Medicine Group . 2022 July 30;3(7):819-826. doi: 10.37871/jbres1518.
Studies on the Anti-Pulmonary Fibrotic Effect of FGF-2-Targeting Binding Peptide G8
Peiji Lan1, Yuan Wang2, Wei Li3 and Ju Wang2*
2Institute of Biomedicine, Guangdong Provincial Key Laboratory of Bioengineering Medicine, National Engineering Research Center of Genetic Medicine, Jinan University, China
3Cell Biology Department, College of Life Science and Technology, Jinan University, China
- FGF-2
- Pulmonary fibrosis
- Biopanning
- α-SMA
Abstract
In our previous research, all the data showed that FGF-2 was very possible to be a new target for inhibiting lung fibrosis. Therefore, we plan to find the specific and small peptides to block FGF-2 signaling, hoping to develop a new scheme to treat pulmonary fibrosis better than FGF
Receptor extracellular domain. As a result, we biopanned several peptides through phage display technology, and finally got G8 which is the strongest peptide binding to FGF-2. Studies have found that G8 is almost non-toxic to L929 mouse lung fibroblast cells. In mouse bleomycin-induced pulmonary fibrosis model, the lung coefficient of the rats decreased significantly after 30 days of G8 treatment, the ground glass shadow decreases in CT scan, also the collagen deposition was alleviated dramatically (Masson staining), which suggests that the pulmonary fibrosis has been inhibited dramatically. And all above results are better than Pirfenidone (PFD) treatment group. The studies suggests obviously that G8 antagonizes the promoting effect of FGF-2 in the lung fibrotic process, which has a predictable applicating prospect.
Introduction
Pulmonary Fibrosis (PF) is caused by alveolar inflammation and the incidence is raising continuously across the world. PF patients typically succumb to respiratory failure secondary to loss of respiratory function because of lung parenchyma fibrosis. The average life expectancy is 3-5 years [1-4]. So far, FDA approved pirfenidone and nintedanib (tyrosine kinase inhibitors both) as a first-line treatment drugs for PF [5,6]. Pirfenidone is a pyridone compound suppressing collagen synthesis and deposition, also growth factors and cytokines expressions such as TGF-β/TNF-α [7,8]. However, neither the quality of life nor survival rates have been improved because of patient noncompliance and multiple side effects [9]. Thus, it is crucial to investigate the detailed molecular mechanism of pulmonary fibrosis and to explore and the effective novel drugs to PF.
Fibroblast Growth Factors (FGFs) represent a family of 22 structurally related growth factors fostering a wide range of biological effects, including mitogenesis and cellular differentiation, as well as increased cell motility and invasiveness in specific contexts [10,11]. FGFs mediate their biological responses by binding to high affinity Tyrosine Kinase (TK) FGF Receptors (FGFRs), designated FGFR-1 to -4 [12].
Transforming Growth Factor-β (TGF-β), the most important profibrogenic cytokine in the progression of pulmonary fibrosis [13], induces differentiation of pulmonary fibroblasts to myofibroblasts that produce high levels of collagen which marked by α-SMA high expression, leading to concomitantly loss of lung elasticity and function [14]. In our previous research, we firstly reported that the proliferation and differentiation of fibroblasts was indirectly achieved through the release of FGF-2 induced by TGF-β1 during the process of fibrosis [15,16]. The extracellular fragment of FGFRs is called soluble FGFR (sFGFR), and it can competitively bind to FGFs and inhibit the FGFR signaling pathway. We also reported in the first time that sFGFR2c (aimed at FGF-1/2/4/8/9) inhibits the expression of α-SMA in primary mouse lung fibroblasts via both the Smad-dependent and -independent signaling pathways that are activated by TGF-β. In vivo, sFGFR2c diminishes the development of Bleomycin (BLM) - induced lung fibrosis in mice. Most importantly, msFGFR2c treatment for 3 weeks beginning at one week after BLM induction could make the lung fibrosis neutralized [17]. These results suggested that blocking FGF-2 signal might convert the lung fibrotic process.
These studies indicated that the development of pulmonary fibrosis requires cell-autonomous mesenchymal Fibroblast Growth Factor (FGF) signaling [18]. Thus, we used the phage peptide library to screen the binding peptide G8 that can target FGF-2, and construct animal models of pulmonary fibrosis to explore the inhibitory effect of FGF-2-targeting binding peptide G8 on pulmonary fibrosis, which aims to test the new target of FGF-2 and provide a new way for the treatment of pulmonary fibrosis.
Materials and Methods
Cell lines, reagents and antibodies
Human non-small cell lung cancer cells (H1299 cells) and Mouse fibroblasts (L929 cells) were purchased from Shanghai Cell Bank of Chinese Academy of Sciences. The following primary antibodies were used. Anti-Collagen Ⅰ Rabbit pAb and Anti-α-SMA antibody were purchased from Abcam (Cambridge, MA, USA). Anti-FGF-2 Rabbit pAb was purchased from R&D Systems (Minneapolis, MN, USA). Bek /FGFR2 polyclonal antibody (Abcam, Cambridge, UK). β-actin (Santa Cruz, CA, USA). Horseradish Peroxidase (HRP)-conjugated anti-mouse IgG and HRP-conjugated anti-rabbit IgG (Cell Signaling Technology, Danvers, MA, USA).
Fmoc-AA-OH/1, 2-ethylene glycol mercaptan (TIS)/N, N-Diisopropylethylamine (DIEA)/N, N-Dimethylamide (DMF)/Diisopropylcarbodiimide (DIC)/ Triisopropylsilane (EDT)/ N, N-Dimethylamide (DMF) were purchased from Anhui Guoping Pharmaceutical Co, Ltd.( Anhui, China). Wang-resin/ 1-Hydroxybenzotriazole (HOBt) was purchased from Nanjing Peptide Industry Biotechnology Co, Ltd. (Nanjing, China).
Panning from peptide libraries: 96-well plate (Corning, New York, NY, USA), Tween 20 and Tris and PEG (Sigma, St. Louis. MO, USA), Glycine (Solarbio, Beijing, China), PBST and PBS (Life, Carlsbad, California, U.S.), BSA (Gibico, Waltham, MA, USA) were used. The Ph.D.™-7 Phage Display Peptide Library kit (New England Biolabs, Ipswich, MA, USA) and Ph.D.™-12 Phage Display Peptide Library Kit (New England Biolabs, Ipswich, MA, USA) were used in a panning protocol to screening binding peptides targeting FGF-2. The whole operation were performed in accordance with the planning kit instructions. Coating tubes with 40 ug/mL FGF-2 (test group) and 0 ug/mL antigens (control group), detecting the titer of eluted phages, and amplifying the eluted phage, positive monoclonal phages were obtained after a total of four rounds of panning.
ELISA assay: Refer to Wang's method and follow the manufacturer's instructions to carry out the test [19]. ELISA kit (Quantikine™; R&D Systems, Minneapolis, MN, USA) and 96-well plates (Corning, New York, NY, USA).
Western blotting: We refer to the method of Jeong DE, et al. [20], and perform Western blot analysis according to standard procedures. α-SMA, E-cadherin and FGF-2 expressions were determined via Western blot analysis of the right lung tissues using the indicated primary antibodies and detected with enhanced chemiluminescence reaction kit (Thermo Fisher Scientific, Waltham, MA, USA), BCA protein determination kit (Thermo Fisher Scientific, Waltham, MA, USA). β-actin protein levels were used as an internal control for equal protein loading. Protein bands were quantified with Image J (NIH, USA) for density analysis.
FMOC solid phase synthesis: All reagents and the resin used in the peptide synthesis are commercially available, were of peptide synthesis grade, and were purchased from the vendors specialized in the supplies for peptide synthesis. CS336X Automatic Peptide Synthesizer (California, USA). Refer to the method of Hansford KA, et al. [21] to synthesize G8. Use PIP/DMF solution as deprotection agent, DMF and DCM as cleaning agent, when cleaving peptide, use TFA:EDT:TIS:H2O = 95:2:2:1 solution as cleavage Liquid, tert-butyl ether is used as a precipitating agent.
RP-HPLC: FGF-2-targeting binding peptide G8 from crude peptide were purified by RP-HPLC with a C18 (Thermo Fisher Scientific, Waltham, MA, USA) reversed-phase column referring to the manual of waters Alliance™ HPLC System (Waters Milford, MA). The gradient of organic pump phase B is 5~95% and the flow rate is 10 ml/min. The purity of G8 is also analyzed by C18 column with the same condition.
Cell culture Human non-small cell lung cancer cells (H1299 cells) and Mouse fibroblasts (L929 cells) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) with standard antibiotics in a 95% air, 5% CO2 humidified atmosphere at 37ºC for all experiments.
CCK-8 test: The Boyden chamber transwell kit from Corning Company was used to evaluate cell viability. Pipet the L929 cell suspension with 3000 cells/well and inoculate it in a 96-well plate. And then performed as the report of our group [22].
Experimental animals: SPF Male SD rats were obtained from the Medical Experimental Animal Centre of Guangdong Province (Guangzhou, China). The animals were allocated to six groups, as follows: control group, BLM group, G8 (0.5 mg/Kg) group, G8 (1 mg/Kg) group, G8 (2 mg/Kg) and pirfenidone (PFD) (100 mg/Kg) group (oral administration). The indicators were tested. Pulmonary fibrosis was induced in male SD rats (170 g ~ 210 g) by intratracheal instillation of Bleomycin (BLM; Hisun Pharmaceutical Co. Ltd., Zhejiang, China) at 5 mg/kg body weight once, then G8 is given QD by intraperitoneal injection from 7 days after. And after 3 weeks of continuous administration, the animals were sacrificed and collected kinds of data needed to evaluate the effects of G8 on lung fibrosis. All animal experiments were performed in accordance with the procedures of the Ethical Committee for Animal Experimentation, Jinan University.
Histology: The mouse lungs were fixed in 4% paraformaldehyde. The lung specimens were then sent to Wuhan saiweier Biotechnology Co., Ltd. for paraffin embedding and sectioning, and the sections were taken back for Hematoxylin and Eosin (H&E). Airspace volume density was measured using minimum five random images from three samples per group and time point.
Hydroxyproline assay: We measured collagen content in the lungs with a conventional hydroxyproline assay, using a hydroxyproline kit (KeyGEN Biochemical Institute, Nanjing, China) according to the manufacturer’s instructions. The experimental results were quantified by comparison to a standard curve of known hydroxyproline concentrations.Masson’s staining was used to detect fibrosis in lung sections [19].
CT scan: After the rats were put to death with an excessive amount of chloral hydrate or isoflurane, the rats were immediately laid flat in the micro-CT (MILabs U-OI/CT small animal imaging system MILabs Holland) scanner, and the lungs were positioned in the control panel of the instrument to start the test. All animal experiments were performed in accordance with the procedures of the Ethical Committee for Animal Experimentation, Jinan University.
Data analysis: All data analyses were performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). The results are presented as mean values ± standard deviation. All data were analyzed using Analysis of Variance (ANOVA) or Student’s t-test to establish differences between the experimental and control groups. Each experiment was repeated three to five times. p values less than 0.05 were considered significant.
Results
Result of biopanning
After 4 rounds of biopanning, the results showed that the phage titer increased from 1.3 × 105 in the first round to 5 × 106 in the fourth round, and this titer increased by 3.8 times. The recovery rate also increased from 4.3 × 10-7 in the first round to 5 × 10-6 in the fourth round, which increased by 11.62 times (Table 1).
| Table 1: Results of biopanning. | ||||
| Rounds | Input (pfu) | Output (pfu) | Recovery rate | |
| Coating Antigen | No Coating | |||
| 1 | 2 × 1011 (12-peptide) | 1.3 × 105 | 8.5 × 105 | 4.3 × 10-7 |
| 1 × 1011 (7-peptide) | ||||
| 2 | 1 × 1012 | 5 × 105 | 4.7 × 104 | 5 × 10-7 |
| 3 | 1 × 1012 | 4.5 × 106 | 3.3 × 105 | 4.5 × 10-6 |
| 4 | 1 × 1012 | 5 × 106 | 4 × 105 | 5 × 10-6 |
Validating the optimal clones
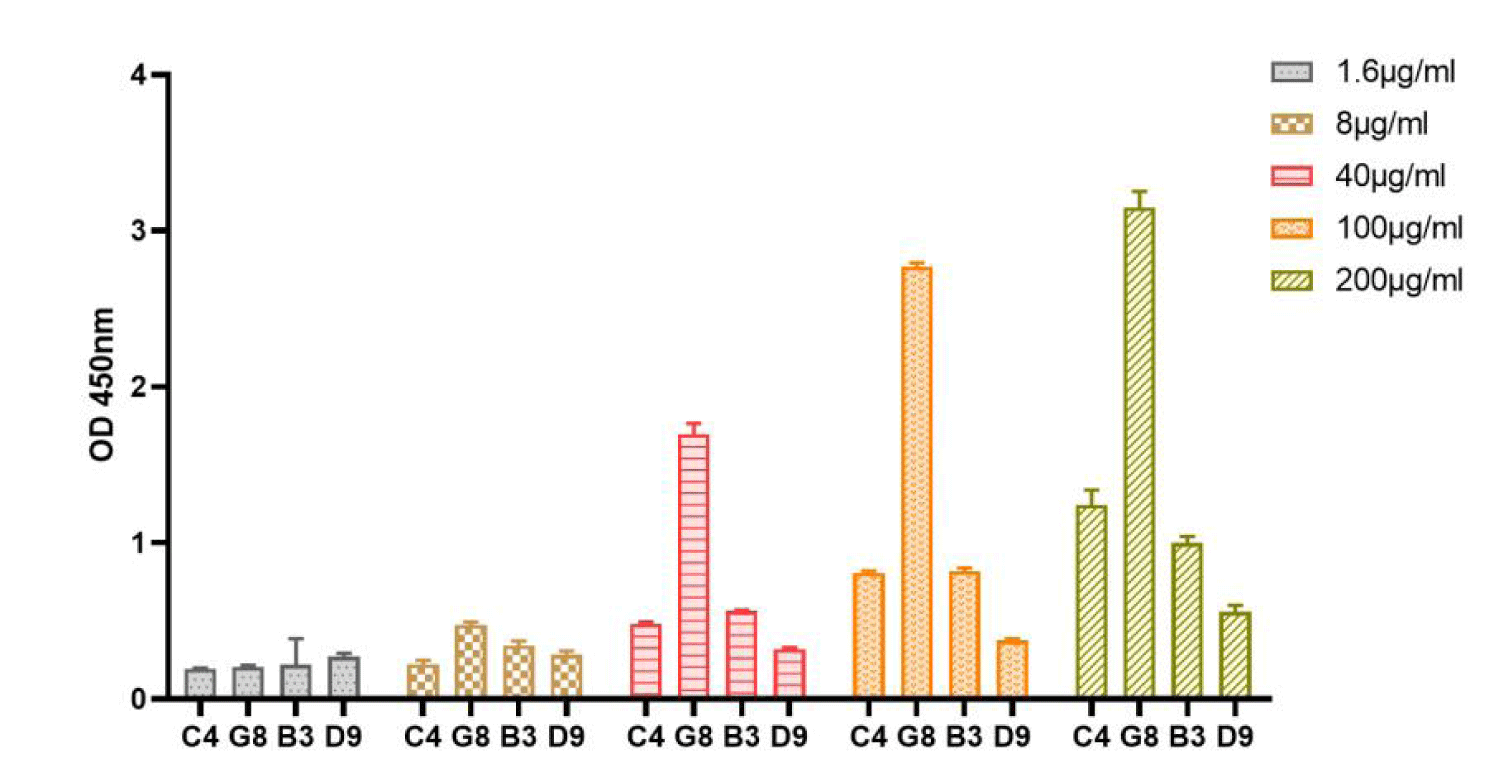
After four rounds of biopanning, we obtained 96 well-growing positive monoclonal plaques, and used ELISA to identify the affinity of the positive monoclonal antibodies to rhFGF-2. The 26 positive phage monoclonals were sequenced and a total of 4 peptide sequences were found (They are C4, G8, B3, D9, data not shown). In order to compare the affinity of these four peptide sequences with FGF-2, they were sent to Nanjing Jetide Biotechnology Co., Ltd. (Nanjing, Jiangsu, China) to synthesize N-terminal biotin-labeled peptides and tested by ELISA. The results show that compared with other peptides (Figure 1), there is a significant difference when G8 reaches 8 μg/ml. It shows that G8 has significant binding specificity with FGF-2. Therefore, the G8 sequence is the short peptide that we screened to target FGF-2.
Purity and molecular weight of G8
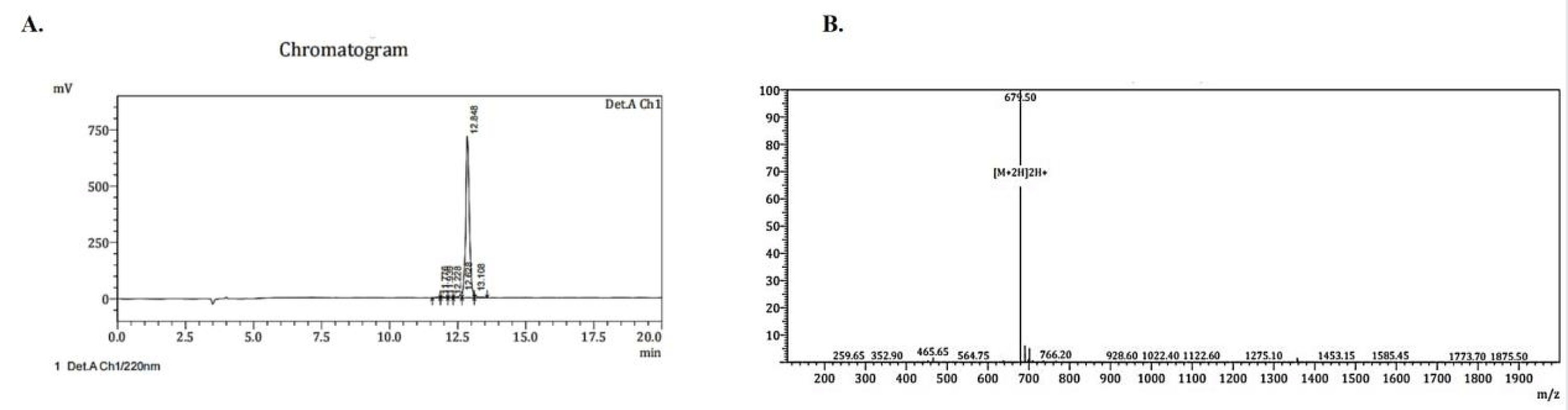
We use Fmoc solid-phase chemical peptide synthesis technology to synthesize FGF-2-targeting binding peptide G8 we screened. The crude G8 peptide was purified by reversed-phase high performance liquid chromatography and the synthesized G8 was identified by mass spectrometry. The results showed that the purity of G8 was 95.445% and the molecular weight was 1357.45 (Figure 2).
The effect of G8 on the proliferation and the phosphorylation of FGFR2 in cells
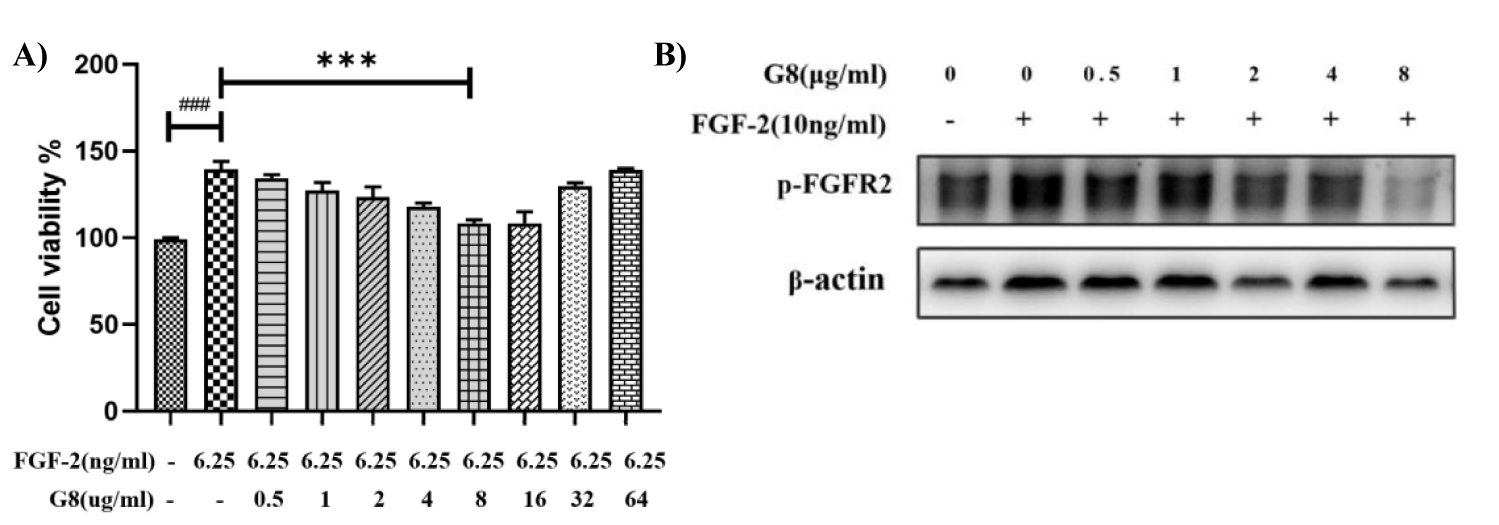
In this CCK-8 and WB assay, L929 mouse fibroblasts and H1299 human cancer cells were used to detect the bioactivity of G8. Compared with the control group, FGF-2(6.25 ng/ml) can significantly promote the proliferation of L929 cells. After the treatment with G8 dose- dependently for 48 h, it neutrolized significantly the proliferation of L929 cells caused by FGF-2, and also the phosphorylation of FGFR2 was absolutely eliminated in H1299 cells when the concentration of G8 reaches 8 μg/ml (Figures 3A,B). This results show that G8 can effectively block the signal pathway of FGFR2 induced by FGF-2.
The inhibitory effect of G8 on the BLM-induced pulmonary fibrosis in SD rats
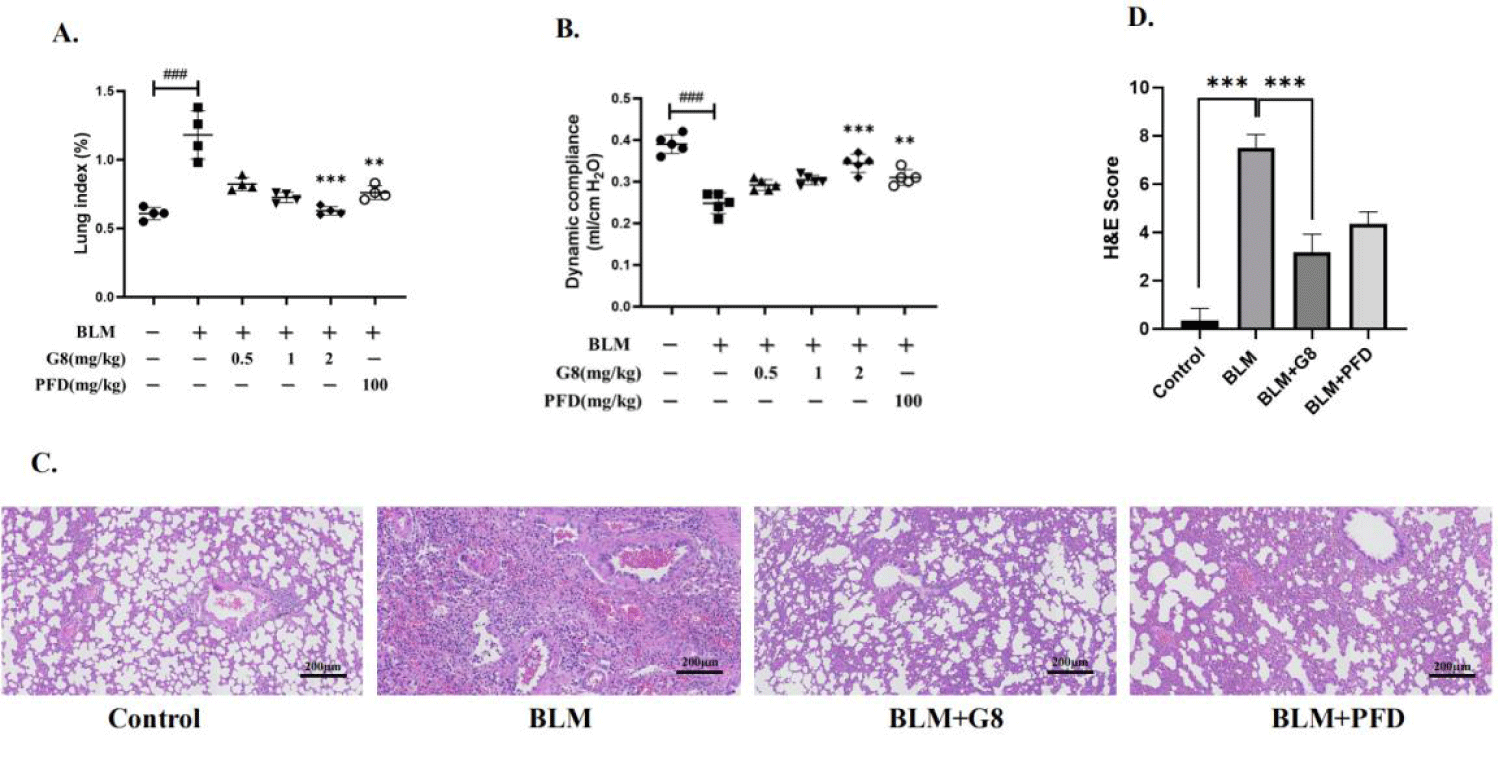
In the BLM-induced pulmonary fibrotic SD rat model, the lung coefficient (lung/body weight) of the rat increased significantly. When BLM-induced SD rats were treated with G8 (2 mg/kg) once a day, the lung coefficient of G8 treatment group decreased significantly, which was lower than PFD treatment group (Figure 4A). These results shows that G8 has a distinguished inhibitory effect on BLM-induced pulmonary fibrosis in SD rats, better than PFD positive control group.
Compared with the blank control group, the dynamic lung compliance of BLM-induced pulmonary fibrotic model group was significantly improved in G8 treatment group, which is better than PFD positive control group (Figure 4B). It shows that G8 can restore the lung function compared with the BLM-induced pulmonary fibrotic model in SD Rats.
Meanwhile HE staining result of G8 treatment group shows that the rat lung tissue sections are intact, and the infiltrations of inflammatory cells significantly reduce compared with the fibrotic model group, also which is better than PFD positive control group (Figure 4C).
Results of micro CT detection
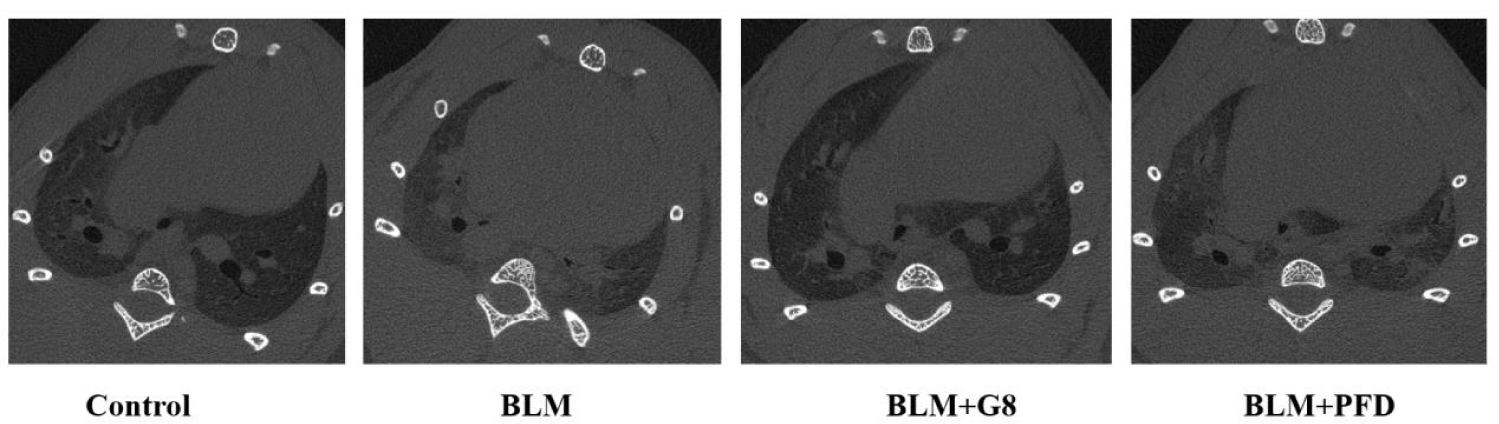
Micro-CT provides a visual assessment of high-resolution images of the lung microstructures. The cross-sectional CT results are shown in figure 5. In the BLM-induced fibrotic model group, it can be seen that the outlines of the lungs in SD rats are collapsed, ground glass shadows appear around the lung airways, and honeycomb shadows appear in the lung lobes. The fibrotic lung tissues in G8 treatment group return almostly to the normal state compared with the model group, which was better than the PFD positive control group (Figure 5).
Effect of G8 on collagen deposition in lung tissue of rats with pulmonary fibrosis
The level of hydroxyproline content reflects the degree of collagen deposition. The right lung of the rat was taken out after 30 days. Compared with the Control group, the hydroxyproline level of the BLM model group was significantly the highest. After continuous treatment with different doses of G8 (0.5 mg/Kg, 1 mg/Kg, 2 mg/Kg) , the content of hydroxyproline decreased to varying degrees, which is better thanthat of the positive control group of 100 mg/Kg of PFD treatment. It can be seen that the drug G8 reduce the accumulation of hydroxyproline in the lung, which further deepens the understanding of the FGF-2 binding peptide in lung fiber formation and treatment.
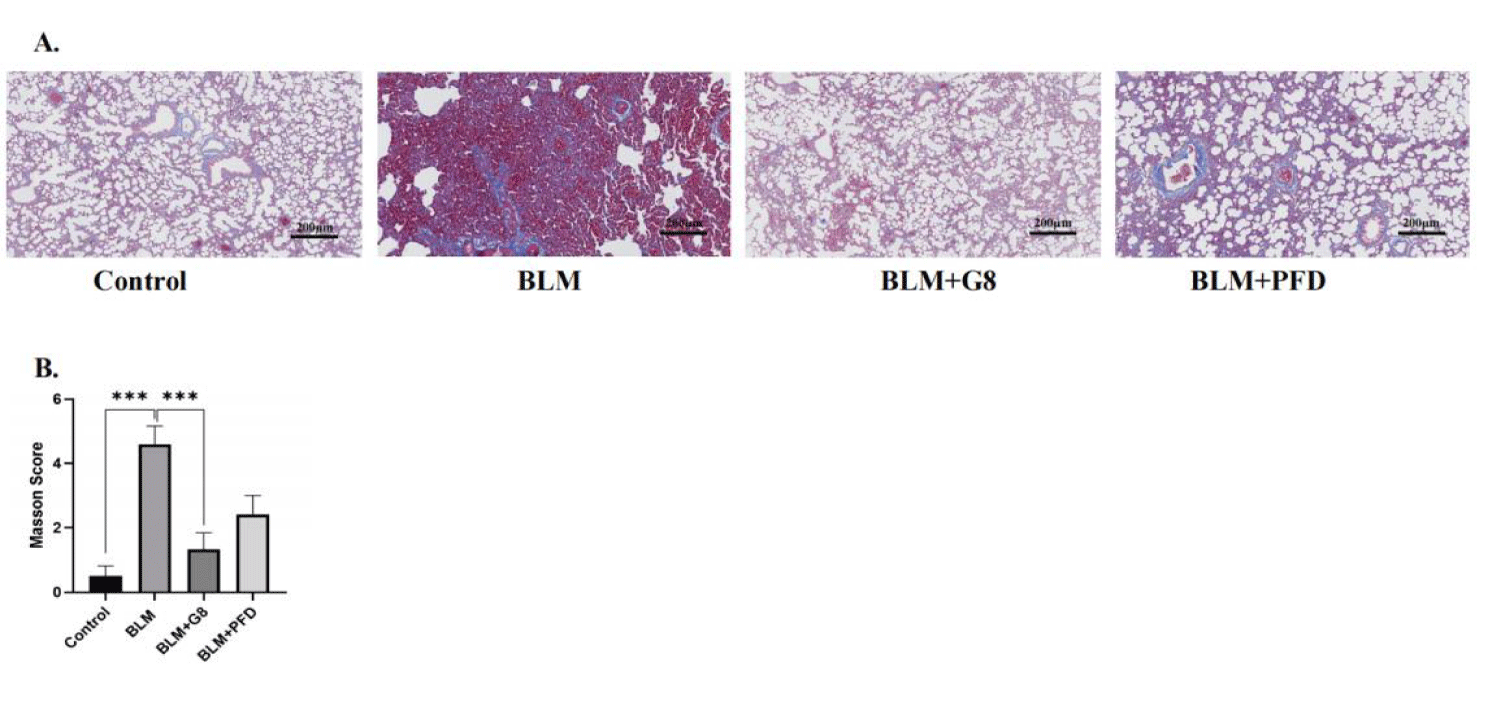
The results of Masson staining are show that the unstained area is the alveolar cavity, the collagen fibers are stained dark blue, and the muscle fibers are red. After BLM induction, a large area of cell proliferation can be seen stained blue in the microscope field, which indicates that a large amount of collagen fibers are deposited, and pulmonary parenchymal lesions are significant, and the degree of fibrosis is obvious. After 30 days of treatment with different doses of G8 (2 mg/Kg), compared with the lung tissue slices of rats in the BLM group, collagen deposition was significantly reduced, which is better than PFD treatment (Figures 6A,B).
WB detection of FGF-2, α-SMA, E-cadherin changes in lung tissues of fibrotic rats
Transforming from epithelium to mesenchyme lungs occurs during the process of fibrosis. Oval alveolar epithelial cells lose their polarity and special connection structure gradually transform into elongated spindle-shaped mesenchymal cells, which leads to the down-regulation of the epithelial marker E-cadherin. α-SMA is regarded as a characteristic protein of myofibroblasts and is the basis of myofibroblast contractility [23,24].
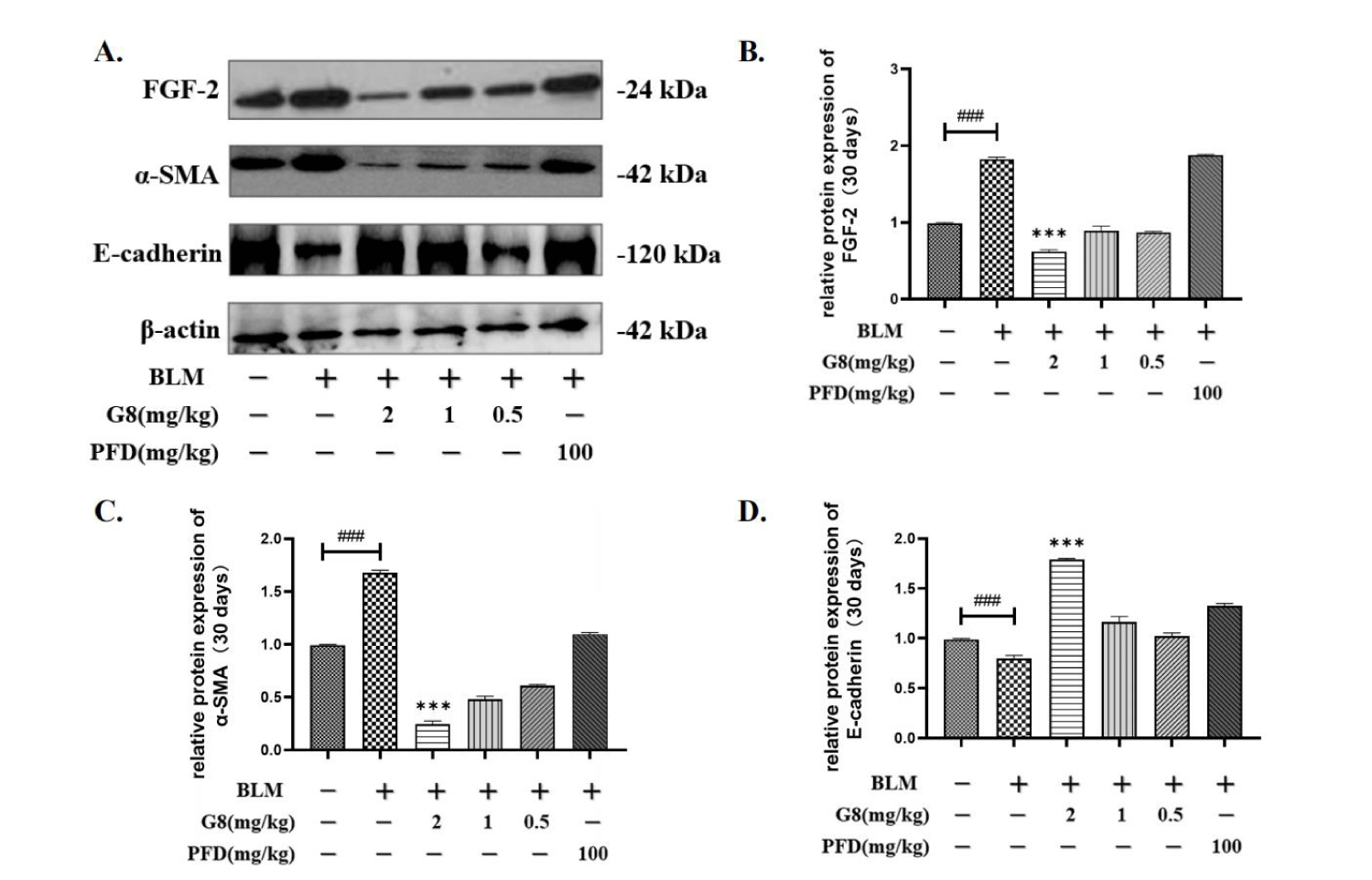
After intraperitoneal injection of G8 with concentrations of 0.5 mg/Kg, 1 mg/Kg, and 2 mg/Kg in SD rats, the WB method was used to detect the expression of FGF-2, α-SMA, and E-cadherin. Compared with the control group, the protein expression of FGF-2 and α-SMA in rats induced by BLM were significantly up-regulated, while E-cadherin was down.
After 30 days of treatment with different concentrations of G8 and oral PFD (100 mg/kg), it can be seen that when the dosage of G8 is 0.5 mg/kg, 1 mg/kg and 2 mg/kg, the protein expression of FGF-2 is significantly lower than that of BLM model group (Figures 7A,B). The expression of FGF-2 in the PFD control group was significantly up-regulated, which may be due to the compensatory effect of FGF-2. Similarly, after 30 days of administration of different doses of G8 and PFD, the expression of α-SMA was significantly down-regulated compared with the model group which is consistent with FGF-2 (Figures 7A,C). Moreover, the expression of E-cadherin was significantly up-regulated compared with the model group (Figures 7A,D).
Discussion
There is currently no drug that can significantly prevent and convert the process of pulmonary fibrosis. Nintanib and pirfenidone can delay the development of pulmonary fibrosis, however, there are still major side effects, and the overall results are still poor [25,26].
In our previous research, We firstly found that TGF-β induced high expressions of FGF-2 and FGFR2c in primary mouse lung fibroblasts which means TGF-β drives the fibrotic process through activating a high level FGF-2 signal pathway [15,16]. The extracellular fragment of FGFRs is called soluble FGFR (sFGFR), and it can competitively bind to FGFs and inhibit the FGFR signaling pathway. We constructed sFGFR2c (including wild-type and mutant sFGFR2c, called wsFGFR2c and msFGFR2c, patent No. ZL 2014 1 0347938.0), their inhibitory effects of lung fibrosis were identified in BLM-induced lung fibrotic model in rats, more importantly they also converted the process of lung fibrosis with 3-weeks treatment of msFGFR2c after one week of the construction of BLM-induced lung fibrotic model in rats. sFGFR2c contains two Ig like domains of the FGFR2IIIc subtype and is the most common receptor of FGF-2. wsFGFR2c can bind to kinds of Fibroblast growth factors, such as FGF1, FGF-2, FGF4, FGF, FGF6, FGF9, msFGFR2c(S252W mutant) can bind more kinds of FGFs. This strategy is involved FGF-2 signal pathway but not pointed at FGF-2 signal pathway. So we biopanned 7 peptides and 12 peptides high-throughputly by phage display technology, and finally got G8 which is the strongest peptide binding to FGF-2.
In this study, all above results indicates that G8 almost absolutely antagonizes the promoting effect of FGF-2 on fibrosis. G8 has a distinguished inhibitory effect on BLM-induced pulmonary fibrosis in SD rats, better than PFD positive control group.
Targeting FGF-2 binding peptide G8 constructed in this experiment is a small molecule polypeptide. Due to the short half-life of short peptide drugs, therefore, we can start from the aspect of modifying the drug to increase the stability of the drug except the oral or spray administration. In addition, since fibrotic diseases not only occur in the lungs, but also in other tissues including the heart, liver, and kidneys, G8 has a broad applicating potential in clinic.
Acknowledgment
This work was funded by the Ministry of Science and Technology of China. (2019ZX09201001-004-003).
References
- Cottin V, Hirani NA, Hotchkin DL, Nambiar AM, Ogura T, Otaola M, Skowasch D, Park JS, Poonyagariyagorn HK, Wuyts W, Wells AU. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur Respir Rev. 2018 Dec 21;27(150):180076. doi: 10.1183/16000617.0076-2018. PMID: 30578335.
- Lederer DJ, Martinez FJ. Idiopathic Pulmonary Fibrosis. N Engl J Med. 2018 May 10;378(19):1811-1823. doi: 10.1056/NEJMra1705751. PMID: 29742380.
- Lee HE, Myong JP, Kim HR, Rhee CK, Yoon HK, Koo JW. Incidence and prevalence of idiopathic interstitial pneumonia and idiopathic pulmonary fibrosis in Korea. Int J Tuberc Lung Dis. 2016 Jul;20(7):978-84. doi: 10.5588/ijtld.16.0003. PMID: 27287654.
- Barratt SL, Creamer A, Hayton C, Chaudhuri N. Idiopathic Pulmonary Fibrosis (IPF): An Overview. J Clin Med. 2018 Aug 6;7(8):201. doi: 10.3390/jcm7080201. PMID: 30082599; PMCID: PMC6111543.
- Sathiyamoorthy G, Sehgal S, Ashton RW. Pirfenidone and Nintedanib for Treatment of Idiopathic Pulmonary Fibrosis. South Med J. 2017 Jun;110(6):393-398. doi: 10.14423/SMJ.0000000000000655. PMID: 28575896.
- Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, Richeldi L, Kolb M, Tetzlaff K, Stowasser S, Coeck C, Clerisme-Beaty E, Rosenstock B, Quaresma M, Haeufel T, Goeldner RG, Schlenker-Herceg R, Brown KK; INBUILD Trial Investigators. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N Engl J Med. 2019 Oct 31;381(18):1718-1727. doi: 10.1056/NEJMoa1908681. Epub 2019 Sep 29. PMID: 31566307.
- Rogliani P, Calzetta L, Cavalli F, Matera MG, Cazzola M. Pirfenidone, nintedanib and N-acetylcysteine for the treatment of idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Pulm Pharmacol Ther. 2016 Oct;40:95-103. doi: 10.1016/j.pupt.2016.07.009. Epub 2016 Jul 29. PMID: 27481628.
- Jin J, Togo S, Kadoya K, Tulafu M, Namba Y, Iwai M, Watanabe J, Nagahama K, Okabe T, Hidayat M, Kodama Y, Kitamura H, Ogura T, Kitamura N, Ikeo K, Sasaki S, Tominaga S, Takahashi K. Pirfenidone attenuates lung fibrotic fibroblast responses to transforming growth factor-β1. Respir Res. 2019 Jun 11;20(1):119. doi: 10.1186/s12931-019-1093-z. PMID: 31185973; PMCID: PMC6558902.
- Moor CC, Mostard RLM, Grutters JC, Bresser P, Aerts JGJV, Dirksen CD, Kimman ML, Wijsenbeek MS. Patient expectations, experiences and satisfaction with nintedanib and pirfenidone in idiopathic pulmonary fibrosis: a quantitative study. Respir Res. 2020 Jul 23;21(1):196. doi: 10.1186/s12931-020-01458-1. PMID: 32703201; PMCID: PMC7376884.
- Hui Q, Jin Z, Li X, Liu C, Wang X. FGF Family: From Drug Development to Clinical Application. Int J Mol Sci. 2018 Jun 26;19(7):1875. doi: 10.3390/ijms19071875. PMID: 29949887; PMCID: PMC6073187.
- Prudovsky I. Cellular Mechanisms of FGF-Stimulated Tissue Repair. Cells. 2021 Jul 20;10(7):1830. doi: 10.3390/cells10071830. PMID: 34360000; PMCID: PMC8304273.
- Ghedini GC, Ronca R, Presta M, Giacomini A. Future applications of FGF/FGFR inhibitors in cancer. Expert Rev Anticancer Ther. 2018 Sep;18(9):861-872. doi: 10.1080/14737140.2018.1491795. Epub 2018 Jul 2. PMID: 29936878.
- Epstein Shochet G, Brook E, Bardenstein-Wald B, Shitrit D. TGF-β pathway activation by idiopathic pulmonary fibrosis (IPF) fibroblast derived soluble factors is mediated by IL-6 trans-signaling. Respir Res. 2020 Feb 18;21(1):56. doi: 10.1186/s12931-020-1319-0. PMID: 32070329; PMCID: PMC7029598.
- Yanagihara T, Sato S, Upagupta C, Kolb M. What have we learned from basic science studies on idiopathic pulmonary fibrosis? Eur Respir Rev. 2019 Sep 11;28(153):190029. doi: 10.1183/16000617.0029-2019. PMID: 31511255.
- Yu ZH, Wang DD, Zhou ZY, He SL, Chen AA, Wang J. Mutant soluble ectodomain of fibroblast growth factor receptor-2 IIIc attenuates bleomycin-induced pulmonary fibrosis in mice. Biol Pharm Bull. 2012;35(5):731-6. doi: 10.1248/bpb.35.731. PMID: 22687409.
- Ju W, Zhihong Y, Zhiyou Z, Qin H, Dingding W, Li S, Baowei Z, Xing W, Ying H, An H. Inhibition of α-SMA by the ectodomain of FGFR2c attenuates lung fibrosis. Mol Med. 2012 Sep 7;18(1):992-1002. doi: 10.2119/molmed.2011.00425. PMID: 22451267; PMCID: PMC3459475.
- El-Baz LMF, Shoukry NM, Hafez HS, Guzy RD, Salem ML. Fibroblast Growth Factor 2 Augments Transforming Growth Factor Beta 1 Induced Epithelial-mesenchymal Transition in Lung Cell Culture Model. Iran J Allergy Asthma Immunol. 2020 Aug 25;19(4):348-361. doi: 10.18502/ijaai.v19i4.4110. PMID: 33463102; PMCID: PMC8366022.
- Guzy RD, Li L, Smith C, Dorry SJ, Koo HY, Chen L, Ornitz DM. Pulmonary fibrosis requires cell-autonomous mesenchymal fibroblast growth factor (FGF) signaling. J Biol Chem. 2017 Jun 23;292(25):10364-10378. doi: 10.1074/jbc.M117.791764. Epub 2017 May 9. PMID: 28487375; PMCID: PMC5481550.
- Jieming G, Liu C, Yang Y, Mo S, Yang X, Wang J. Inhibitory effects of msFGFR2c on the epithelial-to-mesenchymal transition of AE2 cells in pulmonary fibrosis. Biotechnol Lett. 2020 Jun;42(6):1061-1070. doi: 10.1007/s10529-020-02852-x. Epub 2020 Mar 4. PMID: 32130565; PMCID: PMC7211205.
- Jeong DE, Lee Y, Lee SV. Western Blot Analysis of C. elegans Proteins. Methods Mol Biol. 2018;1742:213-225. doi: 10.1007/978-1-4939-7665-2_19. PMID: 29330803.
- Hansford KA, Ziora ZM, Cooper MA, Blaskovich MAT. Solid-Phase Synthesis of Octapeptin Lipopeptides. Methods Mol Biol. 2020;2103:199-213. doi: 10.1007/978-1-0716-0227-0_13. PMID: 31879927.
- Raghu G, Chen SY, Hou Q, Yeh WS, Collard HR. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18-64 years old. Eur Respir J. 2016 Jul;48(1):179-86. doi: 10.1183/13993003.01653-2015. Epub 2016 Apr 28. PMID: 27126689.
- Nagaraja SS, Nagarajan D. Radiation-Induced Pulmonary Epithelial-Mesenchymal Transition: A Review on Targeting Molecular Pathways and Mediators. Curr Drug Targets. 2018;19(10):1191-1204. doi: 10.2174/1389450119666180207092234. PMID: 29412104.
- Veres-Székely A, Pap D, Sziksz E, Jávorszky E, Rokonay R, Lippai R, Tory K, Fekete A, Tulassay T, Szabó AJ, Vannay Á. Selective measurement of α smooth muscle actin: why β-actin can not be used as a housekeeping gene when tissue fibrosis occurs. BMC Mol Biol. 2017 Apr 27;18(1):12. doi: 10.1186/s12867-017-0089-9. PMID: 28449660; PMCID: PMC5408491.
- Meyer KC. Pulmonary fibrosis, part I: epidemiology, pathogenesis, and diagnosis. Expert Rev Respir Med. 2017 May;11(5):343-359. doi: 10.1080/17476348.2017.1312346. Epub 2017 Apr 10. PMID: 28345383.
- Justet A, Klay D, Porcher R, Cottin V, Ahmad K, Molina Molina M, Nunes H, Reynaud-Gaubert M, Naccache JM, Manali E, Froidure A, Jouneau S, Wemeau L, Andrejak C, Gondouin A, Hirschi S, Blanchard E, Bondue B, Bonniaud P, Tromeur C, Prévot G, Marchand-Adam S, Funke-Chambour M, Gamez AS, Ba I, Papiris S, Grutters J, Crestani B, van Moorsel C, Kannengiesser C, Borie R; OrphaLung Network. Safety and efficacy of pirfenidone and nintedanib in patients with idiopathic pulmonary fibrosis and carrying a telomere-related gene mutation. Eur Respir J. 2021 Feb 11;57(2):2003198. doi: 10.1183/13993003.03198-2020. PMID: 33214205.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.