Biology Group . 2022 August 15;3(8):934-940. doi: 10.37871/jbres1535.
Three-Dimensional Electron Microscopy of Human Umbilical Cord Tissue Allograft Pre and Post Processing: A Literature Comparison
Justine M Davis1*, Joseph R Purita2, John Shou3 and Tyler C Barrett1
2Institute of Regenerative Medicine, 200 Glades Road, Suite 1, Boca Raton, FL 33432, USA
3Department of Pharmacology and Chemical Biology, One Baylor Plaza- BCM330 Houston, TX 77030, USA
- Wharton’s jelly
- Minimal manipulation
- Structural tissue defect
Abstract
One in four adults in the US suffer from cartilage degeneration of the Intervertebral Disc (DDD) or load bearing joints (DJD). Since cartilage is avascular, it has a limited regenerative capacity. Conventional non-surgical treatments provide brief symptomatic relief, have sided effects, and do not address the cartilage defect itself. As such, new alternatives are needed. Perinatal birth tissue allografts are a novel frontier for bio-mechanical cartilage engineering research. The tissues of interest include umbilical cord-derived Wharton’s Jelly (WJ). This study assessed WJ tissue samples via ZEISS Supra 55VP FieldEmission Scanning Electron Microscope (SEM) at 100 and 300 nm resolution scales. The captured images of pre and post-processed structural tissue matrices in WJ allografts were analyzed against themselves and peer-reviewed SEM images of articular cartilage, intervertebral disc cartilage, and muscle fascia. SEM images of post-processed WJ structural tissue matrices were found to be comparable to structural tissue matrices in human articular cartilage, intervertebral disc cartilage, and muscle fascia on a qualitative and quantitative level. This is the first study that we are aware of, to demonstrate that structural collagen matrices in post-processed WJ allografts are analogous in structure to the cartilage in articular joints, intervertebral discs, and muscle fascia.
Introduction
Advances in regenerative medicine have increased significantly throughout the past decade. Wharton’s Jelly (WJ) was initially characterized in 1656 by Thomas Wharton [1]. Since its initial discovery, there has been significant interest in the use of WJ in regenerative medicine applications [2]. Located between the blood vessels of the umbilical cord and the amniotic epithelium, WJ spans the entire length of the umbilical cord, providing protection, cushioning, and structural support [2,3]. Initial research centered on WJ as a cellular product, dependent on the metabolic activity of living cells to exert its primary function [3]. However, current research demonstrates that WJ exerts an effect independent of any cellular activity [3]. Initially classified as a mucoid connective tissue, we now know that WJ functions as an ideal system to transplant chemokine and growth factors, in addition to providing a biomechanical microarchitecture for collagen extracellular matrix formation in collagen-based defects [4].
ECM is a collagen-based, cross-linked network that provides tensile strength and distributes load. Damage, degeneration or diminished function in collagen cross-bridging will lead to loss of mechanical properties of the collagen network and result in an impaired ability to resist forces delivered by compression [5]. Collagen-specific structural tissue defects can be identified by physical examination and confirmed with MRI or musculoskeletal ultrasound [6]. A structural tissue defect in numerous skeletal muscles would prove detrimental to both functional outcomes and work productivity. Collagen is the most abundant family of Extracellular Matrix (ECM) proteins and is ubiquitous throughout the body [7].
Collagen fibers are organized into a system of multi-thread ropes, allowing for more flexibility when compared to compact fibers [8]. Human tissue strength is directly dependent upon the sizeable crosslinking of collagen [9]. Collagen types II, III, V, VI, and XII have been isolated from WJ [2,10].
Regeneration of collagen structural tissue defect occurs as ECM is regenerated in the base of the defect, restoring the original relevant characteristics of the damaged tissue [10]. Scar tissue will only achieve a maximum of 80% strength of the native tissue [11]. There is great potential for the WJ matrix derived from the human Umbilical Cord (UC) to act as an ECM scaffold and augment the regenerative process [10]. WJ contains an abundant amount of collagen and hyaluronic acid, both essential components of ECM [2,10]. Since WJ is a comprehensive source of human extracellular matrix proteins and growth factors; there may be numerous clinical applications for collagen-based structural tissue defects [3].
Previously reported uses for the human umbilical cord and its byproducts, including umbilical cord-derived WJ, have focused on the use and proliferation of mesenchymal stem cells [12]. WJ has frequently been studied in terms of cellular activity [13]. However, we have chosen to report on the role of umbilical cord-derived WJ as an extracellular collagen matrix, or micro-architectural framework based on its qualitative and quantitative aspects. Current clinical practice guidelines and research support the homologous use of WJ and its non-cellular role in establishing a microarchitectural framework for ECM in collagen-based structural tissue defects.
Human cell, tissue, and tissue-based products (HCT/Ps) are regulated by the United States Food and Drug Administration (FDA) under title 21, part 1271 of the Code of Federal Regulations (CFR). Under these regulations, the HCT/P must be minimally manipulated, have no systemic effect or be dependent on living cells for its primary function, be intended for homologous use only, and exclude the combination of the product’s cells or tissues with another article except for water, crystalloids, or a sterilizing, preserving, or storage agent provided that the storage agent does not raise new clinical safety concerns with respect to the HCT/P [13]. This study aimed to assess WJ tissue samples via a ZEISS Supra 55VP Field-Emission Scanning Electron Microscope (SEM) at 100 and 300 nm resolution scales.
Materials and Methods
All methods were completed in compliance with the FDA and American Association of Tissue Banks (AATB) standards. The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Institute of Regenerative and Cellular Medicine (IRCM-2022-311) on 12 January 2022.
Donation and collection
Human umbilical cords were obtained from consenting donors following full-term Caesarian section deliveries. Prior to delivery, donors underwent comprehensive medical, social, and blood testing. Qualtex Laboratories in San Antonio, TX tested all donations for infectious disease in accordance with Clinical Laboratory Improvement Amendments (CLIA) of 1988, 42 CFR part 493, and FDA regulations. Each donor was tested for Hepatitis B Core Antibody (HBcAb), Hepatitis B, Surface Antigen (HBsAg), Hepatitis C Antibody (HCV), Human Immunodeficiency Virus Antibody, (HIV-1/HIV-2 Plus O), Human T-Lymphotropic Virus Antibody (HLTV-I/11), Syphilis (RPR), Cytomegalovirus (CMV), HIV-1/HCV/HBV, NAT, and West Nile Virus (WNV). Each test was performed with an FDA-Approved testing kit [Appendix A]. All test results were negative or non-reactive.
Preparation of the pre-processed umbilical cord tissue samples
All procedures were performed in accordance with strict aseptic techniques. In an ISO class 5 biologic safety cabinet, the umbilical cord was rinsed with saline to remove excess blood residue and clots. Various sized cross-sections of the umbilical cord were cut and placed on a sterile drying tray. The cross-sections were then desiccated in a high nitrogen concentration drying chamber.
Preparation of processed umbilical cord tissue samples product
Wharton's jelly was aseptically dissociated from the rinsed umbilical cord. After dissociation, 150 mg of Wharton’s Jelly was suspended in approximately 2mL of sterile Sodium Chloride 0.9% solution (normal saline). The sample was not combined with cells, tissues, or articles other than the exceptions outlined in 21 CFR Part 1271.10(a) (3) (Human Cells, Tissues, and Cellular and Tissue-Based Product Regulation).
The manufacture of the HCT/P does not involve the combination of the cells or tissues with another article, except for water, crystalloids, or a sterilizing, preserving, or storage agent, provided that the addition of water, crystalloids, or the sterilizing, preserving, or storage agent does not raise new clinical safety concerns with the respect to the HCT/P [15].
Scanning electron microscope imaging
Pre-processed and post-processed desiccated tissue samples were received by the University of Montana laboratory for analysis and electron microscope imaging. The tissue samples were transferred to a sticky carbon surface (PELCO Tab, Ted Pella, Inc.) and coated with a thin layer of iridium (60 seconds at 25 mA, Emite K575X Sputter Coater) to mitigate charging in the microscope. The tissue samples were then examined via a ZEISS Supra 55VP Field-Emission Scanning Electron Microscope (SEM) at a 100 and 300 nm scale of resolution.
Results
The present study reports the anatomic structural compatibility of a human Umbilical Cord Tissue (UCT) allograft before and after processing to provide objective evidence of minimal manipulation. The resultant comparison establishes examples of homologous use with objective evidence.
Discussion
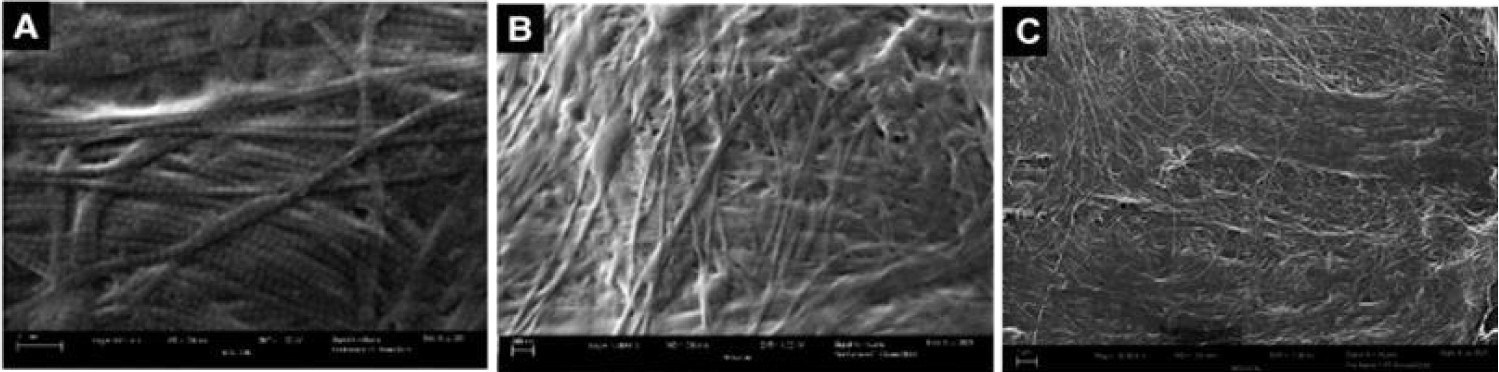
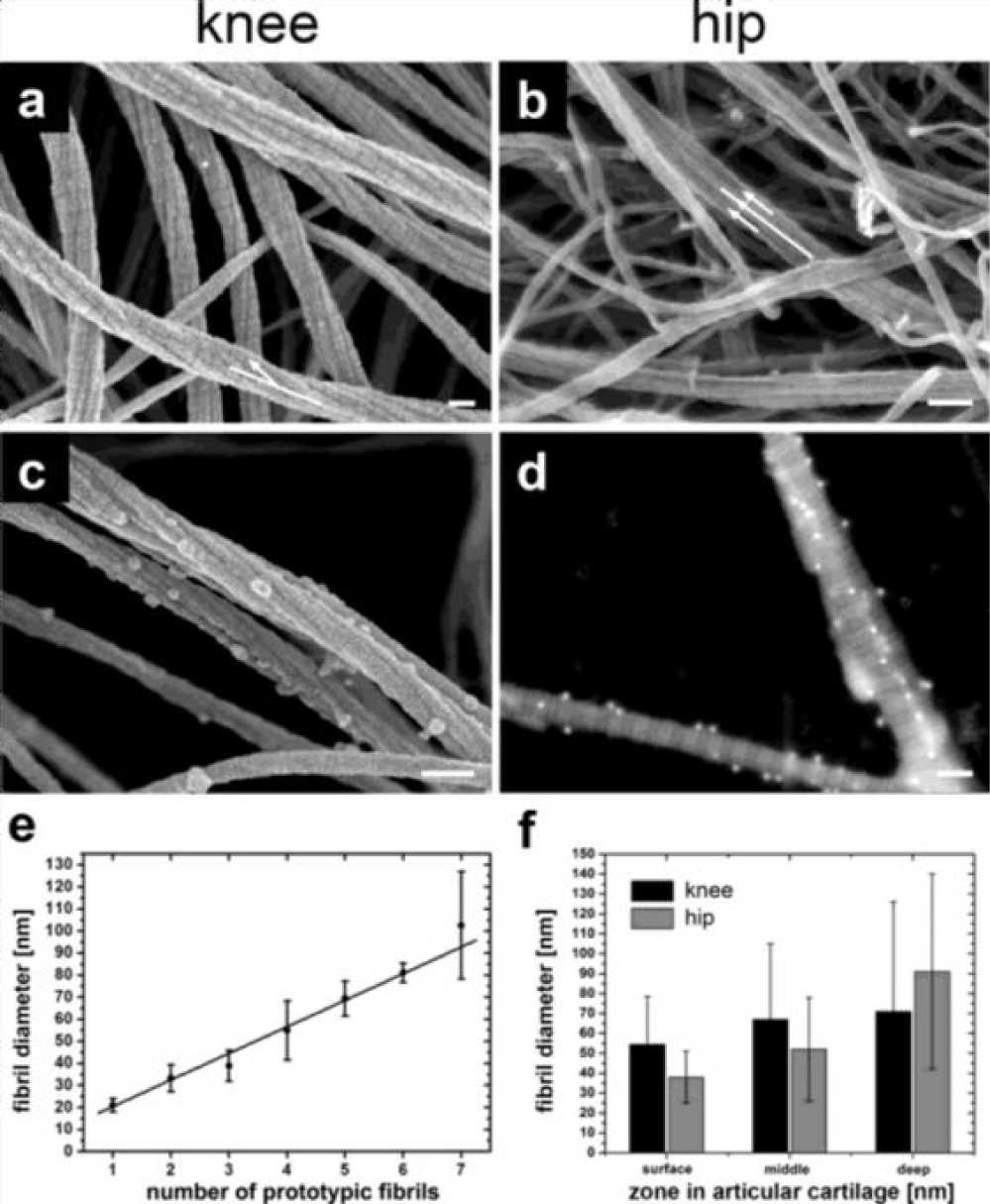
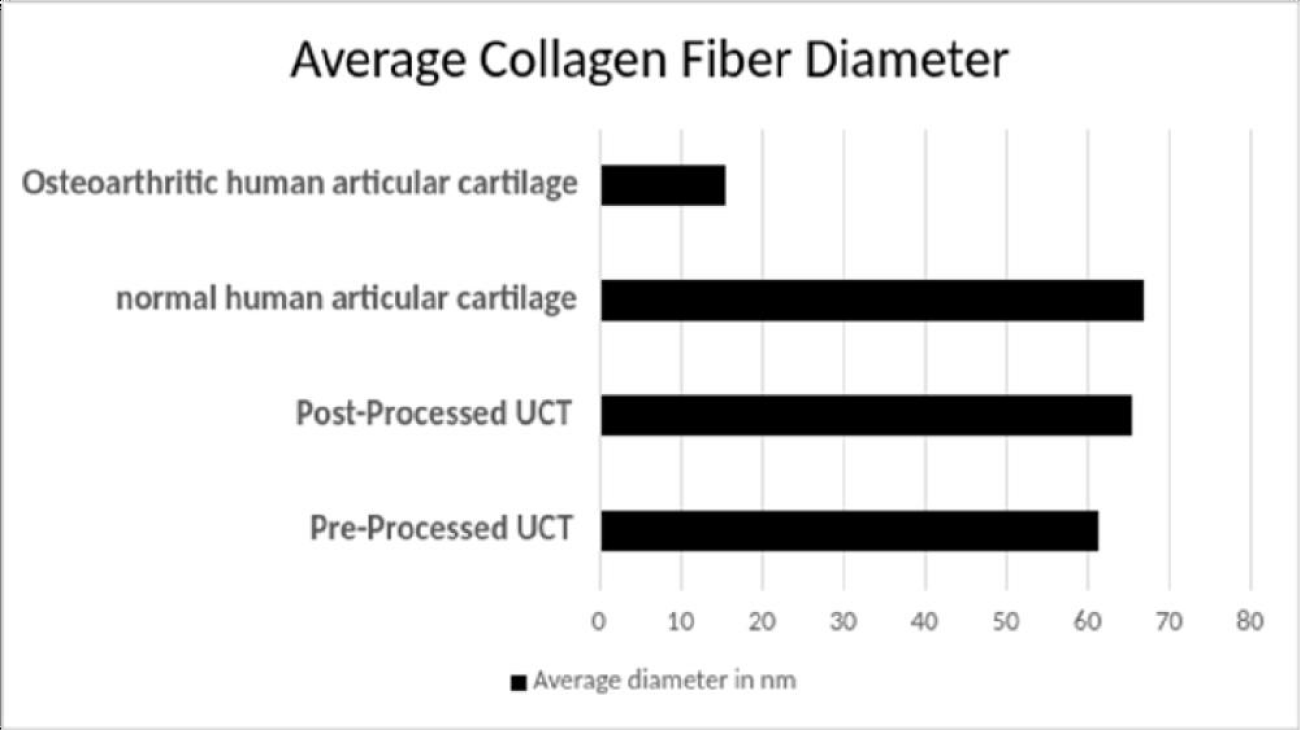
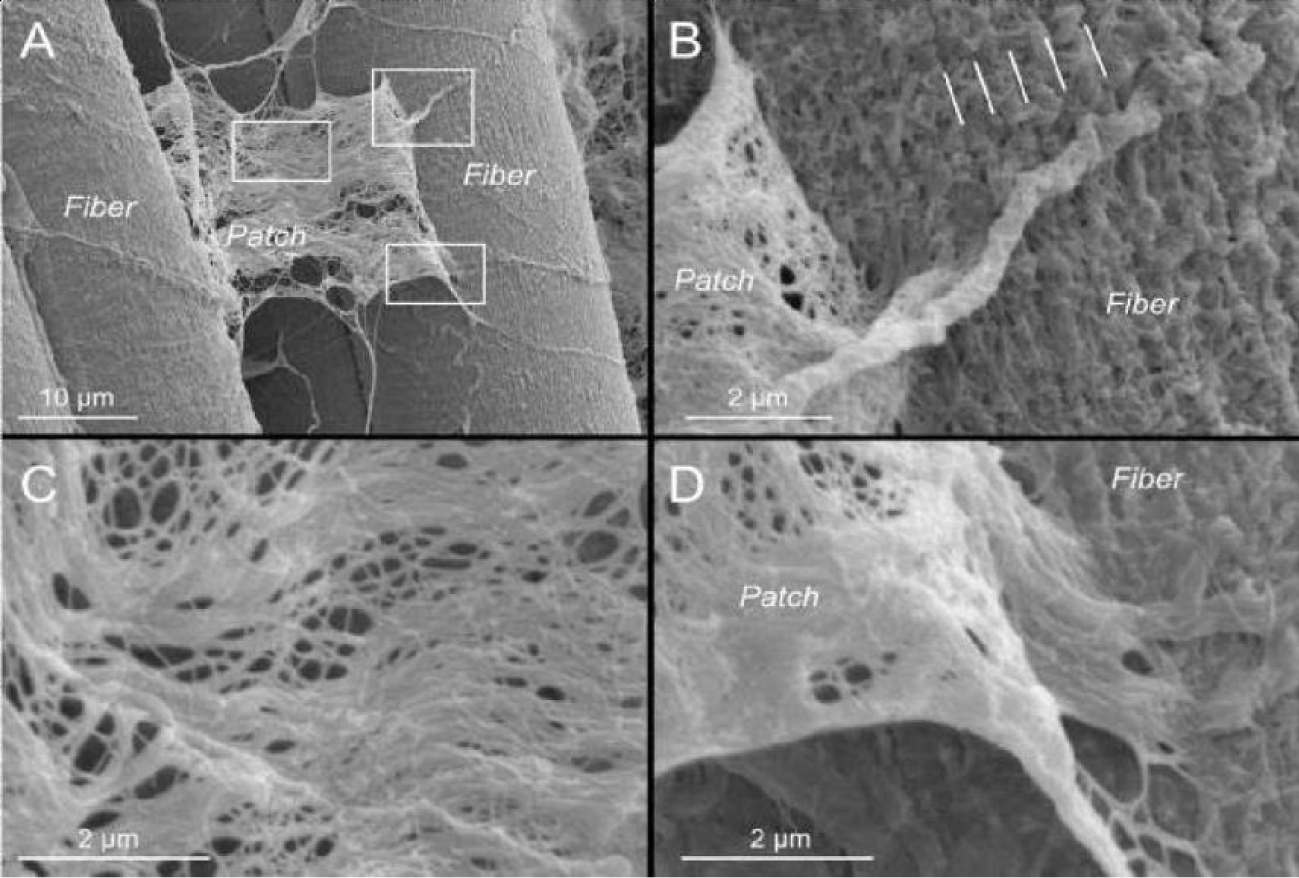
The expanding applications of regenerative medicine have led to increased patient demand, clinical utilization, and substantial marketing of new advancements in regenerative medicine [1]. Despite the well-recognized secondary and tertiary functions of WJ, there has been minimal research on the anatomic structural compatibility of WJ allografts and the sites of application within collagen-based structural tissue defects. In the current study, WJ allografts were produced through minimal manipulation of human umbilical cord tissue samples and are denoted as post-processed umbilical cord tissues. The SEM images of the post-processed samples reveal that the structural integrity of collagen fibers within the ECM is maintained (Figure 2). The diameter of the UCT fibers increased from approximately 61nm pre-processing to 65 nm post-processing. This increase could be due to slight swelling from the saline rinse and is not statistically significant. The average diameter of healthy mid-zone articular cartilage is around 67nm (Figure 3), similar in size and formation to the processed WJ (Figure 5). This characteristic further confirms minimal manipulation of pre- and post-processed tissue.
Collagen is the most prevalent structural macromolecule within the extracellular matrix, arranged in a cross-fiber network [14-16]. The strategic linkage of collagen fibers allows the network to provide protection, cushioning, and structural support, identical to the role of WJ in the umbilical cord [12]. The structure of the collagen fibers remains intact after processing, affording the WJ allograft the intended function of providing cushioning and structural support to the site of the structural tissue defect [17,18]. The structural composition of collagen before processing the umbilical cord tissue samples is shown in Figure 1. It is evident when comparing Figures 1 and 2 that the structural properties of the WJ before and after processing have been retained and are consistent with FDA guidelines defining minimal manipulation of WJ allografts.
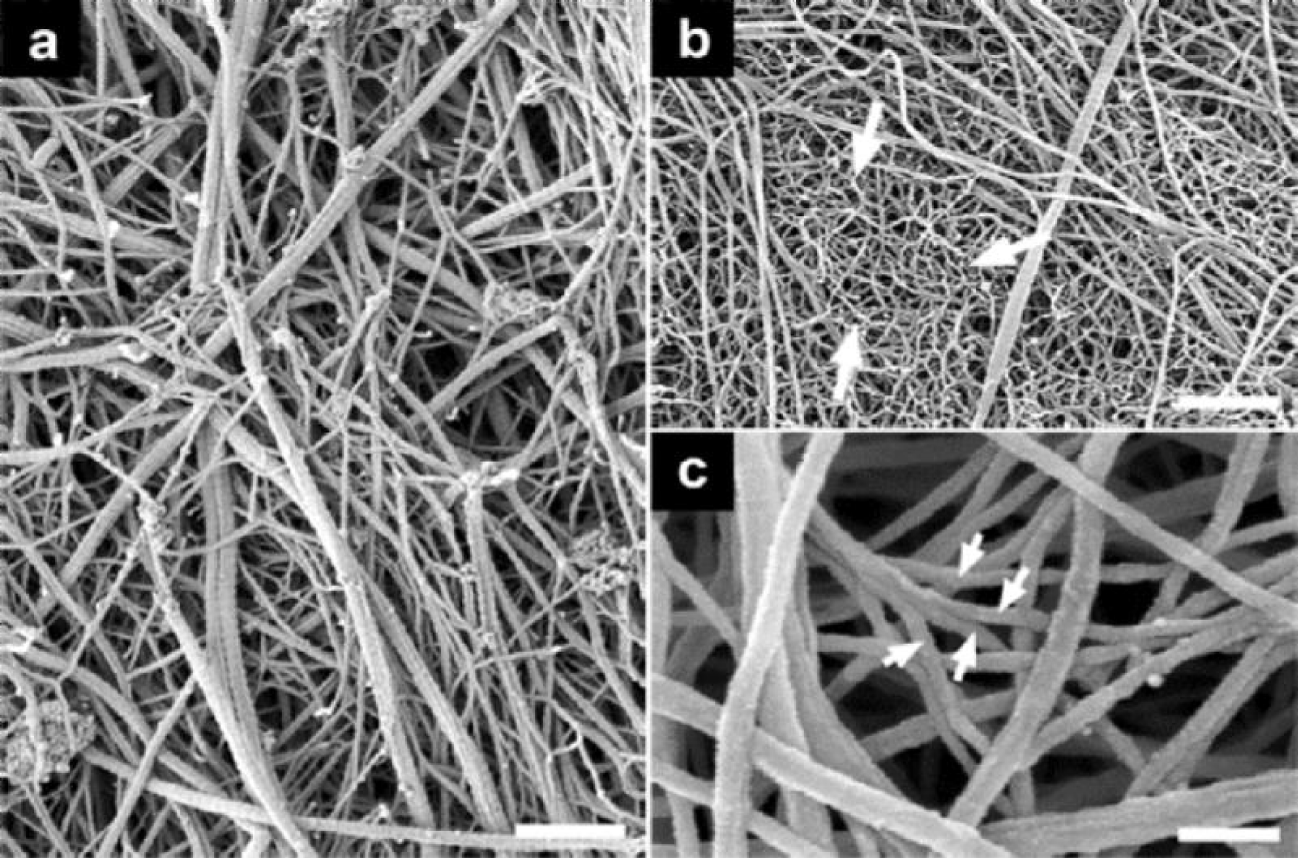
The multidirectional collagen network observed in WJ appears to be structurally comparative to the collagenic organization found in the extracellular matrix of cartilage [16,19]. Previously published research indicates that WJ cells have excellent chondrogenic differentiation capabilities, which could be beneficial in cartilage tissue engineering [19]. Figure 4 illustrates the mesh network of collagen in human articular cartilage. The anatomic structural similarities between the two collagen networks indicate that human umbilical cord tissue allografts are comparatively homologous in structure to the extracellular matrix found in cartilage. The breakdown of collagen (Figure 4C) results in the deterioration of cartilage [8]. Patients with structural tissue defects typically experience impairments in functional outcomes and quality of life. While applications of human umbilical cord tissue allografts have been used extensively in the clinical treatment of cartilage tears, there is also potential for allografts to be used in the pathology of intervertebral discs, as well as muscle tears [20]. As a non-surgical option, applying homologous WJ tissue allografts to structural tissue defects warrants clinical consideration.
Despite WJ being a non-vascular, dense irregular connective tissue, there is also the promise of homologous applications for WJ in vascularized tissue. In figure 6C, the organization of the collagen fibril network in skeletal muscle closely resembles the multidirectional collagen fibers seen in the post-processed umbilical cord tissue samples (Figure 2).
The extracellular matrix of skeletal muscles comprises a three-dimensional architecture consisting primarily of collagen, glycoproteins, and elastin [21]. The comparative composition and organization of collagen between post-processed WJ and skeletal muscle extracellular matrices suggest that WJ can serve as a homologous allograft in structural tissue defects of muscular origin [21,22]. Forty percent of the human body weight is composed of skeletal muscle [22]. The application of WJ as a homologous allograft in patients with structural tissue defects in skeletal muscle provides an opportunity for additional observational and prospective clinical studies. The clinical applications of human umbilical cord tissue allografts are profound in both vascularized and non-vascularized structural defects. The utilization of umbilical cord tissue allografts in regenerative medicine will be continually enhanced with additional discoveries of homologous use applications in clinical research data.
Conclusion
Through observational, collagenic structural comparison from SEM images, WJ demonstrates the potential to be used as an architectural scaffold for ECM supplementation in cartilage-based tissue structures and skeletal muscle tears. Even after processing, WJ retained its pre-processing structural characteristics. Further, the SEM images demonstrate comparative microarchitectural characteristics in post-processing WJ samples and ECM in articular cartilage and peri-muscle fiber fascia. The shared SEM characteristics are consistent between both tissue types, supporting homologous use.
In future work, we intend to use proteomic analysis of collagen fibers to confirm the anatomical comparison between human umbilical cord tissue allografts, skeletal muscle, and cartilage. This analysis would be coupled with high-resolution confocal antibody staining of umbilical cord tissue to allow for differentiation between the different types of collagen in the extracellular matrix. Additional case studies documenting umbilical cord tissue allograft efficacy could include applications in orthopedics, gerontology, sports medicine, general family medicine, and operating rooms. These advancements and commercial coverage of WJ allografts would increase physician and patient access to commercially available umbilical cord tissue allografts. Ease of access to perinatal birth tissue allografts like Wharton’s Jelly could dramatically improve community health, and reduce global surgical healthcare costs.
Acknowledgment
The SEM images in this work were obtained at Montana Nanotechnology Facility, a member of the National Nanotechnology Coordinated Infrastructure (NNCI). The authors would like to thank Naomi Lambert, Eric Vinke, Jessica LaRocco, Anna Colley, Daiyonna Bryd, Gene Bush, and Catherine Becker for their contributions to the authorship of the manuscript. The authors would also like to thank Barbara Krutchkoff for her assistance in coordinating the review of the manuscript.
Supplementary materials
Not applicable.
Author contributions
Conceptualization, J.M.D., J.S., and T.C.B.; methodology, J.M.D.; software, T.C.B.; validation, J.M.D., J.S., and T.C.B.; formal analysis, J.R.P..; investigation, J.M.D., and T.C.B.; resources, T.C.B.; data curation, J.M.D., J.S., and T.C.B.; writing—original draft preparation, J.M.D.; writing—review and editing, J.R.P.. and T.C.B.; visualization, J.S. and T.C.B.; supervision, J.R.P. and T.C.B..; project administration, J.S.; funding acquisition, T.C.B. All authors have read and agreed to the published version of the manuscript.”
Funding
This research received no external funding.
Informed consent statement
Informed Consent was obtained from all consenting mothers during the donation of the umbilical cords.
Data availability statement
Not applicable.
Conflicts of interest
Justine Davis, and Tyler Barrett are associated with Regenative Labs. Regenative Labs was involved in the design of the study, analysis, and manuscript authorship. Regenative Labs influenced the decision to publish.
References
- Kim DW, Staples M, Shinozuka K, Pantcheva P, Kang SD, Borlongan CV. Wharton's jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci. 2013 May 31;14(6):11692-712. doi: 10.3390/ijms140611692. PMID: 23727936; PMCID: PMC3709752.
- Gupta A, El-Amin SF 3rd, Levy HJ, Sze-Tu R, Ibim SE, Maffulli N. Umbilical cord-derived Wharton's jelly for regenerative medicine applications. J Orthop Surg Res. 2020 Feb 13;15(1):49. doi: 10.1186/s13018-020-1553-7. PMID: 32054483; PMCID: PMC7017504.
- Deus IA, Mano JF, Custódio CA. Perinatal tissues and cells in tissue engineering and regenerative medicine. Acta Biomater. 2020 Jul 1;110:1-14. doi: 10.1016/j.actbio.2020.04.035. Epub 2020 May 14. PMID: 32418650.
- Jadalannagari S, Converse G, McFall C, Buse E, Filla M, Villar MT, Artigues A, Mellot AJ, Wang J, Detamore MS, Hopkins RA, Aljitawi OS. Decellularized Wharton's Jelly from human umbilical cord as a novel 3D scaffolding material for tissue engineering applications. PLoS One. 2017 Feb 21;12(2):e0172098. doi: 10.1371/journal.pone.0172098. Erratum in: PLoS One. 2017 Mar 7;12 (3):e0173827. PMID: 28222169; PMCID: PMC5319682.
- Feng H, Danfelter M, Strömqvist B, Heinegård D. Extracellular matrix in disc degeneration. J Bone Joint Surg Am. 2006 Apr;88 Suppl 2:25-9. doi: 10.2106/JBJS.E.01341. PMID: 16595439.
- Mureşan S, Mureşan M, Voidăzan S, Neagoe R. The accuracy of musculoskeletal ultrasound examination for the exploration of meniscus injuries in athletes. J Sports Med Phys Fitness. 2017 May;57(5):589-594. doi: 10.23736/S0022-4707.17.06132-1. Epub 2015 Dec 18. PMID: 26684438.
- Luo Y, Sinkeviciute D, He Y, Karsdal M, Henrotin Y, Mobasheri A, Önnerfjord P, Bay-Jensen A. The minor collagens in articular cartilage. Protein Cell. 2017 Aug;8(8):560-572. doi: 10.1007/s13238-017-0377-7. Epub 2017 Feb 17. PMID: 28213717; PMCID: PMC5546929.
- Gottardi R, Hansen U, Raiteri R, Loparic M, Düggelin M, Mathys D, Friederich NF, Bruckner P, Stolz M. Supramolecular Organization of Collagen Fibrils in Healthy and Osteoarthritic Human Knee and Hip Joint Cartilage. PLoS One. 2016 Oct 25;11(10):e0163552. doi: 10.1371/journal.pone.0163552. PMID: 27780246; PMCID: PMC5079628.
- Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4(1):30-5. doi: 10.1186/ar380. Epub 2001 Oct 5. PMID: 11879535; PMCID: PMC128915.
- Penolazzi L, Pozzobon M, Bergamin LS, D'Agostino S, Francescato R, Bonaccorsi G, De Bonis P, Cavallo M, Lambertini E, Piva R. Extracellular Matrix From Decellularized Wharton's Jelly Improves the Behavior of Cells From Degenerated Intervertebral Disc. Front Bioeng Biotechnol. 2020 Mar 27;8:262. doi: 10.3389/fbioe.2020.00262. PMID: 32292779; PMCID: PMC7118204.
- Xue M, Jackson CJ. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv Wound Care (New Rochelle). 2015 Mar 1;4(3):119-136. doi: 10.1089/wound.2013.0485. PMID: 25785236; PMCID: PMC4352699.
- Velarde F, Castañeda V, Morales E, Ortega M, Ocaña E, Álvarez-Barreto J, Grunauer M, Eguiguren L, Caicedo A. Use of Human Umbilical Cord and Its Byproducts in Tissue Regeneration. Front Bioeng Biotechnol. 2020 Mar 10;8:117. doi: 10.3389/fbioe.2020.00117. PMID: 32211387; PMCID: PMC7075856.
- Kim N, Cho SG. Clinical applications of mesenchymal stem cells. Korean J Intern Med. 2013 Jul;28(4):387-402. doi: 10.3904/kjim.2013.28.4.387. Epub 2013 Jul 1. PMID: 23864795; PMCID: PMC3712145.
- Navani A, Manchikanti L, Albers SL, Latchaw RE, Sanapati J, Kaye AD, Atluri S, Jordan S, Gupta A, Cedeno D, Vallejo A, Fellows B, Knezevic NN, Pappolla M, Diwan S, Trescot AM, Soin A, Kaye AM, Aydin SM, Calodney AK, Candido KD, Bakshi S, Benyamin RM, Vallejo R, Watanabe A, Beall D, Stitik TP, Foye PM, Helander EM, Hirsch JA. Responsible, Safe, and Effective Use of Biologics in the Management of Low Back Pain: American Society of Interventional Pain Physicians (ASIPP) Guidelines. Pain Physician. 2019 Jan;22(1S):S1-S74. PMID: 30717500.
- CFR- Code of Regulations Title 21. US Food and Drug Administration. 2022.
- Zhang Y, Liu S, Guo W, Wang M, Hao C, Gao S, Zhang X, Li X, Chen M, Jing X, Wang Z, Peng J, Lu S, Guo Q. Human umbilical cord Wharton's jelly mesenchymal stem cells combined with an acellular cartilage extracellular matrix scaffold improve cartilage repair compared with microfracture in a caprine model. Osteoarthritis Cartilage. 2018 Jul;26(7):954-965. doi: 10.1016/j.joca.2018.01.019. Epub 2018 Jan 31. PMID: 29391278.
- Li Z, Bi Y, Wu Q, Chen C, Zhou L, Qi J, Xie D, Song H, Han Y, Qu P, Zhang K, Wu Y, Yin Q. A composite scaffold of Wharton's jelly and chondroitin sulphate loaded with human umbilical cord mesenchymal stem cells repairs articular cartilage defects in rat knee. J Mater Sci Mater Med. 2021 Mar 29;32(4):36. doi: 10.1007/s10856-021-06506-w. PMID: 33779853; PMCID: PMC8007499.
- Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009 Nov;1(6):461-8. doi: 10.1177/1941738109350438. PMID: 23015907; PMCID: PMC3445147.
- Majore I, Moretti P, Stahl F, Hass R, Kasper C. Growth and differentiation properties of mesenchymal stromal cell populations derived from whole human umbilical cord. Stem Cell Rev Rep. 2011 Mar;7(1):17-31. doi: 10.1007/s12015-010-9165-y. PMID: 20596801.
- Sadlik B, Jaroslawski G, Puszkarz M, Blasiak A, Oldak T, Gladysz D, Whyte GP. Cartilage Repair in the Knee Using Umbilical Cord Wharton's Jelly-Derived Mesenchymal Stem Cells Embedded Onto Collagen Scaffolding and Implanted Under Dry Arthroscopy. Arthrosc Tech. 2017 Dec 25;7(1):e57-e63. doi: 10.1016/j.eats.2017.08.055. PMID: 29552470; PMCID: PMC5852271.
- Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 2011 Sep;44(3):318-31. doi: 10.1002/mus.22094. PMID: 21949456; PMCID: PMC3177172.
- Csapo R, Gumpenberger M, Wessner B. Skeletal Muscle Extracellular Matrix - What Do We Know About Its Composition, Regulation, and Physiological Roles? A Narrative Review. Front Physiol. 2020 Mar 19;11:253. doi: 10.3389/fphys.2020.00253. PMID: 32265741; PMCID: PMC7096581.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.