The esophageal tears in the delayed presentation appear to be bigger and with irregular edges which lead to a more difficult and invasive procedure [4].
Considering all these factors we suggest to prevent these complications performing the “smart” SCM flap directly during primary procedures in selected cases.
Conclusion
In conclusion, the “smart” SCM flap can be considered a good option in different applications: repair or prevention of esophageal fistula, optional treatment in dural tears after anterior cervical spine surgery, additional mode of antibiotics vehicle in cases of spondylodiscitis. The ease of use of this surgical technique allows performing it in primary procedures without the need of ENT specialist. The use of “smart” SCM flap as a prophylactic measure still needs time to evaluate its efficacy. Further studies are needed.
References
- Jianu. J. The surgical treatment of facial paralysis. Am Hear Assoc. 1909:377-386.
- Laccourreye O, Ménard M, Behm E, Garcia D, Cauchois R, Holsinger FC. Sternocleidomastoid myofascial flap for reconstruction after composite resection of invasive squamous cell carcinoma of the tonsillar region: Technique and outcome. Laryngoscope. 2006 Nov;116(11):2001-2006. doi: 10.1097/01.mlg.0000236845.51421.03. PMID: 17075422.
- Nofal AA, Mohamed M. Sternocleidomastoid muscle flap after parotidectomy. Int Arch Otorhinolaryngol. 2015 Oct;19(4):319-324. doi: 10.1055/s-0035-1549155. Epub 2015 Mar 27. PMID: 26491478; PMCID: PMC4593913.
- Halani SH, Baum GR, Riley JP, Pradilla G, Refai D, Rodts GE Jr, Ahmad FU. Esophageal perforation after anterior cervical spine surgery: A systematic review of the literature. J Neurosurg Spine. 2016 Sep;25(3):285-291. doi: 10.3171/2016.1.SPINE15898. Epub 2016 Apr 15. PMID: 27081708.
- Urken M. Atlas of head and neck flap. Lippincott Willimas and Wilkins. 2012:53.
- Benazzo M, Spasiano R, Bertino G, Occhini A, Gatti P. Sternocleidomastoid muscle flap in esophageal perforation repair after cervical spine surgery: Concepts, techniques, and personal experience. J Spinal Disord Tech. 2008 Dec;21(8):597-605. doi: 10.1097/BSD.0b013e31815c5f96. PMID: 19057255.
- Lien JR, Patel RD, Graziano GP. Sternocleidomastoid muscular flap: treatment of persistent cerebrospinal fluid leak after anterior cervical spine surgery. J Spinal Disord Tech. 2013 Dec;26(8):449-453. doi: 10.1097/BSD.0b013e31825f6fbf. PMID: 22643186.
- Ellabban MA. The sternocleidomastoid muscle flap: A versatile local method for repair of external penetrating injuries of hypopharyngeal-cervical esophageal funnel. World J Surg. 2016 Apr;40(4):870-880. doi: 10.1007/s00268-015-3306-z. PMID: 26578319.
- Boulahroud O, Choho A, Ajja A. Successfull management of a cervical oesophageal injury after an anterior cervical approach: A case report. Pan Afr Med J. 2017 Nov 28;28:274. doi: 10.11604/pamj.2017.28.274.13870. PMID: 29881514; PMCID: PMC5989256.
- Perrone O, Tassi V, Mattioli B, Daddi N, Uneddu M, Borghesi I, Mattioli S. Pharyngo-oesophageal perforation following anterior cervical discectomy and fusion: management and results. Eur J Cardiothorac Surg. 2017 Jan;51(1):160-168. doi: 10.1093/ejcts/ezw292. Epub 2016 Sep 11. PMID: 27615268.
- Solerio D, Ruffini E, Gargiulo G, Camandona M, Raggio E, Solini A, Dei Poli M. Successful surgical management of a delayed pharyngo-esophageal perforation after anterior cervical spine plating. Eur Spine J. 2008 Sep;17 Suppl 2(Suppl 2):S280-284. doi: 10.1007/s00586-007-0578-5. Epub 2008 Jan 26. PMID: 18224356; PMCID: PMC2525898.
- Orlando ER, Caroli E, Ferrante L. Management of the cervical esophagus and hypofarinx perforations complicating anterior cervical spine surgery. Spine (Phila Pa 1976). 2003 Aug 1;28(15):E290-295. doi: 10.1097/01.BRS.0000087093.89889.0A. PMID: 12897507.
- Pompili A, Canitano S, Caroli F, Caterino M, Crecco M, Raus L, Occhipinti E. Asymptomatic esophageal perforation caused by late screw migration after anterior cervical plating: Report of a case and review of relevant literature. Spine (Phila Pa 1976). 2002 Dec 1;27(23):E499-502. doi: 10.1097/00007632-200212010-00016. PMID: 12461406.
- Sharma RR, Sethu AU, Lad SD, Turel KE, Pawar SJ. Pharyngeal perforation and spontaneous extrusion of the cervical graft with its fixation device: A late complication of C2-C3 fusion via anterior approach. J Clin Neurosci. 2001 Sep;8(5):464-468. doi: 10.1054/jocn.2000.0826. PMID: 11535022.
- Navarro R, Javahery R, Eismont F, Arnold DJ, Bhatia NN, Vanni S, Levi AD. The role of the sternocleidomastoid muscle flap for esophageal fistula repair in anterior cervical spine surgery. Spine (Phila Pa 1976). 2005 Oct 15;30(20):E617-622. doi: 10.1097/01.brs.0000182309.97403.ca. PMID: 16227880.
- Brinster CJ, Singhal S, Lee L, Marshall MB, Kaiser LR, Kucharczuk JC. Evolving options in the management of esophageal perforation. Ann Thorac Surg. 2004 Apr;77(4):1475-1483. doi: 10.1016/j.athoracsur.2003.08.037. PMID: 15063302.
- Kierner AC, Zelenka I, Gstoettner W. The sternocleidomastoid flap--its indications and limitations. Laryngoscope. 2001 Dec;111(12):2201-2204. doi: 10.1097/00005537-200112000-00025. PMID: 11802026.
- Kierner AC, Aigner M, Zelenka I, Riedl G, Burian M. The blood supply of the sternocleidomastoid muscle and its clinical implications. Arch Surg. 1999 Feb;134(2):144-147. doi: 10.1001/archsurg.134.2.144. PMID: 10025452.
- Epstein NE. A review article on the diagnosis and treatment of cerebrospinal fluid fistulas and dural tears occurring during spinal surgery. Surg Neurol Int. 2013 May 6;4(Suppl 5):S301-317. doi: 10.4103/2152-7806.111427. PMID: 24163783; PMCID: PMC3801173.
- Hannallah D, Lee J, Khan M, Donaldson WF, Kang JD. Cerebrospinal fluid leaks following cervical spine surgery. J Bone Joint Surg Am. 2008 May;90(5):1101-1105. doi: 10.2106/JBJS.F.01114. PMID: 18451403.
- Bosacco SJ, Gardner MJ, Guille JT. Evaluation and treatment of dural tears in lumbar spine surgery: A review. Clin Orthop Relat Res. 2001 Aug;(389):238-247. doi: 10.1097/00003086-200108000-00033. PMID: 11501817.
- Gentile L, Benazzo F, De Rosa F, Boriani S, Dallagiacoma G, Franceschetti G, Gaeta M, Cuzzocrea F. A systematic review: Characteristics, complications and treatment of spondylodiscitis. Eur Rev Med Pharmacol Sci. 2019 Apr;23(2 Suppl):117-128. doi: 10.26355/eurrev_201904_17481. PMID: 30977878.
- Herren C, Jung N, Pishnamaz M, Breuninger M, Siewe J, Sobottke R. Spondylodiscitis: Diagnosis and treatment options. Dtsch Arztebl Int. 2017 Dec 25;114(51-52):875-882. doi: 10.3238/arztebl.2017.0875. PMID: 29321098; PMCID: PMC5769318.
- Leclère FM, Vacher C, Benchaa T. Blood supply to the human sternocleidomastoid muscle and its clinical implications for mandible reconstruction. Laryngoscope. 2012 Nov;122(11):2402-2406. doi: 10.1002/lary.23430. Epub 2012 Sep 24. PMID: 23007956.
- Cuzzocrea F, Ghiara M, Vanelli R, Medetti M, Lombardini AA, Benazzo F, Mauramati S, Mossinelli C, Herman I, Benazzo M. Smart flap of sternocleidomastoid muscle in anterior cervical spine surgery: Surgical anatomical dissection technique. Head Neck. 2020 Mar;42(3):587-589. doi: 10.1002/hed.25976. Epub 2019 Nov 1. PMID: 31675162.
- Yang SY, Lee SB, Cho KS. Delayed esophagus perforation after anterior cervical spine surgery. Korean J Neurotrauma. 2015 Oct;11(2):191-194. doi: 10.13004/kjnt.2015.11.2.191. Epub 2015 Oct 31. PMID: 27169093; PMCID: PMC4847517.
- Witwer BP, Resnick DK. Delayed esophageal injury without instrumentation failure: Complication of anterior cervical instrumentation. J Spinal Disord Tech. 2003 Dec;16(6):519-523. doi: 10.1097/00024720-200312000-00006. PMID: 14657748.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.