Medicine Group . 2022 July 23;3(7):797-801. doi: 10.37871/jbres1516.
A Single Amino Acid Change in Perforin-1: Two Patients with Severe and Recurrent HLH
Cunill V1,2, Molina-Fuentes A1,2, Jiménez MR1,2, Iglesias J1,2, Estevez M1,2, Daza-Cajigal V1,2, Gual C3, Ballester C4, Durán MA4 and Martínez-Pomar N1,2*
2Human Immunopathology Research Laboratory, Institut d'Investigació Sanitària de les Illes Balears (IdISBa), Palma, Spain
3Hematology Department, Hospital Son Llatzer, Palma, Spain
4HematologyDepartment, Hospital Universitari Son Espases, Palma, Spain
- Hemophagocytic lymphohistiocytosis (HLH)
- Familial HLH
- Natural Killer (NK) cells
- Cytotoxic T lymphocytes (CTLs);
- (PRF1) gene
Abstract
Biallelic mutations in several genes associated with cytotoxic lymphocytes function has been related to Familial HLH (FHLH). The clinical presentation of primary and acquired HLH may be indistinguishable, and both forms are commonly triggered by infections. FHLH is well described in children but in the last decade several late-onset cases have been reported.
We report the clinical and immunological characteristics of two adult patients carrying a single variant in Perforin-1. Patient-1, p.A91V carrier, was diagnosed of B-cell Chronic Lymphocytic Leukemia (CLL) and suffered leishmaniosis with severe progression of HLH disease that leads to death. Patient-2, carrier of p.R4H, suffered two HLH episodes in the context of HIV infection. The severity and the recurrence of the disease may suggest that these single PRF1 variants could predispose to immune-mediated disease.
Future studies are needed to understand the role of these variants in the development of the disease.
Abbreviations
HLH: Hemophagocytic Lymphohistiocytosis; FHLH: Familial HLH; NK: Natural Killer; CTLs: Cytotoxic T Lymphocytes; CLL: Chronic Lymphocytic Leukemia; AIDS: Acquired Immune Deficiency Syndrome; CADD: Combined Annotation Dependent Depletion; ExAC: Exome Aggregation Consortium
Introduction
Hemophagocytic Lymphohistiocytosis (HLH) is a heterogeneous life-threatening inflammatory multiorganic symdrome. Patients suffer an hyperinflammatory syndrome with fever, hepatosplenomegaly, cytopenias and, less commonly, central nervous system involvement. Hemophagocytosis is the hallmark of HLH. Others common findings include hypofibrinogenemia, hypertriglyceridemia and increased levels of ferritin, aminotransferases and the alpha-chain of the soluble IL-2 receptor [1,2].
HLH can be divided into primary (underlying genetic basis) and secondary (acquired) forms, which can overlap in their clinical presentation. Patients with Familial HLH (FHLH) present defects related to granule-dependent cytotoxicity of Natural Killer (NK) cells and Cytotoxic T Lymphocytes (CTLs) [2]. Five subtypes of FHLH have been described associated with the following genes: PRF1 (FHL2), UNC13D (FHL3), STX11 (FHL4), and STXBP2 (FHL5) [3-5]. The estimated incidence of FHLH is 0.1 to 1 cases per 100,000 children per year, but it is more common in regions of high consanguinity due to the autosomal-recessive inheritance [1]. Patients with familial form typically develop symptoms within the first months of life [2]. Nevertheless, up to 20% of patients present the first episode after 2 years of age and a few of them develop FHLH in adulthood.
Secondary HLH is probably more frequent than primary and it can be typically triggered by infection, malignancy, metabolic or autoimmune diseases [2]. In these cases, patients do not have disease-causing mutations in related genes and it may become apparent at a later age. EBV and leishmania infections are the most frequent triggers of the disease [2]. However, finding an infectious agent does not help to distinguish the etiology of HLH, because the primary forms are often triggered by infections.
Clinical diagnosis of HLH is made on the basis of cardinal signs and symptoms including prolonged fever, hepatosplenomegaly and characteristic laboratory findings [6]. Even though, therapy with immunosuppressive agents is addressed at the severity of symptoms, prompt diagnosis of primary HLH is mandatory. It is difficult to distinguish between secondary or primary HLH forms by clinical symptoms. In fact, it is necessary to perform specialized functional tests to evaluate granule-dependent cytotoxicity and mutational study. FHLH is considered an autosomal recessive immune disorder but nowadays some studies show that hypomorphic mutations in related genes correlate to late-onset presentation and/or mild symptoms and other reports suggest that heterozygous defects in two different genes (PRF1, UNC13D, STX11, and STXBP2) involved in cytotoxic pathway may have a synergistic deleterious effect [7].
We describe genetic and immunological findings of two adult patients who developed clinical features of HLH in the context of infection that presented a monoallelic variant in the PRF1 locus.
Materials and Methods
Case reports
P1: A 45-year-old asymptomatic man was incidentally discovered to have a white blood cell count of 33.70 × 103/µL [normal range: 3.50-12*103 /µL] (neutrophils 5.5%, lymphocytes 93.20% and monocytes 0.9%) during a routine annual visit to his primary care physician.
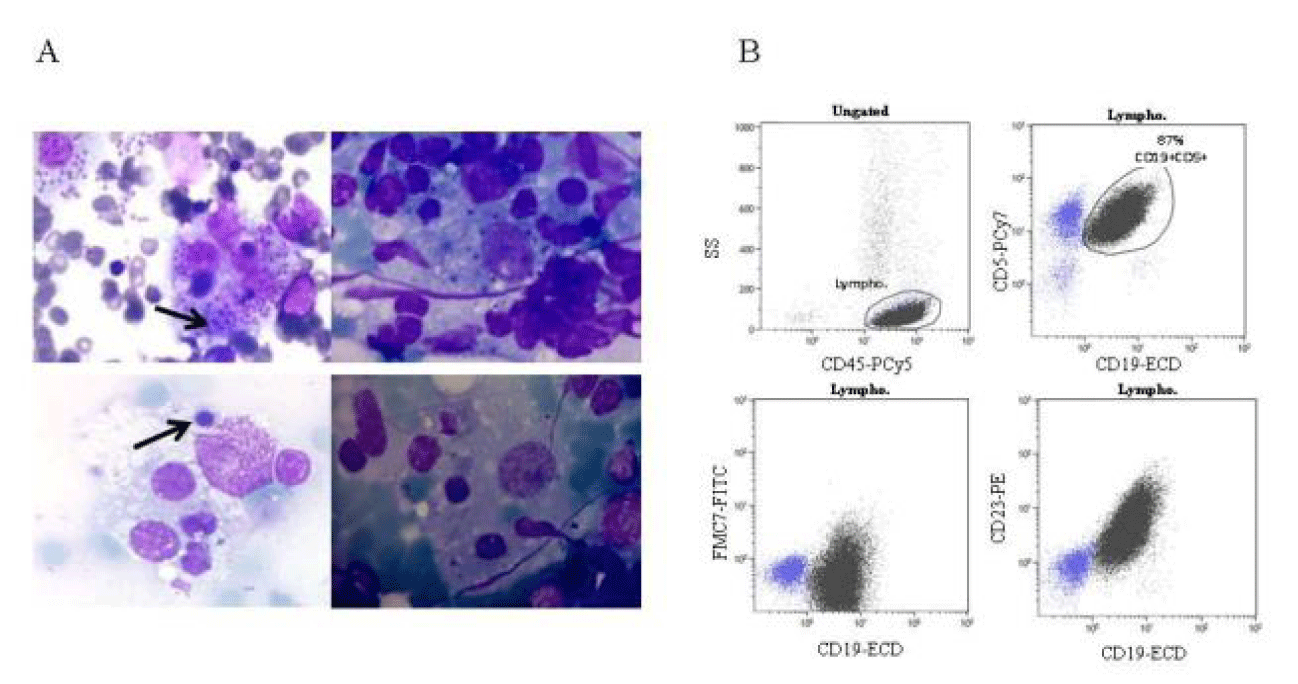
Peripheral blood flow cytometry revealed a clonal B cell population CD19+ CD5+ FMC7- and CD23+, consistent with B-cell Chronic Lymphocytic Leukemia (CLL) (Figure 1). The physical examination did not reveal neither organomegaly or lymphadenopathy and the CLL was classified as Rai state 0. Fluorescence in situ hybridization (FISH) to detect common chromosome abnormalities with prognostic significance in CLL showed del(13) q(14.3), associated with a relatively good prognosis.
Five months after the diagnosis, he presented sweating and subfebrile temperature, associated to hepatosplenomegaly and lymphadenopathies. The patient suffered a rapid progression to stage C of the disease.
Complete blood count demonstrated leukocytosis (5.93* 103 / µL) with persistent lymphocytosis (4.92* 103 /µL [normal range: 1.5-4.5*103/µL]), anemia (hemoglobin 10 g/dL [normal range: 12.0-18.0 g/dL]) and thrombocytopenia (platelets 87*103/µL [normal range: 130– 450*103/µL]). Chemotherapy (bendamustina and Obinutuzumab) was started.
Over the next month, the patient developed an episode consistent with HLH. Bone Marrow (BM) aspirate revealed active hemophagocytosis (Figure 1) and laboratory studies showed anemia, hypertriglyceridemia and hyperferritinemia. The severity of symptoms required initiating treatment according to HLH-04 protocol. Leishmania infection was detected by PCR and amastigote forms of Leishmania were observed in BM (Figure 1). Chloroquine treatment was started. The parasitic infection was eradicated but the patient died due to progressive multiple-organ failure.
P2: 39-year-old male from Senegal was diagnosed with stage B3 of Acquired Immune Deficiency Syndrome (AIDS). Persistent low levels of CD4 lymphocytes meant a forced change of antiretroviral therapy to emtricitabine, rilpivirine and tenofovir.
Three years after diagnosis, the patient was re-admitted to the Hospital due to pneumonia by P. Aaeruginosa, that was treated with Cefepime. On admission he presented splenomegaly and a pulmonary infiltrate. Laboratory analysis revealed anemia [Hgb 8 g/dL (normal range: 12.0-18.0 g/dL)], hypertriglyceridemia (199 mg/dl [normal range: 50-150 mg/dl]) and hyperferritinemia (453 ng/mL [normal range: 20-275 ng/mL]). Immunological analysis showed a T CD4+ lymphopenia (461 cell/µl) and low percentage of NK (CD3- CD56+) cells (< 1%). BM aspirate and spleen biopsy showed abundant histiocytes with active hemophagocytosis. The computer tomography scan showed splenomegaly and several lymphadenopathies and biopsy of inguinal lymph nodes showed Kaposi’s sarcoma. He required splenectomy and chemotherapy was established.
Four years later the patient was readmitted with fever and fatigue. The anamnesis showed several adenopathies, hemolytic anemia, hepatomegaly, hypertriglyceridemia (777 mg/dL) and hyperferritinemia (4533 ng/mL). Castleman disease was diagnosed secondary to Human Herpesvirus Type 8 and hemophagocytosis was present in BM and peripheral blood. Corticoids, rituximab and ganciclovir were added to the treatment regimen.
Laboratory tests to evaluate the cytoxicity machinery in P2 showed normal Perforin-1 expression and normal degranulation function in CTLs, test based on surface up-regulation of CD107a (data not shown).
Genetic analysis
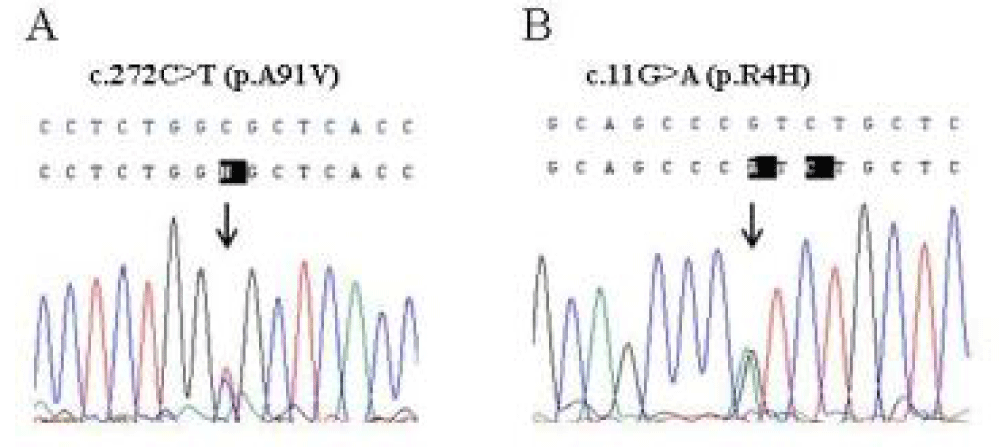
The severity and the recurrence of the disease in P1 and in P2 may suggest a genetic disease, predisposing to HLH. Sequence analysis of the PRF1, UNC13D and STX11 genes revealed the monoallelic p.A91V and p.R4H variants in Perforin-1, in patients P1 and P2 respectively (Figure 2). Written informed consent was obtained from all subjects.
Discussion and Conclusion
Classically, Familial Hemophagocytic Lymphohistiocytosis (FHLH2-5), is considered an autosomal recessive immune disorder caused by mutations in PRF1, UNC13D, STX11, and STXBP2, respectively. These genes all encode proteins essentials for granule mediated cytotoxicity [1].
Perforin-1, codifying by PRF1 gene, is a pore-forming cytolytic protein synthesized and stored in secretory granules of CTLs and NK. Up to 60% of cases of FHLH are associated with mutations in PRF1 gene. However, the contribution of some variants has not been fully clarified [8]. The p.R4H change detected in P2 is a poor documented substitution and it has not been associated to defined Perforin-1 activity in the literature. This variant, located in the leader signal peptide, shows ambiguous results in silico prediction tools of human sNPS: it was predicted to be benign and deleterious using PolyPhen-2 and SIFT tools, respectively. The Combined Annotation Dependent Depletion (CADD) score was 19.06 for this variant, indicating that it is predicted to be damaging. According to ExAC Browser database, the allele frequency in the total population is 1.1%, however, it has been found in up to 11% of African population [9]. The p.A91V variant found in P1 is one of the most controversial variants of the PRF1 gene. It is high frequency, around 3% and 17% of healthy study populations, suggests that it may represent a neutral polymorphism of Perforin-1 [10,11]. However, different studies have shown that p.A91V in homozygous state or together with other nonfunctional allele is associated with impaired function [8] and/or different clinical presentations, ranging from an atypical FHLH to hematological malignancies [12,13]. The significant question about this high frequent variant is whether heterozygosity may predispose to an immune mediated disease. Some authors have made an effort studying the functional effect of p.A91V variant in the cytolytic activity of Perforin-1. Ilia Voskoboinik, et al. [14] showed that the p.A91V substitution results in impaired protein function. Jenny, Chia et al. [15] established a link between the p.A91V and partial loss function, delayed onset of FHL, and increased susceptibility to hematological malignancies. Finally, a relevant study evaluated the cytotoxicity capacity of cells from healthy individual carriers of p.A91V [16]. Surprisingly their assays revealed that these healthy carriers had at least a 35% reduction in NK cytotoxicity due to a protein misfolding. These relevant data suggest that the p.A91V variant could be a risk factor associated to HLH, even in heterozygous state.
Patient 1, with asymptomatic CLL, developed rapid progression of the disease although he did not present apparent high-risk biological features. The association between HLH and hematological malignancies has been well described [17-19] but only a few cases of CLL have been associated with HLH. This patient suffered a severe HLH in the context of Leishmania infection and hematological malignancies that led to death. Patient 2, carrier of the monoallelic p.R4H substitution was HIV infected since 2010 and suffered from 2 episodes of HLH over 5 years.
The presence of these genetic variants, one of them with recognized functional effect in heterozygous cytotoxic cells, may indicate that monoallelic mutations in PRF1 could be a risk factor for HLH.
Early differential diagnosis of primary and secondary forms of HLH is clinically important to decide the treatment strategy. However, the establishment of the etiology may be difficult because there are not enough specific clinical or laboratory parameters.
FHLH disease usually appears in the first years of life, however, it has been described several cases of late onset. For this reason, exclusion of a genetic disease predisposing to HLH is important for childhood and adult-onset forms of the disease.
Genetic tests are useful for early and late onset presentations of FHLH but, unfortunately, results are not available in a short period of time. Expression and functional tests may rapidly help differentiate between inherited and acquired forms of HLH. However, these tests are only available in specialized clinical laboratories and have limitations detecting partial defects.
Therefore, the mutational study of PRF1 is also recommended despite obtaining a normal Perforin-1 expression level, in order to evaluate the possible effects of these common variants in a heterozygous state, mainly in patients with indicators for a genetic predisposition.
In summary, our results further support that this frequent variants may predispose to suffer a milder clinical phenotype or later onset of the disease. Further studies are needed to reveal the effect of these monoallelic variants on HLH.
References
- Degar B. Familial Hemophagocytic Lymphohistiocytosis. Hematol Oncol Clin North Am. 2015;29:903.
- Morimoto A, Nakazawa Y, Ishii E. Hemophagocytic lymphohistiocytosis: Pathogenesis, diagnosis, and management. Pediatr Int. 2016 Sep;58(9):817-25. doi: 10.1111/ped.13064. PMID: 27289085.
- Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, Lambert N, Ouachée-Chardin M, Chedeville G, Tamary H, Minard-Colin V, Vilmer E, Blanche S, Le Deist F, Fischer A, de Saint Basile G. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell. 2003 Nov 14;115(4):461-73. doi: 10.1016/s0092-8674(03)00855-9. PMID: 14622600.
- Bryceson YT, Rudd E, Zheng C, Edner J, Ma D, Wood SM, Bechensteen AG, Boelens JJ, Celkan T, Farah RA, Hultenby K, Winiarski J, Roche PA, Nordenskjöld M, Henter JI, Long EO, Ljunggren HG. Defective cytotoxic lymphocyte degranulation in syntaxin-11 deficient familial hemophagocytic lymphohistiocytosis 4 (FHL4) patients. Blood. 2007 Sep 15;110(6):1906-15. doi: 10.1182/blood-2007-02-074468. Epub 2007 May 24. PMID: 17525286; PMCID: PMC1976360.
- zur Stadt U, Rohr J, Seifert W, Koch F, Grieve S, Pagel J, Strauss J, Kasper B, Nürnberg G, Becker C, Maul-Pavicic A, Beutel K, Janka G, Griffiths G, Ehl S, Hennies HC. Familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) is caused by mutations in Munc18-2 and impaired binding to syntaxin 11. Am J Hum Genet. 2009 Oct;85(4):482-92. doi: 10.1016/j.ajhg.2009.09.005. PMID: 19804848; PMCID: PMC2756548.
- Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, Janka G. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007 Feb;48(2):124-31. doi: 10.1002/pbc.21039. PMID: 16937360.
- Zhang K, Chandrakasan S, Chapman H, Valencia CA, Husami A, Kissell D, Johnson JA, Filipovich AH. Synergistic defects of different molecules in the cytotoxic pathway lead to clinical familial hemophagocytic lymphohistiocytosis. Blood. 2014 Aug 21;124(8):1331-4. doi: 10.1182/blood-2014-05-573105. Epub 2014 Jun 10. PMID: 24916509; PMCID: PMC4141517.
- Martínez-Pomar N, Lanio N, Romo N, Lopez-Botet M, Matamoros N. Functional impact of A91V mutation of the PRF1 perforin gene. Hum Immunol. 2013 Jan;74(1):14-7. doi: 10.1016/j.humimm.2012.10.011. Epub 2012 Oct 13. PMID: 23073290.
- Willenbring RC, Ikeda Y, Pease LR, Johnson AJ. Human perforin gene variation is geographically distributed. Mol Genet Genomic Med. 2018 Jan;6(1):44-55. doi: 10.1002/mgg3.344. Epub 2017 Dec 7. PMID: 29216683; PMCID: PMC5823683.
- Busiello R, Fimiani G, Miano MG, Aricò M, Santoro A, Ursini MV, Pignata C. A91V perforin variation in healthy subjects and FHLH patients. Int J Immunogenet. 2006 Apr;33(2):123-5. doi: 10.1111/j.1744-313X.2006.00582.x. PMID: 16611257.
- Zur Stadt U, Beutel K, Weber B, Kabisch H, Schneppenheim R, Janka G. A91V is a polymorphism in the perforin gene not causative of an FHLH phenotype. Blood. 2004 Sep 15;104(6):1909; author reply 1910. doi: 10.1182/blood-2004-02-0733. PMID: 15342365.
- Clementi R, Emmi L, Maccario R, Liotta F, Moretta L, Danesino C, Aricó M. Adult onset and atypical presentation of hemophagocytic lymphohistiocytosis in siblings carrying PRF1 mutations. Blood. 2002 Sep 15;100(6):2266-7. doi: 10.1182/blood-2002-04-1030. PMID: 12229880.
- Manso R, Rodríguez-Pinilla SM, Lombardia L, Ruiz de Garibay G, Del Mar López M, Requena L, Sánchez L, Sánchez-Beato M, Piris MÁ. An A91V SNP in the perforin gene is frequently found in NK/T-cell lymphomas. PLoS One. 2014 Mar 14;9(3):e91521. doi: 10.1371/journal.pone.0091521. PMID: 24632576; PMCID: PMC3954696.
- Voskoboinik I, Sutton VR, Ciccone A, House CM, Chia J, Darcy PK, Yagita H, Trapani JA. Perforin activity and immune homeostasis: the common A91V polymorphism in perforin results in both presynaptic and postsynaptic defects in function. Blood. 2007 Aug 15;110(4):1184-90. doi: 10.1182/blood-2007-02-072850. Epub 2007 May 2. PMID: 17475905.
- Chia J, Yeo KP, Whisstock JC, Dunstone MA, Trapani JA, Voskoboinik I. Temperature sensitivity of human perforin mutants unmasks subtotal loss of cytotoxicity, delayed FHL, and a predisposition to cancer. Proc Natl Acad Sci U S A. 2009 Jun 16;106(24):9809-14. doi: 10.1073/pnas.0903815106. Epub 2009 Jun 1. PMID: 19487666; PMCID: PMC2701033.
- House IG, Thia K, Brennan AJ, Tothill R, Dobrovic A, Yeh WZ, Saffery R, Chatterton Z, Trapani JA, Voskoboinik I. Heterozygosity for the common perforin mutation, p.A91V, impairs the cytotoxicity of primary natural killer cells from healthy individuals. Immunol Cell Biol. 2015 Jul;93(6):575-80. doi: 10.1038/icb.2015.1. Epub 2015 Mar 17. PMID: 25776844.
- Daver N, McClain K, Allen CE, Parikh SA, Otrock Z, Rojas-Hernandez C, Blechacz B, Wang S, Minkov M, Jordan MB, La Rosée P, Kantarjian HM. A consensus review on malignancy-associated hemophagocytic lymphohistiocytosis in adults. Cancer. 2017 Sep 1;123(17):3229-3240. doi: 10.1002/cncr.30826. Epub 2017 Jun 16. PMID: 28621800; PMCID: PMC5568927.
- Roe C, Bennett J, Zhang L, Chavez J, Shah B, Sokol L, Komrokji R. Hemophagocytic Lymphohistiocytosis in Malignant Hematology: Uncommon but Should Not Be Forgotten? Clin Lymphoma Myeloma Leuk. 2015 Jun;15 Suppl:S147-50. doi: 10.1016/j.clml.2015.03.009. PMID: 26297268.
- El Abed R, Bourdon V, Voskoboinik I, Omri H, Youssef YB, Laatiri MA, Huiart L, Eisinger F, Rabayrol L, Frenay M, Gesta P, Demange L, Dreyfus H, Bonadona V, Dugast C, Zattara H, Faivre L, Zaier M, Jemni SY, Noguchi T, Sobol H, Soua Z. Molecular study of the perforin gene in familial hematological malignancies. Hered Cancer Clin Pract. 2011 Sep 21;9(1):9. doi: 10.1186/1897-4287-9-9. PMID: 21936944; PMCID: PMC3197553.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.