Medicine Group . 2022 July 30;3(7):833-841. doi: 10.37871/jbres1520.
Functional Foods: A Glimpse into Beneficial Microorganisms in Gastrointestinal Diseases
Daniela Castillo1, Mario Cruz2*, Rosa M Rodríguez-Jasso1, Héctor Ruiz1 and Ruth Belmares1*
2Food Science and Technology Department, Antonio Narro Autonomous Agrarian University, Saltillo, Coahuila, Mexico
Mario Cruz, Food Science and Technology Department, Antonio Narro Autonomous Agrarian University, Saltillo, Coahuila, Mexico E-mail:
- Gut microbiota
- Functional foods
- Probiotics
Abstract
Gut microbiota the “virtual organ” plays a contributory role in the maintaining of human health as well as in the development of gastrointestinal diseases due to intervenes in digestion and metabolism. In this context is relevant to clarify the mechanisms of interaction of the intestinal microbiota with macromolecules for potential therapeutic applications and how bacterial growth or inhibition affect human health. This article reviewed the interaction of beneficial gut microbiota bacteria such as the Bifidobacterium, eubacterium, roseburia, Bacteroides, faecalibacteriummand Lactobacillus genera with food macromolecules (carbohydrates, protein, lipids). It also summarized the way in which gastrointestinal diseases and gut microbiota are related in diabetes, obesity and irritable bowel syndrome as well as the perspectives of how functional foods such as prebiotics, probiotics and synbiotics can be used as a dietary therapy for the modulation of gut microbiota.
Introduction
The understanding of microbial host colonization and interactions with other microorganisms in the gastrointestinal tract has been even more relevant in latest years due to evidence that gut microbiota play an important role in maintaining human health, or leading to illness, like in the progression of obesity as well as diabetes, metabolic disease and irritable bowel syndrome [1,2]; also intervenes in digestion, metabolism, immune function and in the gut-brain axis. What can we find inside the human gut have the power to change our health. In the past decades it has been reported that bacteria, fungi, viruses, and helminthes are the vast number of microorganisms which conform the microbiota [3] whatever in recent years the trends are directed to how we can manipulate it for our benefit.
Between the host and the microbiota exist a symbiotic relationship that constitute a regulator of many physiology processes related to the human intestine like metabolism of nutrients, mucosal immunity, systemic inflammation, barrier function, energy extraction of ingested foods, intestinal permeability and transit-time [2,4]. This association human gut-microbiota start at birth with the acquirer of a diverse spectrum of microbes, which is influenced by several factors like prenatal antibiotic use, birth via caesarean or from vaginal delivery and breastfeeding, also diet plays a huge role in its composition that allow or not to have a healthy gut microbiota, which is the one that has a varied system of metabolically interacting members, so when its composition changes it has consequences on the host health [5,6], even the glucose response is influenced by their gut microbiota composition [7].
This review summarized the recent work available about the ways in which gut microbiota and gastrointestinal health are related and the current perspectives of how functional foods can affect the metabolism of gut microbiota.
Beneficial microorganisms in the gut microbiota
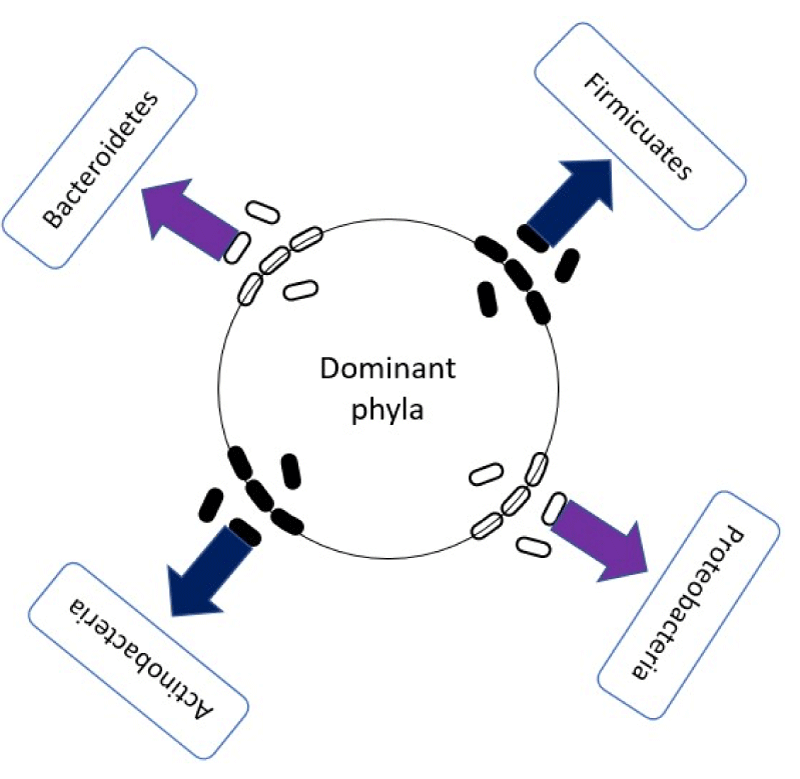
The gut microbial consist in trillions of microorganisms and comprises a huge ecology of bacteria, yeasts, and parasites like helminths, viruses, and protozoa and is often called a “virtual organ” [8], it has been estimated that the biomass of it can reach up to 1.5 Kg mainly made up of the 4 dominant phyla: firmicutes, bacteroidetes, actinobacteria and proteobacteria (Figure 1). Just in the large intestine we can find a community of around 100 trillions of commensal bacteria that comprise about 500 to 1000 species both pathogenic and health-promoting, species from the genus Bacteroidesalone constitute about 30% which is fundamental for the functioning of the host; the major contributor to the bacterial population is the colon [9-11].
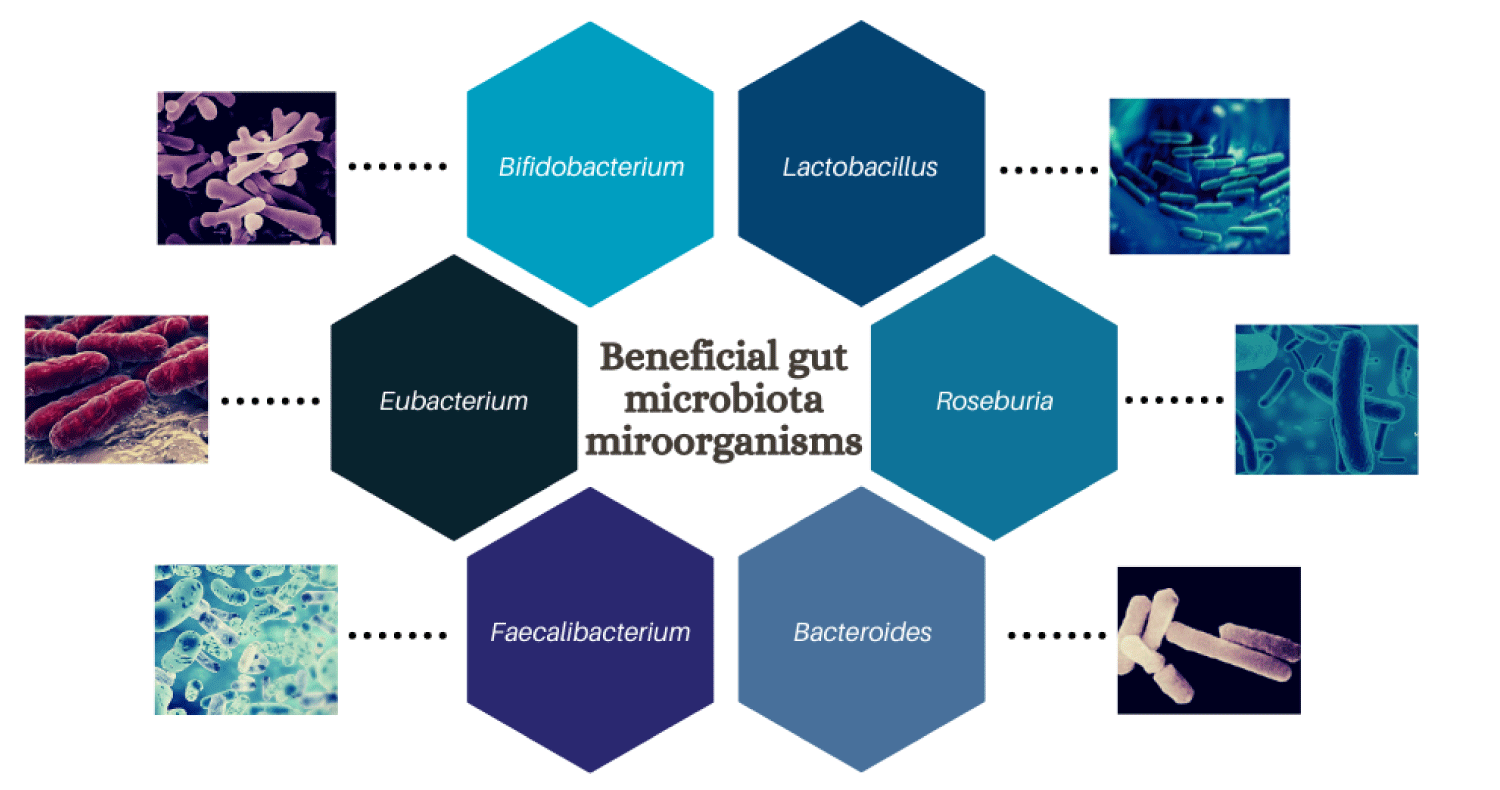
Due to the relationship between gut microbiota and the individual's health status, it is important to identify the beneficial species in order to manipulate it in favor of the host’ health [12]. It has been reported that several of these microorganisms in the gastrointestinal tract show a beneficial function for the host health such as certain genera of bacteria like Bifidobacterium, eubacterium, roseburia, Bacteroides, faecalibacterium, and Lactobacillus (Figure 2), as well as some bacterial species for example Roseburia intestinalis, Faecalibacterium prausnitzii and Akkermansia muciniphila, which have been suggested to be present in healthy individuals (Table 1) [13]. Bifidobacteria has a special spot within of the Actinobacteria memebers begin one of the main bacteria present in the healthy human gut and even some strains have been classified as probiotics [14]. Some of the most studied probiotic bacterias like lactic acid bacteria and Bifidobacteria can bring benefits to human health, they have been reported to be beneficial in the treatment or prevention of diverse conditions, both of them inhibit the growth of pathogens due to their ability to use their association with gut epithelial cells and the capacity to induce host mucosal defense systems as well as tissue repair mechanisms also the production of organic acids is important for antimicrobial activity against gram negative pathogens [12], besides Clostridium butyricum and Akkermansia muciniphila ferment polysaccharides and can produce short-chain fatty acids who works like anti-inflammatories and some strains from Bacilli and Clostridia can inhibit the propagation of pathogens [15].
| Table 1: Gut bacteria and its function. The table summarized the characteristics and gastrointestinal function of gut bacteria. | |||
| Bacteria | Characteristics | Function | References |
| C. butyricum | Gram positive Anaerobic Heat and acid resistant |
Fermentation of carbohydrates and production of short-chain fatty acid (butyric acid). Secretion of amylase. Reduce sputum and amines. Inhibition of the development of pathogenic bacteria. Promotes the growth of Bifidobacterium. |
[16] |
| A. muciniphila | Gram negative Strict anaerobic Non-motile Non-spore forming Represent about 0.5-5% of intestinal microbiota |
Degradation of mucins. Fermentation of polysaccharides. Production of short.chain fatty acids. Intervention in host metabolic modulation. Production of bioactive lipids with anti-inflammatory activities. |
[17-20] |
| R. intestinalis | Gram positive Anaerobic |
Production of butyrate Intervention in the control of gut inflammatory processes. Interference in the maduration of the immune system. Increase the abundance of T regulatory cells |
[21,22] |
Interaction of gut microbiota-macromolecules
The interaction between gut microbiota and macromolecules have been recently studied in due to the relevance that it has in relation to the way in which microorganisms act and survive and also because it can be modified by diet in order to maintain and promote health benefits.
Carbohydrates: The relation between gut microbiota and carbohydrates is mainly appreciated in those that colonise the colon, they metabolize the digestion resistant carbohydrates. Most of these microorganisms ferment carbohydrates and the products obtained from the fermentation like hydrolysed carbohydrate fractions and end-products can altered the health of both microbiota and host and it can be appreciated in the fact that about 70-80% of polysaccharides in the colon are used to produce short chain fatty acids. However, the impact of it is related to the carbohydrate structure which determines if it is or not soluble and gel-forming in the gut [24]. Also changes in the gut microbiota can be observed in relation to the source of the carbohydrates [25].
We can divide the carbohydrate structures in tree main groups. Macrostructure, mesostructure and molecular structure. Just like it was reported back in the 2000’s the macrostructure correlates with physical properties including solubility, which is a final characteristic since some mechanisms for degrading carbohydrates vary depending on the microorganism ability to metabolize soluble and insoluble carbs. For example because of their extracellular enzymes, Gram positive Firmicutes can degrade insoluble carbohydrates; Ruminococcus bromii is able to degrade raw starch particles meanwhile R. champanellensis is the only reported bacteria capable of degrading crystalline cellusose [26]. As regards mesostructure, these are ingested from food components like cellulose who is arranged in mesostructures. The organization of its individual polymers in microfibrils affects the microbial cross-feeding making it less accessible to enzymatic hydrolysis while in the case of starch the effect of the mesostructure is quite the opposite when it’s in its gelatinized form which makes the starch polymers more accessible to digestion. Finally the molecular structure refers to the way the units are ordered in polymer chains which is related to the metabolism of bacteria end how the enzymatic hydrolysis is carried out [24].
Protein: Proteins are one of the essential nutrients in diet, therefore the metabolism of it by the gut microbial community and the diet itself play a key role in the host health, just like Mafra, et al. [27] describes: “the principal metabolic activities of colonic microorganisms are associated with carbohydrate and protein digestion”. Recently has been described by some researches that there is a complex relation between proteins and microorganisms in the gut, even related to chronic kidney disease when the composition and function of gut microbiota are altered [28]. Meanwhile, other authors have approached the subject from a more general angle such as Wu, et al. [29], where the interaction mechanisms are described not only in humans but in different host, this due to the importance of colonic microorganisms in the breakdown of proteins and peptides.
Gut bacteria have the ability to synthesize proteinogenic amino acids as well as the capacity to produce metabolites that could or not begin beneficial to the host. There is a wide variety of the resulting end-products covering since short chain fatty acids, ammonia, amines and phenols to indoles, thiols, CO2, H2 and H2S [27,30]. The proteolysis occurs mainly in the distal colon and it depends of several factors such as the microbiota composition, proteolytic bacteria have been identified in human faces where Clostridium spp. and Bacteroidesspp. are in greater proportion and they both have an important peptidase activity on the other hand Desulfomonas spp. and Desulfovibrio spp. can oxidize organic substrates, like methionine and cysteine and also produce sulfur compounds, such as Hydrogen Sulfide (H2S) who has been reported to have toxic or beneficial effect depending of the concentration.
Lipids: The way in which the gut microbiota and lipid metabolism are related has been recently studied and the evidence suggest that lipid metabolism can affect the development of metabolic diseases, however the disorders attributed to it are multifactorial and are influenced by several factors such as nutritional ones, insulin resistance and hormonal disorders; also genetic and epigenetic factors can be involve [31].
In a recent study performed on mice, Lui, et al. [32] investigated the effect that a high fat diet has on lipid metabolism and gut microbiota, in their experiment the population of mice was divided in four groups: a control group and three groups that were fed with high fat diets consisting of olive oil, lard oil and soybean oil respectively. They found that the high fat diet cause alterations in the gut microbiota such as an increased population of Actinobacteria and Enterococcaceae and also decreased Bacteroidetes, Proteobacteria, Lactobacillales as well as the microbiota diversity and also based on the correlation analysis, it was observed that Actinbactera and Lactobacillales may play an important role in serum total cholesterol and liver Malondialdehyde (MDA) levels. They conclude that a high fat diet (in this case regardless of the type of oil) cause harmful effects on gut microbiota, lipid metabolism and oxidative stress, they also refer to the importance of limiting the consumption of fats in the diet as a method to prevent diseases.
Despite the majority of research have been done on mice and rats the correlations with the human gut microbiota are enormous. In another study performed in mice [33], it was found that alone with the compound they used in their investigation (celastrol), the gut microbiota composition could mediate the anti-obesity role of celastrol under a high fat diet. In this experiment the results shown that a compound of interest can antagonize obesity by resetting the gut microbiota profile under a specific feeding treatment.
Microbiota and gastrointestinal diseases
The relationship between gut microbiota and diseases it's clearer now than it was two decades ago but there is still much to discover about this complex interaction. Recently research has been directed to clarify the way in which gastrointestinal diseases and gut microbiota are related. Some of these mechanisms will be discussed below.
Diabetes: During the last two decades the prevalence of individuals with prediabetes and type 2 diabetes has increased. While in the state of prediabetes can be found an intermediate hyperglycemia and insulin resistance, the diabetes condition is defined like a metabolic disorder characterized by “a blood glucose levels gradually rise due to increasing insulin resistance and decreasing beta cell function” [34] in which the hyperglycemia is persistent and a low-grade inflammation is present [35].
In recent years, it has been reported that there are changes in the composition of the intestinal microbiota in patients with type 2 diabetes, especially with regard to the reduction of butyrate-producing bacteria, this being associated as the main factor of dysbiosis [36]. Butyrate is generally produced as a consequence of the fermentation of dietary fibers and a low population of butyrate producers has been observed not only in diabetes but also in prediabetes [37].
Several research articles have been published that seek to clarify the relationship between diabetes and the absence or presence of certain bacterial strains in the disease, for example a study of Larsen, at al. [38] shows that the presence of the Firmicutes phylum and the Clostridia class were reduced in the diabetic group of the experiment in contrast with the control group used for the investigation. In another study carried out with a Chinese population it was found that a moderate dysbiosis by a reduction of butyrate-producing bacteria like Roseburia intestinalis was present in diabetic individuals [39,40]. In more recent studies the role of metformin in the gut microbiota of type 2 diabetes patients has begun to be studied and the evidence revels the metformin’s power to change it. In the investigation of Wu, et al. [41] it was reported that after four months of treatment metformin caused changes in the abundance of several bacterial strains, mostly observed in the Firmicutes and Proteobacteria phyla.
The reduction of butyrate-producing bacteria may have considerable consequences in human health due to butyrate’s anti-inflammatory properties and signaling capacities via G protein–coupled receptors (some SCFAs have that capacity as well) [42]. A diet that considers these factors could contribute to the gut microbiota healthy environment and therefore to the patient’s health.
Obesity: Alterations in the intestinal microbiota have a great impact on the development of obesity; poor microbial diversity is linked to inflammation as well as insulin-resistance and adiposity [36].
There are some differences between the composition of the microbiota of people with obesity compared to lean people for example, as mentioned earlier, one of the most abundant species in the gut microbiota is Akkermansia muciniphila, which has been more relevant since a decreased population of it have been associated to obesity, insulin resistance and diabetes as well as cardiometabolic disorders. Another change in the population of gut microbiota in patients with obesity that has been reported is the higher level of Lactobacillus reuteri, a reduction of Lactobacillus casei and Lactobacillus plantarum [43], and a lower presence of butyrate-producing Firmicutes.
Furthermore alterations in the bacterial phylum Bifidobacterium are also associated to obesity, specifically low levels of Bifidobacteria [44]. Meanwhile in regard to the phylum Enterobacteriaceae, the levels of Faecalibacterium prausnitzii has been reported notably higher in children with obesity [45].
Irritable bowel syndrome: Irritable Bowel Syndrome (IBS) is a chronic functional gastrointestinal disorder which affects around 10 % of the population worldwide [46], it is characterized by abdominal pain and altered bowel habits that reduce quality of life.
Several factors are involve in the pathophysiology of IBS, like abnormalities of gastrointestinal motility, visceral hypersensitivity, genetic factors and alterations in the gut microbiota but recent contributions suggest that it’s multifactorial and also depends on environmental factors [47,48].
Furthermore, there is a growing evidence of the interaction between the gut microbiota and IBS, such as the difference in the microbiota in healthy population versus that of people with the disease or that IBS has been associated with small intestinal bacterial overgrowth as well as to develop after an intestinal infection [49].
It has been observed that IBS patients show lower concentration of enteric Lactobacillus, Bifidobacterium and Faecalibacterium prausnitzii [46] and it seem to be less population of SCFA-producing bacteria which promotes epithelial integrity [50]. Following this, it can be addressed the relevance of the FODMAPs diet (low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) which is used as dietary therapy for IBS patients as well as the use of probiotics [51]. The FODMAPs diet can contribute to alleviate some symptoms in IBS patients but at the same time it has been observed that reduce luminal Bifidobacteria concentration. In this particular case combine the FODMAPs diet with probiotic supplementation is key to maintain the Bifidobacteria population controlled [46].
However, there is still much to comprehend about the mechanisms in which diet can reshape gut microbiota and the way it impact on the IBS patient’s health and symptoms.
Diet and gut microbiota: The role of functional foods
Nowadays, the importance of diet and its impact on the composition and health of the gut microbiota has been pointed out, from probiotics to synbiotics and functional foods of all kinds, the opportunities are there.
The relation of gut microbiota with several diseases (not only gastrointestinal) becomes clearer little by little and that allows the development of new ways to reshape the intestinal microbiota for the benefit of human health and food is an indispensable part of achieving this. Functional foods may help gastrointestinal diseases’ patients, but for that is crucial to elucidate how the gut microbiota react to a certain food ingredient.
Functional foods are often defined as dietary supplements that have potentially positive effects on health beyond basic nutrition [52,53]. Recently, the modulation of the intestinal microbiota from functional foods such as probiotics, prebiotics and synbiotics is becoming even more attractive with the aim of being used as a tool to fight against diseases such as obesity or diabetes among others.
Prebiotics: The term prebiotic was described by Gibson, et al. [54] in 1995 as “a nondigestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, and thus improves host health”, and later taken up by the same author as “a substrate that is selectively utilized by host microorganisms, conferring a health benefit” [55].
The non-digestible carbohydrates like fructans and galactans are usually classified as prebiotics and increase Bifidobacteria in the human gut, due to these carbohydrates have linkage bonds that are break down by enzymes produced by bifidobateria and lactobacilli like b-fructosidase and b-galactosidase [56].
This selective fermentation can induce both, microbiological and metabolic changes that may be beneficial for the host, this can been seen in some of the potential benefits that have been attributed to dietary fiber and prebiotics on bowel health, like higher resistance against pathogenic colonization as well as reductions in the levels of toxins and also carcinogens in the gut, but not only that, is also expected that thorough its impact on epithelial layers the formation of SCFAs can contribute to the human health by the anti-inflammatory and anti-apoptotic effects that have been attributed to SCFAs [57].
As mentioned above, in IBS there is a decrease in the presence of Lactobacillus and Bifidobacterium while in obesity a reduction in the Bifidobacteria population is observed, so a diet that includes prebiotics can be beneficial in the right conditions, for example, Bifidobacteria prefers metabolise substrates of oligosaccharides’ size [55].
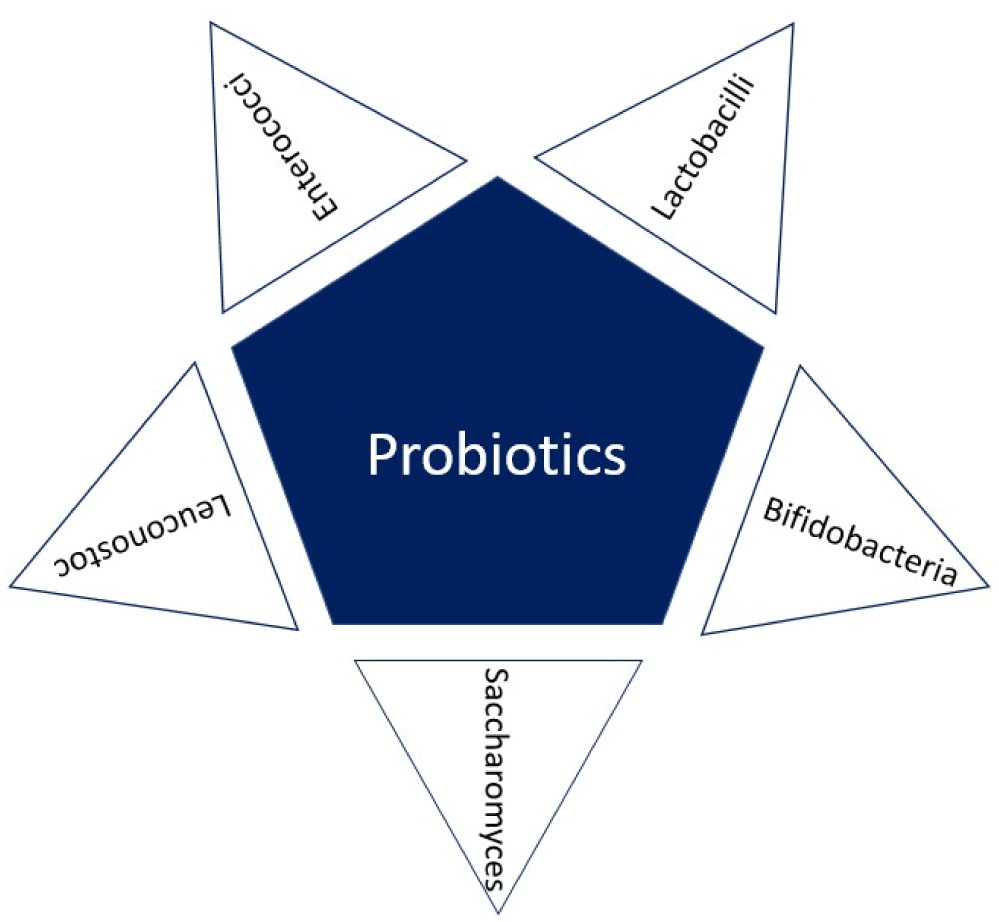
Probiotics: The modern definition of probiotics is that “they are living microorganisms that administered in adequate amounts are beneficial to the host’ health” and also have a key role in inflammation modulation by modifying the gut microbiota (Figure 3) [58]. An important parameter to consider is its ability to adhere to the gastrointestinal tract, since this allows them to perform a competitive suppression of pathogens [59].
Some of the most used probiotics in functional foods include Lactobacillus, Bifidobacterium, Saccharomyces, Enterococcus and Leuconostoc [59,60]. Bifidobacterium and Lactobacillus seem to be positioned as the most promising health-promoting dairy food formulations [61].
Lactobacillus: Lactobacillus species have been used in many dairy preparations due to the health benefits associated to its fermentation ability. L. acidophilus has been studied as both, antimicrobial and antiviral, and toward eradication of diarrhea. It also has been reported that L. acidophilus Pul13_14 may breakdown the starchy branched a-glucan oligomers which allows that those resultant short parts act as prebiotics for the intestinal microorganisms as these are degraded by the gut microbiota enzymes, while L. acidophilus ATCC 4357 can produce short chain fatty acids which improves its functional property [62]. L. fermentumhas also been shown to have the ability to contribute to the health of the host. In animals treated with L. fermentumATCC 11976 it was observed that insulin resistance decreased [63].
On the other hand, besides the well-known benefits of L. casei Shirota, the effects that it has on the glucose intolerance, insulin resistance and lipid metabolism have been studied in rats with a high fat diet and the results showed that it might affect the tissue-specific autonomic nerves through the afferent vagal nerve pathway to modulate glucose and lipid metabolism [64], this kind of behavior or L. casei Shirota is promising.
Bifidobacterium: Bifidobacterium species has earned a place alongside LAB as one of the top predominant cultures in the human colon [65] and it is no surprise to anyone that bifidobacteruim possesses a significant ability to exert beneficial effects on human health.
The commonest species that are used in probiotic formulations are B. animalis, B. adolescentis, B. bifidum, B. infantis, B. breve, and B. longum [58].
One strain which has been widely used in fermented dairy products is Bifidobacterium animalis. The subsp. lactis JCM 10602 showed a great adhesion potential to dietary fibers [66] and in a sudy of Wang, et al. [67] the subsp. Lactis MN-Gup improved constipation-related issues like stool consistency and straining.
In addition, the beneficial contributions of Bifidobacterium with regard to prevention of diseases has been observed like in inflammatory bowel disease in model animals; B. bifidum ATCC 29521 demonstrated an anti-inflammatory behavior in a mouse model [68] and in a study performed in rats subjected to maternal separation, Bifidobacterium bifidum G9-1 showed a beneficial role in irritated bowel syndrome by reducing the hypersensitivity to restraint stress (serum corticosterone level) and defecation’ frequency [69].
The fermentation capacity of Bifidobacterium strains may boost the bioactivity of functional foods [58]. Due to several strains are considered as GRAS (generally recognized as safe), its use as prebiotic agents in fermented dairy products is wide as well as the investigation of new strains. In a study of Awasti, et al. [65] they found three strains of Bifidobacterium with prebiotic and functional activities and the search for new confirmed probiotic strains continues.
Furthermore, in order to innovate in the probiotic market as well as to add new confirmed strains with confirmed probiotic capacity that could act as a dietary therapy in gastrointestinal diseases is important to develop more investigation both, in animal models as in humans.
Synbiotics: What we know as synbiotics is nothing but the combination of prebiotics and prebiotics which have been shown positive health effects and may contribute to beneficial growth of bacteria in human intestine. The synbiotics are divided into two groups: complementary and synergistic. The first ones are those that contain pre and probiotics that were chose independently of the other and where each one carries a specific health effect. And in the second one, the prebiotic is selected with the intention of support the growth of the specific probiotic [70].
Synergistic ones present the advantage that they can be used to clarify the responder-nonresponder phenomenon, due not every single subject will react in the same way to a certain probiotic since it also depends on host abiotic factors. The studies about synergistic synbiotics mostly includes included lactobacilli and Bifidobacteria as the probiotic and oligosaccharides or dietary fibers as the prebiotic; and the majoriy of them are also performed in animals [42].
The consumption of synbiotics is usually associated with a higher SCFA production, and the stimulation of beneficial gut bacteria [71], but more research in humans is needed. However, the most recent investigations begin to point to new possibilities.
Effects of functional foods on gut microbiota
The great majority of studies are about how gut microbes can bio transform functional foods, but there is not much about how gut microbiota metabolism is altered by these specific foods.
In a recent study of Farag, et al. [53] extracts prepared through functional foods were tested in a gut consortium culture of 8 microbes which allow to simulate the metabolic activities found in the human gut, the samples were analyzed using gas chromatography coupled to mass spectrometry detection after 0.5 and 24 hours after the extracts were added from which it was possible to identify different metabolites like organic acids, amino acids, fatty acids, nitrogenous compounds, nucleic acids as well as phenolics, steroids and sugars. That research aimed to elucidate the way in which certain food supplements can affect the metabolism of the intestinal microbiota, they observed that tea phenolics exhibited an inhibitory effect in species like Bacteroidesspp., and Clostridium spp, which form part of the human gut microbiota. Their results provide a glimpse into how the intestinal microbiota reacts and its metabolism can be altered through the use of functional food extracts in terms of metabolites. They describe two forms in which this happens: one way begin that functional food components can serve as a substrate to microbiota metabolism and the other is that functional food components modify the extent, existing metabolic pathways are activated within microbiota.
The research field is enormous since are many functional foods out there to evaluate.
Conclusion
Resent research on gut microbiota are directed to how we can manipulate it for our benefit, which is why it is important to analyze the information available about the symbiotic relationship that exists between host, microbiota and the macromolecules
Although there are gut microbiota and functional foods investigations, many of them, if not most, are carried out in animal models and continue to be far from going to clinical studies that allow clarifying the potential therapeutic applications that they could have in gastrointestinal diseases (and other types of diseases).
In the years to come the biotechnological use of microorganisms could improve health by focusing on the alterations present in the intestinal microbiota of patients such as the Clostridia class reduced in diabetic patients.
This review, through a substantial bibliography research, showed that to clarify how food and functional foods effects on the intestinal microbiota are essential to take advantage of the full potential of its interactions with specific food compounds like carbohydrates, proteins or lipids and use that information for maintain or reshape a healthy gut microbiota as well as the implications it has on some of the most recurrent gastrin-intestinal diseases worldwide.
References
- Musso G, Gambino R, Cassader M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu Rev Med. 2011;62:361-80. doi: 10.1146/annurev-med-012510-175505. PMID: 21226616.
- Bordalo Tonucci L, Dos Santos KM, De Luces Fortes Ferreira CL, Ribeiro SM, De Oliveira LL, Martino HS. Gut microbiota and probiotics: Focus on diabetes mellitus. Crit Rev Food Sci Nutr. 2017 Jul 24;57(11):2296-2309. doi: 10.1080/10408398.2014.934438. PMID: 26499995.
- Vemuri R, Shankar EM, Chieppa M, Eri R, Kavanagh K. Beyond Just Bacteria: Functional Biomes in the Gut Ecosystem Including Virome, Mycobiome, Archaeome and Helminths. Microorganisms. 2020 Mar 28;8(4):483. doi: 10.3390/microorganisms8040483. PMID: 32231141; PMCID: PMC7232386.
- Stacchiotti V, Rezzi S, Eggersdorfer M, Galli F. Metabolic and functional interplay between gut microbiota and fat-soluble vitamins. Crit Rev Food Sci Nutr. 2021;61(19):3211-3232. doi: 10.1080/10408398.2020.1793728. Epub 2020 Jul 25. PMID: 32715724.
- Van den Abbeele P, Verstraete W, El Aidy S, Geirnaert A, Van de Wiele T. Prebiotics, faecal transplants and microbial network units to stimulate biodiversity of the human gut microbiome. Microb Biotechnol. 2013 Jul;6(4):335-40. doi: 10.1111/1751-7915.12049. Epub 2013 Apr 18. PMID: 23594389; PMCID: PMC3917468.
- Long-Smith C, O'Riordan KJ, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota-Gut-Brain Axis: New Therapeutic Opportunities. Annu Rev Pharmacol Toxicol. 2020 Jan 6;60:477-502. doi: 10.1146/annurev-pharmtox-010919-023628. Epub 2019 Sep 10. PMID: 31506009.
- Mendes-Soares H, Raveh-Sadka T, Azulay S, Edens K, Ben-Shlomo Y, Cohen Y, Ofek T, Bachrach D, Stevens J, Colibaseanu D, Segal L, Kashyap P, Nelson H. Assessment of a Personalized Approach to Predicting Postprandial Glycemic Responses to Food Among Individuals Without Diabetes. JAMA Netw Open. 2019 Feb 1;2(2):e188102. doi: 10.1001/jamanetworkopen.2018.8102. PMID: 30735238; PMCID: PMC6484621.
- Koç F, Mills S, Strain C, Ross RP, Stanton C. The public health rationale for increasing dietary fibre: Health benefits with a focus on gut microbiota. Nutr Bull. 2020;45:294-308. doi: 10.1111/nbu.12448.
- Sender R, Fuchs S, Milo R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell. 2016 Jan 28;164(3):337-40. doi: 10.1016/j.cell.2016.01.013. PMID: 26824647.
- Linares DM, Ross P, Stanton C. Beneficial Microbes: The pharmacy in the gut. Bioengineered. 2016;7(1):11-20. doi: 10.1080/21655979.2015.1126015. PMID: 26709457; PMCID: PMC4878258.
- Wan MLY, Ling KH, El-Nezami H, Wang MF. Influence of functional food components on gut health. Crit Rev Food Sci Nutr. 2019;59(12):1927-1936. doi: 10.1080/10408398.2018.1433629. Epub 2018 Feb 23. PMID: 29381385.
- Lukic J, Chen V, Strahinic I, Begovic J, Lev-Tov H, Davis SC, Tomic-Canic M, Pastar I. Probiotics or pro-healers: the role of beneficial bacteria in tissue repair. Wound Repair Regen. 2017 Nov;25(6):912-922. doi: 10.1111/wrr.12607. Epub 2018 Feb 9. PMID: 29315980; PMCID: PMC5854537.
- Cao Y, Liu H, Qin N, Ren X, Zhu B, Xia X. Impact of food additives on the composition and function of gut microbiota: A review. Trends Food Sci Technol. 2020;295-310. doi: 10.1016/j.tifs.2020.03.006.
- González-Rodríguez I, Ruiz L, Gueimonde M, Margolles A, Sánchez B. Factors involved in the colonization and survival of Bifidobacteria in the gastrointestinal tract. FEMS Microbiol Lett. 2013 Mar;340(1):1-10. doi: 10.1111/1574-6968.12056. Epub 2012 Dec 17. PMID: 23181549.
- Zheng DW, et al. Prebiotics-Encapsulated Probiotic Spores Regulate Gut Microbiota and Suppress Colon Cancer. Adv Mater. 2020. doi: 10.1002/adma.202004529.
- Zhang M, et al. Effects of Clostridium butyricum on growth, digestive enzyme activity, antioxidant capacity and gut microbiota in farmed tilapia (Oreochromis niloticus). Aquac Res. 2020. doi: 10.1111/are.15009.
- Cani PD, de Vos VM. Next-generation beneficial microbes: The case of Akkermansia muciniphila. Front Microbiol. 2017. doi: 10.3389/fmicb.2017.01765.
- Machado D, Almeida D, Seabra CL, Andrade JC, Gomes AM, Freitas AC. Uncovering Akkermansia muciniphila resilience or susceptibility to different temperatures, atmospheres and gastrointestinal conditions. Anaerobe. 2020 Feb;61:102135. doi: 10.1016/j.anaerobe.2019.102135. Epub 2019 Dec 14. PMID: 31875576.
- Kim HB, Isaacson RE. Salmonella in Swine: Microbiota Interactions. Annu Rev Anim Biosci. 2017 Feb 8;5:43-63. doi: 10.1146/annurev-animal-022516-022834. Epub 2016 Nov 9. PMID: 27860494.
- Lopetuso LR, Quagliariello A, Schiavoni M, Petito V, Russo A, Reddel S, Del Chierico F, Ianiro G, Scaldaferri F, Neri M, Cammarota G, Putignani L, Gasbarrini A. Towards a disease-associated common trait of gut microbiota dysbiosis: The pivotal role of Akkermansia muciniphila. Dig Liver Dis. 2020 Sep;52(9):1002-1010. doi: 10.1016/j.dld.2020.05.020. Epub 2020 Jun 20. PMID: 32576522.
- La Rosa SL, Leth ML, Michalak L, Hansen ME, Pudlo NA, Glowacki R, Pereira G, Workman CT, Arntzen MØ, Pope PB, Martens EC, Hachem MA, Westereng B. The human gut Firmicute Roseburia intestinalis is a primary degrader of dietary β-mannans. Nat Commun. 2019 Feb 22;10(1):905. doi: 10.1038/s41467-019-08812-y. PMID: 30796211; PMCID: PMC6385246.
- Luo W, Shen Z, Deng M, Li X, Tan B, Xiao M, Wu S, Yang Z, Zhu C, Tian L, Wu X, Meng X, Quan Y, Wang X. Roseburia intestinalis supernatant ameliorates colitis induced in mice by regulating the immune response. Mol Med Rep. 2019 Aug;20(2):1007-1016. doi: 10.3892/mmr.2019.10327. Epub 2019 Jun 4. PMID: 31173202; PMCID: PMC6625378.
- Ferreira-Halder CV, Faria AVS, Andrade SS. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract Res Clin Gastroenterol. 2017 Dec;31(6):643-648. doi: 10.1016/j.bpg.2017.09.011. Epub 2017 Sep 18. PMID: 29566907.
- Payling L, Fraser K, Loveday SM, Sims I, Roy N, McNabb W. The effects of carbohydrate structure on the composition and functionality of the human gut microbiota. Trends Food Sci Technol. 2020. doi: 10.1016/j.tifs.2020.01.009.
- Scott KP, Duncan SH, Flint HJ. Dietary fibre and the gut microbiota. Nutr Bull. 2008;33:201-211. doi: 10.1111/j.1467-3010.2008.00706.x.
- Moraïs S, Ben David Y, Bensoussan L, Duncan SH, Koropatkin NM, Martens EC, Flint HJ, Bayer EA. Enzymatic profiling of cellulosomal enzymes from the human gut bacterium, Ruminococcus champanellensis, reveals a fine-tuned system for cohesin-dockerin recognition. Environ Microbiol. 2016 Feb;18(2):542-56. doi: 10.1111/1462-2920.13047. Epub 2015 Oct 14. PMID: 26347002.
- Mafra D, Barros AF, Fouque D. Dietary protein metabolism by gut microbiota and its consequences for chronic kidney disease patients. Future Microbiol. 2013 Oct;8(10):1317-23. doi: 10.2217/fmb.13.103. PMID: 24059921.
- Gryp T, De Paepe K, Vanholder R, Kerckhof FM, Van Biesen W, Van de Wiele T, Verbeke F, Speeckaert M, Joossens M, Couttenye MM, Vaneechoutte M, Glorieux G. Gut microbiota generation of protein-bound uremic toxins and related metabolites is not altered at different stages of chronic kidney disease. Kidney Int. 2020 Jun;97(6):1230-1242. doi: 10.1016/j.kint.2020.01.028. Epub 2020 Feb 17. PMID: 32317112.
- Wu L, Tang Z, Chen H, Ren Z, Ding Q, Liang K, Sun Z. Mutual interaction between gut microbiota and protein/amino acid metabolism for host mucosal immunity and health. Anim Nutr. 2021 Mar;7(1):11-16. doi: 10.1016/j.aninu.2020.11.003. Epub 2020 Dec 21. PMID: 33997326; PMCID: PMC8110859.
- Portune KJ, Beaumont M, Davila AM, Tomé D, Blachier F, Sanz Y. Gut microbiota role in dietary protein metabolism and health-related outcomes: The two sides of the coin. Trends Food Sci Technol. 2016. doi: 10.1016/j.tifs.2016.08.011.
- Wang K, Liang X, Pang Y, Jiang C. The Role of Gut Microbiota in Host Lipid Metabolism: An Eye on Causation and Connection. Small Methods. 2020. doi: 10.1002/smtd.201900604.
- Liu H, Zhu H, Xia H, Yang X, Yang L, Wang S, Wen J, Sun G. Different effects of high-fat diets rich in different oils on lipids metabolism, oxidative stress and gut microbiota. Food Res Int. 2021 Mar;141:110078. doi: 10.1016/j.foodres.2020.110078. Epub 2020 Dec 28. PMID: 33641963.
- Hua H, Zhang Y, Zhao F, Chen K, Wu T, Liu Q, Huang S, Zhang A, Jia Z. Celastrol inhibits intestinal lipid absorption by reprofiling the gut microbiota to attenuate high-fat diet-induced obesity. iScience. 2021 Jan 20;24(2):102077. doi: 10.1016/j.isci.2021.102077. PMID: 33598642; PMCID: PMC7868996.
- Allin KH, Tremaroli V, Caesar R, Jensen BAH, Damgaard MTF, Bahl MI, Licht TR, Hansen TH, Nielsen T, Dantoft TM, Linneberg A, Jørgensen T, Vestergaard H, Kristiansen K, Franks PW; IMI-DIRECT consortium, Hansen T, Bäckhed F, Pedersen O. Aberrant intestinal microbiota in individuals with prediabetes. Diabetologia. 2018 Apr;61(4):810-820. doi: 10.1007/s00125-018-4550-1. Epub 2018 Jan 29. PMID: 29379988; PMCID: PMC6448993.
- Ebrahimpour S, Zakeri M, Esmaeili A. Crosstalk between obesity, diabetes, and alzheimer's disease: Introducing quercetin as an effective triple herbal medicine. Ageing Res Rev. 2020 Sep;62:101095. doi: 10.1016/j.arr.2020.101095. Epub 2020 Jun 11. PMID: 32535272.
- Pascale A, Marchesi N, Govoni S, Coppola A, Gazzaruso C. The role of gut microbiota in obesity, diabetes mellitus, and effect of metformin: new insights into old diseases. Curr Opin Pharmacol. 2019 Dec;49:1-5. doi: 10.1016/j.coph.2019.03.011. Epub 2019 Apr 20. PMID: 31015106.
- Wu H, Tremaroli V, Schmidt C, Lundqvist A, Olsson LM, Krämer M, Gummesson A, Perkins R, Bergström G, Bäckhed F. The Gut Microbiota in Prediabetes and Diabetes: A Population-Based Cross-Sectional Study. Cell Metab. 2020 Sep 1;32(3):379-390.e3. doi: 10.1016/j.cmet.2020.06.011. Epub 2020 Jul 10. PMID: 32652044.
- Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010 Feb 5;5(2):e9085. doi: 10.1371/journal.pone.0009085. PMID: 20140211; PMCID: PMC2816710.
- Qin J, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012. doi: 10.1038/nature11450.
- Miele L, Giorgio V, Alberelli MA, De Candia E, Gasbarrini A, Grieco A. Impact of Gut Microbiota on Obesity, Diabetes, and Cardiovascular Disease Risk. Curr Cardiol Rep. 2015 Dec;17(12):120. doi: 10.1007/s11886-015-0671-z. PMID: 26497040.
- Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, Ståhlman M, Olsson LM, Serino M, Planas-Fèlix M, Xifra G, Mercader JM, Torrents D, Burcelin R, Ricart W, Perkins R, Fernàndez-Real JM, Bäckhed F. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017 Jul;23(7):850-858. doi: 10.1038/nm.4345. Epub 2017 May 22. PMID: 28530702.
- Krumbeck JA, Walter J, Hutkins RW. Synbiotics for Improved Human Health: Recent Developments, Challenges, and Opportunities. Annu Rev Food Sci Technol. 2018 Mar 25;9:451-479. doi: 10.1146/annurev-food-030117-012757. Epub 2018 Jan 18. PMID: 29350558.
- Million M, Lagier JC, Yahav D, Paul M. Gut bacterial microbiota and obesity. Clin Microbiol Infect. 2013 Apr;19(4):305-13. doi: 10.1111/1469-0691.12172. Epub 2013 Mar 2. PMID: 23452229.
- Stephens RW, Arhire L, Covasa M. Gut Microbiota: From Microorganisms to Metabolic Organ Influencing Obesity. Obesity (Silver Spring). 2018 May;26(5):801-809. doi: 10.1002/oby.22179. PMID: 29687647.
- Balamurugan R, George G, Kabeerdoss J, Hepsiba J, Chandragunasekaran AM, Ramakrishna BS. Quantitative differences in intestinal Faecalibacterium prausnitzii in obese Indian children. Br J Nutr. 2010 Feb;103(3):335-8. doi: 10.1017/S0007114509992182. Epub 2009 Oct 23. PMID: 19849869.
- Herndon CC, Wang YP, Lu CL. Targeting the gut microbiota for the treatment of irritable bowel syndrome. Kaohsiung J Med Sci. 2020 Mar;36(3):160-170. doi: 10.1002/kjm2.12154. Epub 2019 Nov 29. PMID: 31782606.
- Liu J, Chey WD, Haller E, Eswaran S. Low-FODMAP Diet for Irritable Bowel Syndrome: What We Know and What We Have Yet to Learn. Annu Rev Med. 2020 Jan 27;71:303-314. doi: 10.1146/annurev-med-050218-013625. PMID: 31986083.
- Mei L, et al. Gut microbiota composition and functional prediction in diarrhea-predominant irritable bowel syndrome. BMC Gastroenterol. 2021;21. doi: 10.1186/s12876-021-01693-w.
- Liu HN, Wu H, Chen YZ, Chen YJ, Shen XZ, Liu TT. Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: A systematic review and meta-analysis. Dig Liver Dis. 2017 Apr;49(4):331-337. doi: 10.1016/j.dld.2017.01.142. Epub 2017 Jan 21. PMID: 28179092.
- Ooijevaar RE, Terveer EM, Verspaget HW, Kuijper EJ, Keller JJ. Clinical Application and Potential of Fecal Microbiota Transplantation. Annu Rev Med. 2019 Jan 27;70:335-351. doi: 10.1146/annurev-med-111717-122956. Epub 2018 Nov 7. PMID: 30403550.
- Staudacher HM, Scholz M, Lomer MC, Ralph FS, Irving PM, Lindsay JO, Fava F, Tuohy K, Whelan K. Gut microbiota associations with diet in irritable bowel syndrome and the effect of low FODMAP diet and probiotics. Clin Nutr. 2021 Apr;40(4):1861-1870. doi: 10.1016/j.clnu.2020.10.013. Epub 2020 Oct 23. PMID: 33183883.
- Granato D, Barba FJ, Bursać Kovačević D, Lorenzo JM, Cruz AG, Putnik P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu Rev Food Sci Technol. 2020 Mar 25;11:93-118. doi: 10.1146/annurev-food-032519-051708. Epub 2020 Jan 6. PMID: 31905019.
- Farag MA, Abdelwareth A, Sallam IE, El Shorbagi M, Jehmlich N, Fritz-Wallace K, Serena Schäpe S, Rolle-Kampczyk U, Ehrlich A, Wessjohann LA, von Bergen M. Metabolomics reveals impact of seven functional foods on metabolic pathways in a gut microbiota model. J Adv Res. 2020 Jan 3;23:47-59. doi: 10.1016/j.jare.2020.01.001. PMID: 32071791; PMCID: PMC7016031.
- Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995 Jun;125(6):1401-12. doi: 10.1093/jn/125.6.1401. PMID: 7782892.
- Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, Reid G. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017 Aug;14(8):491-502. doi: 10.1038/nrgastro.2017.75. Epub 2017 Jun 14. PMID: 28611480.
- Lockyer S, Stanner S. Prebiotics - an added benefit of some fibre types. Nutr Bull. 2019;44:74-91. doi: 10.1111/nbu.12366.
- Verspreet J, Damen B, Broekaert WF, Verbeke K, Delcour JA, Courtin CM. A Critical Look at Prebiotics Within the Dietary Fiber Concept. Annu Rev Food Sci Technol. 2016;7:167-90. doi: 10.1146/annurev-food-081315-032749. Epub 2016 Jan 6. PMID: 26735801.
- Sharma M, Wasan A, Sharma RK. Recent developments in probiotics: an emphasis on Bifidobacterium. Food Biosci. 2021;41. doi: 10.1016/j.fbio.2021.100993.
- Ashaolu TJ. Immune boosting functional foods and their mechanisms: A critical evaluation of probiotics and prebiotics. Biomed Pharmacother. 2020 Oct;130:110625. doi: 10.1016/j.biopha.2020.110625. Epub 2020 Aug 11. PMID: 32795926.
- Yang R, Zhao X, Wu W, Shi J. Potential of probiotics for use as functional foods in patients with non-infectious gastric ulcer. Trends Food Sci Technol. 2020;111:463-474. doi: 10.1016/j.tifs.2021.02.070.
- Linares DM, Gómez C, Renes E, Fresno JM, Tornadijo ME, Ross RP, Stanton C. Lactic Acid Bacteria and Bifidobacteria with Potential to Design Natural Biofunctional Health-Promoting Dairy Foods. Front Microbiol. 2017 May 18;8:846. doi: 10.3389/fmicb.2017.00846. PMID: 28572792; PMCID: PMC5435742.
- Minj J, Chandra P, Paul C, Sharma RK. Bio-functional properties of probiotic Lactobacillus: current applications and research perspectives. Crit Rev Food Sci Nutr. 2021;61(13):2207-2224. doi: 10.1080/10408398.2020.1774496. Epub 2020 Jun 10. PMID: 32519883.
- Bhathena J, Martoni C, Kulamarva A, Tomaro-Duchesneau C, Malhotra M, Paul A, Urbanska AM, Prakash S. Oral probiotic microcapsule formulation ameliorates non-alcoholic fatty liver disease in Bio F1B Golden Syrian hamsters. PLoS One. 2013;8(3):e58394. doi: 10.1371/journal.pone.0058394. Epub 2013 Mar 12. PMID: 23554890; PMCID: PMC3595252.
- Tanida M, Imanishi K, Akashi H, Kurata Y, Chonan O, Naito E, Kunihiro S, Kawai M, Kato-Kataoka A, Shibamoto T. Injection of Lactobacillus casei strain Shirota affects autonomic nerve activities in a tissue-specific manner, and regulates glucose and lipid metabolism in rats. J Diabetes Investig. 2014 Mar 23;5(2):153-61. doi: 10.1111/jdi.12141. Epub 2013 Nov 5. PMID: 24843755; PMCID: PMC4023578.
- Awasti N, Tomar SK, Pophaly SD, Poonam, Lule VK, Singh TP, Anand S. Probiotic and functional characterization of Bifidobacteria of Indian human origin. J Appl Microbiol. 2016 Apr;120(4):1021-32. doi: 10.1111/jam.13086. PMID: 26849092.
- Taniguchi M, Nambu M, Katakura Y, Yamasaki-Yashiki S. Adhesion mechanisms of Bifidobacterium animalis subsp. lactis JCM 10602 to dietary fiber. Biosci Microbiota Food Health. 2021;40(1):59-64. doi: 10.12938/bmfh.2020-003. Epub 2020 Oct 3. PMID: 33520570; PMCID: PMC7817516.
- Wang R, Sun J, Li G, Zhang M, Niu T, Kang X, Zhao H, Chen J, Sun E, Li Y. Effect of Bifidobacterium animalis subsp. lactis MN-Gup on constipation and the composition of gut microbiota. Benef Microbes. 2021 Feb 24;12(1):31-42. doi: 10.3920/BM2020.0023. Epub 2020 Dec 14. PMID: 33308038.
- Din AU, et al. Inhibitory effect of Bifidobacterium bifidum ATCC 29521 on colitis and its mechanism. J Nutr Biochem. 2020;79:108353. doi: 10.1016/j.jnutbio.2020.108353.
- Fukui H, Oshima T, Tanaka Y, Oikawa Y, Makizaki Y, Ohno H, Tomita T, Watari J, Miwa H. Effect of probiotic Bifidobacterium bifidum G9-1 on the relationship between gut microbiota profile and stress sensitivity in maternally separated rats. Sci Rep. 2018 Aug 17;8(1):12384. doi: 10.1038/s41598-018-30943-3. Erratum in: Sci Rep. 2021 Oct 28;11(1):21554. PMID: 30120330; PMCID: PMC6098190.
- Kolida S, Gibson GR. Synbiotics in health and disease. Annu Rev Food Sci Technol. 2011;2:373-93. doi: 10.1146/annurev-food-022510-133739. PMID: 22129388.
- Zepeda-Hernández A, Garcia-Amezquita LE, Requena T, García-Cayuela T. Probiotics, prebiotics, and synbiotics added to dairy products: Uses and applications to manage type 2 diabetes. Food Res Int. 2021;14. doi: 10.1016/j.foodres.2021.110208.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.