Medicine Group . 2022 July 19;3(7):779-786. doi: 10.37871/jbres1513.
Inflammation and Diabetic Kidney Disease: New Perspectives
Rico-Fontalvo Jorge1*, Daza-Arnedo Rodrigo1, Rodriguez-Yanez Tomás2, Martinez-Avila María Cristina3, Cabrales José4, Cardona-Blanco Maria Ximena1, Almanza-Hurtado Amilkar2, Uparella-Gulfo Isabella5 and Vergara-Serpa Oscar6
2Internal Medicine, University Cartagena, Colombia
3Epidemiologist, BIOTOXAM Group, Universidad de Cartagena, Colombia
4Nephrology Fellow, Stanford University School of Medicine, Palo Alto, USA
5General Physician, University of Sinu, Cartagena, Colombia
6Internal Medicine, Colombian Association of Internal Medicine, Cartagena, Colombia
Abstract
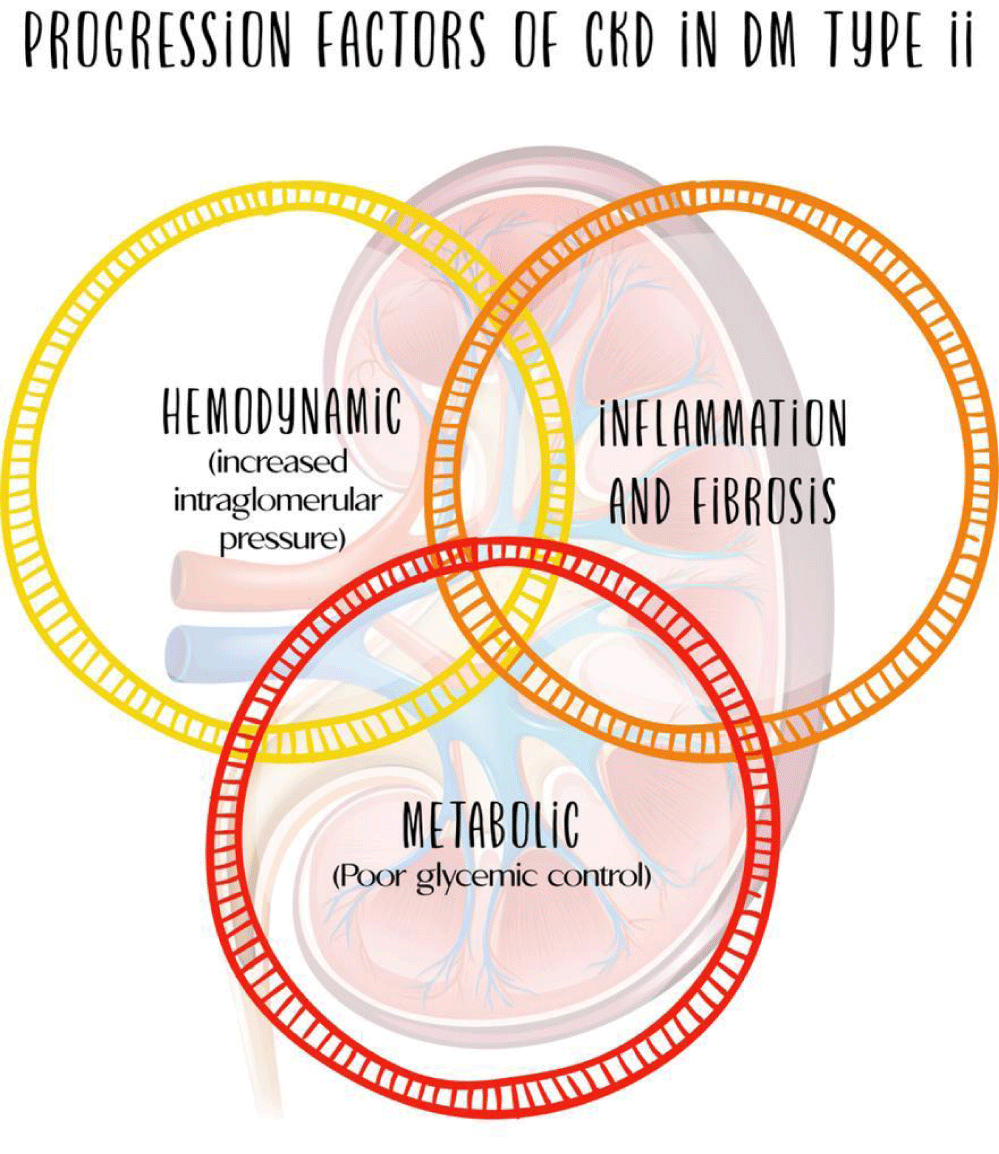
Diabetic Kidney Disease (DKD) can occur in approximately 30-40% of the population with type 1 or 2 diabetes mellitus around the world. In the pathogenesis and progression the Diabetic Kidney Disease (DKD), three fundamental axes are distinguished: hemodynamic, metabolic and inflammatory.
The primary purpose of the review is to describe the role and mechanisms related to inflammation in the course of this disease. The pathophysiological mechanisms involved in the development and progression of DKD include different pathways present long before the clinical diagnosis of the disease. Inflammation is a complex mechanism where innate and acquired immunity play an important role, in addition to other factors such as oxidative stress and end products of glycation that in one way or another would be linked to this process of inflammation. We cannot forget the contribution in this axis of inflammation of the well-known aldosterone escape phenomenon. The blockade of the Mineralocorticoid Receptor (MRA) inactivates the action of aldosterone and prevents the genomic and non-genomic response from interacting with the receptor, thus decreasing the degree of inflammation and remodeling in the heart and kidney. Finally, there are many territories to explore in this fascinating universe of inflammation as a mechanism of kidney damage in diabetic patients. Current management of DKD with innovative interventions point to this multitarget approach.
Introduction
Diabetic Kidney Disease (DKD) can occur in approximately 30-40% of the population with type 1 or 2 diabetes mellitus around the world [1-4]. In the pathogenesis and progression of this condition, three fundamental axes are distinguished: hemodynamic, metabolic and inflammatory, the latter with importance and growing evidence oriented to a possible therapeutic target (Figure 1).
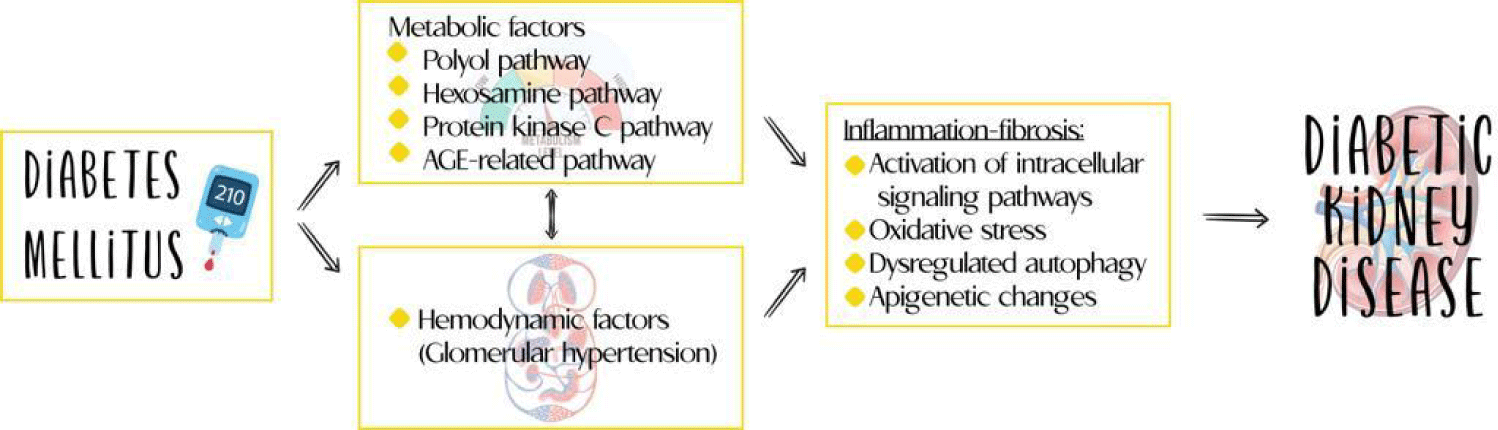
Consequently, the immune and inflammatory response play a central role in the pathogenesis of DKD [5]. However, in the traditional view of this disease it is considered a non-inflammatory glomerular disease, considering it a condition induced primarily by metabolic and hemodynamic changes, a situation that has been changing with the information constructed from the new available evidence [6], being inflammation a main phenomenon for the onset and progression of DKD. Therefore, we plan to review the role and mechanisms related to inflammation in the course of this disease.
Several studies and reports that have contributed to the development of the molecular pathways involved in DKD are emphasized, highlighting the role of inflammatory mediators such as inflammatory cytokines, especially Interleukin 1 (IL-1), IL-6 and IL-18. On the contrary, the participation of different cell populations such as monocytes and macrophages stands out, whose activation has been identified as a risk factor for the development of DKD [7]. In the following pages we will develop the different mechanisms involved in the pathogenesis of DKD and that can guide future therapeutic targets.
Hemodynamic Axis in DKD
Changes due to glomerular hyperfiltration lead to the development and appearance of diabetic kidney disease [8]. Hyperglycemia is related to the release of vasoactive mediators, with secondary dilation of the afferent arterioles. Among these mediators are insulin-like Growth Factor 1 (IGF-1), glucagon, Nitric Oxide (NO), Vascular Endothelial Growth Factor (VEGF) and prostaglandin [8].
Furthermore, alterations in renal tubular function occur in the early stages of diabetes mellitus, related to the degree of glycemic control [8]. Due to the high filtered load of glucose, tubular reabsorption of sodium and glucose is increased by upregulation of sodium glucose cotransporter 2 (SGLT2) in the proximal tubule [9]. This phenomenon leads to a decrease in the concentration of sodium in the distal tubular fluid, which alters the tubuloglomerular balance, the consequence of which is the dilation of the afferent arteriole with increased intraglomerular pressures and, therefore, a state of hyperfiltration [9]. This represents a mechanism parallel to those classically described derived from dysregulation in the vascular tone of the glomerular capillaries.
Within the understanding of the pathogenesis of DKD, Hostetter's work has made it possible to understand the interaction between hemodynamic and metabolic disturbances. In these publications, it was confirmed that hyperglycemia alters the self-regulation mechanisms of the glomerular capillaries, producing a secondary reduction in the arterial tone of the afferent glomerular arterioles and, to a lesser extent, the efferent one [9]. This dysregulation of vascular tone leads to glomerular hypertension and an initial increase in glomerular filtration rate, which contributes to the damage to the renal structure typical of type 2 diabetes mellitus [10].
Additionally, the increase in the production of angiotensin 2 mediated by hyperglycemia and with activation of the Renin Angiotensin Aldosterone System (RAAS), play an additional role in the dysregulation present in the glomerular capillaries [9]. These would be some fundamental hemodynamic mechanisms, a detailed description is beyond the scope of this publication.
Metabolic Axis in DKD
Exposure to chronic hyperglycemia, present in the course of diabetes mellitus, leads to increased absorption and oxidation of this by the cell, interfering with mitochondrial energy metabolism [9]. Electron donors drive a disproportionate increase in the mitochondrial membrane voltage gradient that results in inhibition of the complex III electron transport chain and increased synthesis of superoxide and Reactive Oxygen Species (ROS) [9].
Consequently, this increased production of free radicals and reactive oxygen species leads to inhibition of the activity of the enzyme glyceraldehyde-3-phosphate dehydrogenase, a key enzyme in glycolysis [11]. This inhibition of glycolysis activates the metabolic pathways involved in the pathogenesis of DKD; these include the hexosamine pathway, the pathway involved in Advanced Glycation End product (AGE) synthesis, the sorbitol pathway, and protein kinase-C activation [9].
Among the factors involved in the metabolic disturbances typical of diabetes mellitus and that lead to DKD are Angiotensin (ANG) 2, Vascular Endothelial Growth Factor (VEGF) A, Angiopoietin (ANGPT) 2, and Transforming Growth Factor (TGF) β1 [9]. The participation and contribution of ROS in the pathogenesis of DKD has been questioned, with variable results, it continues to be a point of research interest, without being able to clearly establish their participation. Recently, the parallel participation of lipid metabolism and the contribution of lipotoxicity in the development of this disease has been included, assuming that lipids are central elements in the progression of kidney disease [9]. The reader is referred to texts that fully address this basic problem in the development of diabetic kidney disease.
The Role of Inflammation in the Pathogenesis of Diabetic Kidney Disease
The pathophysiological mechanisms involved in the development and progression of DKD are complex and include different pathways present long before the clinical diagnosis of the disease. The processes that lead to stimulation of inflammation and fibrosis are the product of the intervention of metabolic alterations, glomerular hyperfiltration, oxidative stress and production of ROS, as well as the activation of innate immunity with secondary development of inflammation and fibrosis [12]. However, it was considered a non-inflammatory glomerular disease, indicating that it was a disease induced primarily by metabolic and hemodynamic changes, a situation that is reconsidered in light of the recent evidence of inflammation as the third pillar of the development of the disease [6] (Figure 2).
ARN sequencing studies of the nucleus of kidney cells taken from biopsies of patients with type 2 diabetes mellitus support the activation of signaling pathways involved in inflammation [12,13]. On the other hand, the study of the diabetic kidney shows an increase in the presence of inflammatory cells, leukocytes in an order of 7 to 8 times in relation to the kidney of healthy subjects [12]. Predominating some subpopulations of interest such as monocytes, B cells and plasma cells [12].
The traditional approach to DKD directs it towards a predominantly metabolic pathology. However, it is now recognized that hyperglycemia triggers an inflammatory response and induces oxidative stress in the different organs and tissues affected by diabetes [1,14]. These inflammatory mechanisms will be reviewed in the following pages and are presented as a therapeutic target for interventions derived from recent RCTs involving the use of SGLT2i, GLP-1RA and finerenone.
Consequently, hyperglycemia correlates with the development of inflammation, a situation precipitated by multiple mechanisms, among which there are some that are related to immunity [6]. Hyperglycemia generates activation of different pathways beyond the classical, hemodynamic, and metabolic axes. Within these, as previously noted, the inflammatory cascade generates interest; there is a correlation between inflammatory and hemodynamic phenomena [12]. There is an interaction between the inflammatory and non-inflammatory mechanisms in diabetes, these will generate the development of the natural history of DKD.
Toll-like receptors
Membrane Toll-Like Receptors (TLRs) and cytoplasmic Nod-Like Receptors (NLRs) are the main sensors present in immune cells that play a fundamental role in the initiation of the innate immune response through detection by sensing Pathogen-Associated Molecular Patterns (PAMPs) and Damage-Associated Molecular Patterns (DAMPs).
TLRs are encoded in a unique germline of Pattern Recognition Receptors (PRRs), which participate in the recognition and activation of the innate immune response, triggering a cascade of inflammatory events that lead to the production of cytokines. These types of receptors are expressed on a large number of immune cells, including macrophages, dendritic cells, T cells, B cells, and Natural Killer (NK) cells, as well as by non-immune cells, including renal tubular epithelial cells, endothelial cells, podocytes and mesangial cells [6]. The genome human encodes about 10 TLRs (1-10). In DRD, the participation of TLR2 and TLR4 has been reported contributing to the pathogenesis of inflammation [15].
There is a correlation between the metabolic and inflammatory pathways mediated by TLRs, circulating monocytes have a high expression of TLR2 and TLR4, especially within the population with diabetes mellitus type 1 and 2, and these levels of expression of TLRs are positively correlated with glycosylated hemoglobin levels and insulin resistance [15,16]. TLR4s are overexpressed at the renal tubular level and are associated with interstitial infiltration by macrophages in patients with type 2 diabetes mellitus [12]. The expression of the latter correlates with progression of chronic kidney disease and negative correlation with the glomerular filtration rate [12].
Nod-Like Receptors (NLRs)
Nod-Like Receptors (NLRs) are expressed in the cytoplasm and detect intracellular PAMPs and DAMPs [12]. NLRs included nucleotide-binding oligomerization domain, leucine rich repeat-containing, and pyrin domain-containing 1 (NLRP1), NLRP3, NLRP6, NLRP12, and NLRC4 (C for CARD, caspase activation and recruitment domain), will form the inflammasome, which plays a central role in the detection of inflammatory and microbiota disturbances, fundamental in the innate immune response [6].
The participation of renal resident mononuclear cells, such as macrophages and dendritic cells, stands out. They contain all the components of the inflammasome and precipitate the activation of the inflammatory cascade. The NLRP3 inflammasome complex is the most widely studied and implicated in different diseases in humans, including cancer, liver diseases and those of interest in the current document, kidney diseases [17,18]. This complex is composed of 3 components: an adapter protein ASC (apoptosis-associated speck-like protein containing a caspase-activating recruitment domain) and an effector protein, pro-CASP1 [6]. Finally, it is activated by different components fatty acid, uric acid, uromodulin, extracellular adenosine triphosphate, hyperglycemia, serum amyloid A, and mitochondrial ROS [12].
Innate immunity cells
The effector cells of innate immunity involved in the immune response and inflammation correspond to macrophages and dendritic cells, these infiltrate the kidney of patients with diabetes mellitus, mediating histological damage and a drop in the glomerular filtration rate [6,12]. The inflammatory response in the setting of type 2 diabetes mellitus is precipitated as a consequence of the presence of multiple metabolic products derived from prolonged exposure to hyperglycemia and hemodynamic changes. Among these products, ROS stand out, hyperuricemia and lipid metabolites which act as DAMPs detected by TLR and NLR inducing secondary activation of innate immune cells [19,20].
On the other hand, the overproduction of deleterious metabolic products could have an influence on non-immune renal cells, which would participate in the release of chemokines and cytokines with effects on the recruitment of immune cells themselves, as discussed in other sections [6]. The proliferation and maintenance of these inflammatory mononuclear cells in the kidney requires the presence and participation of Colony-Stimulating Factor-1 (CSF-1), which acts through the C-FMS receptor [12]. Consequently, the increased production of renal CSF-1 contributes to the proliferation and activation of renal mononuclear cells, a key part of the innate immune response in DKD [6].
Another relevant pathophysiological event is the increased expression of adhesion molecules such as Intercellular Adhesion Molecule 1 (ICAM-1) and Monocyte Chemoattractant Protein 1 (MCP-1)/CC motif chemokine ligand 2 (CCL2), which promote migration and arrival of immune cells to the kidney of the diabetic patient [21,22]. Other cells have been identified beyond macrophages and detrital cells, identified with markers such as D11b, F4/80, and CD68 for macrophages or CD11c, myosin heavy chain II, and CD80/86 for dentritic cells [12].
Inflammatory cytokines
Inflammatory cytokines correspond to polypeptide molecules produced by immune cells, endothelial cells, epithelial cells, and fibroblasts in an autocrine, paracrine, and juxtacrine manner [12]. Among the inflammatory cytokines, IL-1, IL-18, IL-6, Tumor Necrosis Factor (TNF), and IL-17 stand out with powerful proinflammatory effects in the pathogenesis of DKD [23]. In the study of the role of cytokines in inflammation, IL-1 and the determination of elevated urinary levels of IL-1 have been correlated with injury to the epithelial cells of the proximal tubules and podocytes [6].
Moreover, IL-18 is a member of the IL-1 superfamily and mediates the stimulation of Interferon-γ (IFN-γ) release, secondarily modulating the function of innate and adaptive immune cells [12]. In patients with type 2 diabetes mellitus and renal involvement, it has been possible to identify elevated levels of this cytokine in urine and serum, which may be a marker of renal inflammation.
IL-6 functions as a co-stimulatory molecule and an acute phase reactant mediating the activation of T and B cells [12]. Elevated levels of this molecule have been determined in patients with type 2 diabetes mellitus and DKD [24]. The determination of inflammatory markers such as the cytokine IL-6 and IL-10 allows the identification of diabetic patients with DKD even before deterioration in glomerular function or filtration appears [25].
Tumor Necrosis Factor α (TNF-α), acts as a proinflammatory molecule in DKD, within these functions the induction and differentiation of inflammatory cells, renal cytotoxicity, induction of apoptosis, hemodynamic alteration and autoregulation of the glomeruli, increased permeability of the vascular endothelium and the production of reactive oxygen species and oxidative stress [12]. Genomic sequencing studies have been able to establish an inverse correlation between the expression of TNF-α and the drop in the glomerular filtration rate [26]. Different population studies carried out in patients with type 2 diabetes mellitus have oriented the relationship between the renal expression of TNF-α and its receptors associated with impaired glomerular filtration rate and the appearance of DKD [27,28].
Finally, the role of IL-17 is fundamental in the production of CD4+ IL-17+ cells, referred to as T helper 17 cells, which interact with the IL-17 receptor. IL-17 dysregulation in autoimmune diseases activates various immune pathways leading to induction of IL-6, TNF-α, CCL2, and CCL5 expression. Activation of these molecules contributes to the recruitment of monocytes and neutrophils to the site of inflammation [6]. IL-17 levels are particularly elevated in patients with type 1 diabetes mellitus and correlate with glomerular filtration rate. The participation of this group of cytokines correlates as well as the activation and progression of inflammation, keeping a close relationship with the remaining complementary pathways and the deterioration in glomerular filtration.
Chemokines and their receptors
Chemokines are small cytokines with the ability to recruit different cell populations through chemotaxis [12]. These proteins are activated in non-immune renal cells in response to the hemodynamic and metabolic changes associated with DKD. This results in the recruitment, migration and adhesion of inflammatory cells [29]. The main chemokines involved in DKD correspond to CCL2 (MCP-1), CCL5 (RANTES), and C-X3-C motif chemokine 1 (CX3CL1, Fractalkine) with growing evidence in this regard [12].
The participation of CCL2 (MCP-1) is highlighted, produced by renal tubular epithelial cells and podocytes in the kidney of patients with diabetes mellitus, these favor the recruitment of mononuclear cells of innate immunity and memory T lymphocytes in the foci of inflammation renal [30]. Urine levels of CCL2 have been correlated with progression of chronic kidney disease in healthy adults and those with DKD [12]. Additionally, the degree of infiltration by inflammatory cells would correlate with urinary levels of CCL2, for example in the case of the presence of CD68+ cells in the interstitium, markers of cells such as inflammatory cells such as macrophages [12].
Besides, other chemokines such as CCL5 are involved in the recruitment of monocytes and T cells, playing a central role in the marginalization and localization of leukocytes at sites of inflammation. Elevated levels of CCL5 (RANTES) have been shown to participate as markers of progression to type 2 diabetes mellitus in patients with obesity and intolerance to carbohydrates when compared with healthy subjects [31]. Kidney biopsy studies of patients with type 2 diabetes mellitus and DKD have been able to identify a strong upregulation in the concentrations of CCL2 and CCL5, which would be related to the progression of renal complications derived from diabetes mellitus.
Complement system
Two main complement-mediated mechanisms have been identified in the development of DKD [32]. In the first instance, the activation of the lectin pathway in response to the presence of glycosylated proteins, present on the surface of cells in response to hyperglycemia [6]. The second mechanism involved is mediated by the glycation of complement regulatory proteins in response to hyperglycemia, with dysfunction of the regulatory mechanisms of the complement pathways [6]. In the histopathological study of the kidney of patients with DKD, the presence of complement deposits has been confirmed [6].
The complement system is an effector organ of innate immunity, with great participation in the pathogenesis of different inflammatory and infectious diseases [12]. At the renal level, different components of this system are expressed, among which the expression by the epithelial cells of the proximal tubule complement C3 and membrane-bound C3 convertase are distinguished, which could activate the intrarenal complement pathway in various kidney diseases [33,34].
Variations in concentrations of complement components have been correlated with increased risk of diabetic retinopathy, nephropathy, and neuropathy, especially increased levels of complement C3 [35]. Also, the concentrations of complement proteins such as Mannose-Binding Lectin (MBL) have been associated with an OR 2.6 for the development of proteinuria and decreased renal function in patients with type 2 diabetes mellitus [36]. Changes in MBL concentrations could correlate with progression to end-stage kidney disease and thus progression to DKD. Increased renal tubular expression of C5a in patients with CKD has been correlated with the progression of this disease [37]. The role of innate immunity is fundamental in the development of DKD, with a growing participation of complement pathways both in the onset of the disease and in its progression.
Adaptive immunity cells
Infiltration by inflammatory cells in the kidney of diabetic patients occurs predominantly by cells of innate immunity; however, the presence of T and B lymphocytes has been confirmed to a lesser extent [12]. The participation of cells of adaptive immunity has been documented in recent reports, participating in the pathogenesis of metabolic diseases, highlighting their participation in DKD. In this scenario, the response mediated by Th1 and Th17 cells predominates, with the consequence of a reduction in the expression of Treg lymphocytes as an adaptive response to hyperglycemia [38].
In the setting of glomerular diseases, the presence of proteinuria in patients with type 2 diabetes mellitus is related to increased circulation of Th1 and Th17 cells [39]. Th1 cells are characterized by the production of cytokines such as IFN-γ, TNF-α, IL-2 and in the case of Th17 cells, the production of IL-17 type cytokines predominates [12]. Moon, et al. [40] managed to demonstrate an increase in the expression of CD4+, CD8+ and CD20+ cells in the glomerular and tubulointerstitial areas; additionally, that the expression of CD4+ and CD20+ cells correlates with the severity and amount of proteinuria. The participation of cells of adaptive immunity is a piece to consider within the articulated inflammatory response that leads to the onset and progression of CKD.
Aldosterone
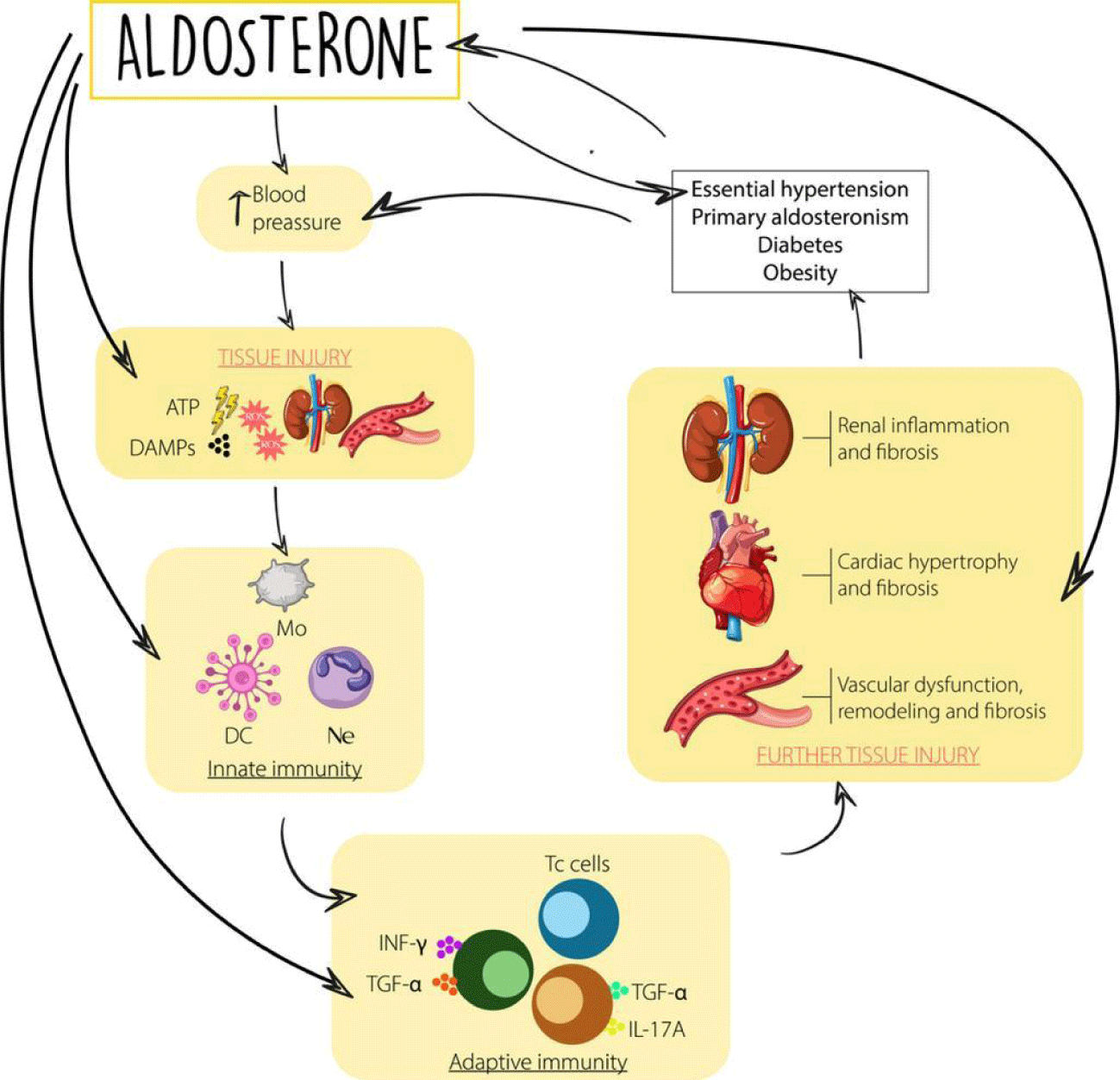
Finally, we cannot forget the contribution in this axis of inflammation of the well-known aldosterone escape phenomenon. From preclinical models, the antifibrotic and anti-inflammatory effects of mineralocorticoid receptor blockade have been documented [41,42]. This is a very active field of current research and much progress is expected in the coming years (Figure 3).
Aldosterone acts directly on the Na/K/ATP-ase pumps located in the basolateral membrane of the principal cells of the kidney [42]; however, Mineralocorticoid (MR) receptors are present in multiple tissues, including vascular and endothelial smooth muscle cells, cardiomyocytes, fibroblasts, kidney (mesangial cells and podocytes), adipocytes, macrophages, and brain (hypothalamus), activating remodeling in response to inflammation and damage [42]. This wide distribution explains why aldosterone exerts multiple cardiac, vascular, and renal effects including endothelial dysfunction, vasoconstriction, natriuresis, K+ retention, sympathetic activation, adverse cardiovascular (hypertrophy, fibrosis), and renal (glomerular and tubular sclerosis) remodeling and oxidative stress; it increases vascular stress, stiffness and exerts proarrhythmic, proinflammatory and prothrombotic effects [42].
In summary, blockade of the Mineralocorticoid Receptor (MRA) inactivates the action of aldosterone and prevents the genomic and non-genomic response from interacting with the receptor, thus decreasing the degree of inflammation and remodeling in the heart and kidney.
Innovative therapies emerging for the management of diabetes and DKD significantly engage and impact the inflammatory axis. We must remember that these therapies act at different points of this inflammatory axis which, as has been reviewed, is quite complex and there are still aspects to be elucidated. These innovative therapies would be SGLT-2 co-transporter inhibitors, some GLP-1 agonists and mineralocorticoid receptor blockers, of which we highlight Finerenone.
Conclusion
Previously, diabetic glomerulopathy was considered a non-inflammatory disorder, highlighting the metabolic and hemodynamic components as the main substrates. Nevertheless, with the passage of time and new advances in the pathophysiological mechanisms of DKD, it has been understood more and more, the fundamental role of inflammation in the onset and progression of kidney damage. Inflammation is a complex mechanism where innate and acquired immunity play an important role, in addition to other factors such as oxidative stress and end products of glycation that in one way or another would be linked to this process of inflammation.
In light of these new findings, we must consider DKD as an inflammatory process where multiple axes act in its development and progression. There are many territories to explore in this fascinating universe of inflammation as a mechanism of kidney damage in diabetic patients. Current management of DRD with innovative interventions point to this multitarget approach.
References
- Turkmen K. Inflammation, oxidative stress, apoptosis, and autophagy in diabetes mellitus and diabetic kidney disease: the Four Horsemen of the Apocalypse. Int Urol Nephrol. 2017 May;49(5):837-844. doi: 10.1007/s11255-016-1488-4. Epub 2016 Dec 29. PMID: 28035619.
- Castillo GA, Aroca G, Buelvas J, Buitrago AF, Carballo V, Cárdenas JM, Gómez EA, Fériz K, Lopera JM, Melgarejo E, Restrepo K, Montejo JD, Pinzón JB, Quintero A, Ricoy JE, Rosero R. Recomendaciones para el manejo del riesgo cardiorrenal en el paciente con diabetes mellitus tipo 2. Rev Colomb Cardiol. 2020;27(S3):3-22. doi: 10.1016/j.rccar.2020.07.005.
- Rico Fontalvo JE. Guía de práctica clínica para la enfermedad renal diabética. Rev Colomb Nefrol. 2022;8(2). doi: 10.22265/acnef.8.2.561.
- Rico Fontalvo JE. Enfermedad renal diabética: de cara a la prevención, diagnóstico e intervención temprana. Rev Colomb Nefrol. diciembre de 2020;7(2):15-16. doi: 10.22265/acnef.7.2.506.
- Samsu N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. Biomed Res Int. 2021 Jul 8;2021:1497449. doi: 10.1155/2021/1497449. PMID: 34307650; PMCID: PMC8285185.
- Tang SCW, Yiu WH. Innate immunity in diabetic kidney disease. Nat Rev Nephrol. 2020 Apr;16(4):206-222. doi: 10.1038/s41581-019-0234-4. Epub 2020 Jan 15. PMID: 31942046.
- Kiritoshi S, Nishikawa T, Sonoda K, Kukidome D, Senokuchi T, Matsuo T, Matsumura T, Tokunaga H, Brownlee M, Araki E. Reactive oxygen species from mitochondria induce cyclooxygenase-2 gene expression in human mesangial cells: potential role in diabetic nephropathy. Diabetes. 2003 Oct;52(10):2570-7. doi: 10.2337/diabetes.52.10.2570. PMID: 14514642.
- Lin YC, Chang YH, Yang SY, Wu KD, Chu TS. Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc. 2018 Aug;117(8):662-675. doi: 10.1016/j.jfma.2018.02.007. Epub 2018 Mar 2. PMID: 29486908.
- Ricciardi CA, Gnudi L. Kidney disease in diabetes: From mechanisms to clinical presentation and treatment strategies. Metabolism. 2021 Nov;124:154890. doi: 10.1016/j.metabol.2021.154890. Epub 2021 Sep 22. PMID: 34560098.
- De Vriese AS, Stoenoiu MS, Elger M, Devuyst O, Vanholder R, Kriz W, Lameire NH. Diabetes-induced microvascular dysfunction in the hydronephrotic kidney: role of nitric oxide. Kidney Int. 2001 Jul;60(1):202-10. doi: 10.1046/j.1523-1755.2001.00787.x. PMID: 11422752.
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005 Jun;54(6):1615-25. doi: 10.2337/diabetes.54.6.1615. PMID: 15919781.
- Jung SW, Moon JY. The role of inflammation in diabetic kidney disease. Korean J Intern Med. 2021 Jul;36(4):753-766. doi: 10.3904/kjim.2021.174. Epub 2021 Jul 1. PMID: 34237822; PMCID: PMC8273831.
- Woroniecka KI, Park AS, Mohtat D, Thomas DB, Pullman JM, Susztak K. Transcriptome analysis of human diabetic kidney disease. Diabetes. 2011 Sep;60(9):2354-69. doi: 10.2337/db10-1181. Epub 2011 Jul 13. PMID: 21752957; PMCID: PMC3161334.
- Winiarska A, Knysak M, Nabrdalik K, Gumprecht J, Stompór T. Inflammation and Oxidative Stress in Diabetic Kidney Disease: The Targets for SGLT2 Inhibitors and GLP-1 Receptor Agonists. Int J Mol Sci. 2021 Oct 6;22(19):10822. doi: 10.3390/ijms221910822. PMID: 34639160; PMCID: PMC8509708.
- Devaraj S, Dasu MR, Rockwood J, Winter W, Griffen SC, Jialal I. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab. 2008 Feb;93(2):578-83. doi: 10.1210/jc.2007-2185. Epub 2007 Nov 20. PMID: 18029454; PMCID: PMC2243229.
- Dasu MR, Devaraj S, Park S, Jialal I. Increased toll-Like Receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care. abril de 2010;33(4):861-8.
- Mulay SR. Multifactorial functions of the inflammasome component NLRP3 in pathogenesis of chronic kidney diseases. Kidney Int. 2019 Jul;96(1):58-66. doi: 10.1016/j.kint.2019.01.014. Epub 2019 Mar 4. PMID: 30922667.
- Moossavi M, Parsamanesh N, Bahrami A, Atkin SL, Sahebkar A. Role of the NLRP3 inflammasome in cancer. Mol Cancer. 2018 Nov 17;17(1):158. doi: 10.1186/s12943-018-0900-3. PMID: 30447690; PMCID: PMC6240225.
- Susztak K, Raff AC, Schiffer M, Böttinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006 Jan;55(1):225-33. PMID: 16380497.
- Har R, Scholey JW, Daneman D, Mahmud FH, Dekker R, Lai V, Elia Y, Fritzler ML, Sochett EB, Reich HN, Cherney DZ. The effect of renal hyperfiltration on urinary inflammatory cytokines/chemokines in patients with uncomplicated type 1 diabetes mellitus. Diabetologia. 2013 May;56(5):1166-73. doi: 10.1007/s00125-013-2857-5. Epub 2013 Feb 15. PMID: 23412605.
- Chow FY, Nikolic-Paterson DJ, Ma FY, Ozols E, Rollins BJ, Tesch GH. Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia. 2007 Feb;50(2):471-80. doi: 10.1007/s00125-006-0497-8. Epub 2006 Dec 12. PMID: 17160673.
- Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Rollin BJ, Tesch GH. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006 Jan;69(1):73-80. doi: 10.1038/sj.ki.5000014. PMID: 16374426.
- Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, García-Pérez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011 Jun;7(6):327-40. doi: 10.1038/nrneph.2011.51. Epub 2011 May 3. PMID: 21537349.
- Pickup JC, Chusney GD, Thomas SM, Burt D. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci. 2000 Jun 8;67(3):291-300. doi: 10.1016/s0024-3205(00)00622-6. PMID: 10983873.
- Sangoi MB, de Carvalho JA, Tatsch E, Hausen BS, Bollick YS, Londero SW, Duarte T, Scolari R, Duarte MM, Premaor MO, Comim FV, Moretto MB, Moresco RN. Urinary inflammatory cytokines as indicators of kidney damage in type 2 diabetic patients. Clin Chim Acta. 2016 Sep 1;460:178-83. doi: 10.1016/j.cca.2016.06.028. Epub 2016 Jun 25. PMID: 27353644.
- Park J, Guan Y, Sheng X, Gluck C, Seasock MJ, Hakimi AA, Qiu C, Pullman J, Verma A, Li H, Palmer M, Susztak K. Functional methylome analysis of human diabetic kidney disease. JCI Insight. 2019 Jun 6;4(11):e128886. doi: 10.1172/jci.insight.128886. PMID: 31167971; PMCID: PMC6629092.
- Niewczas MA, Ficociello LH, Johnson AC, Walker W, Rosolowsky ET, Roshan B, Warram JH, Krolewski AS. Serum concentrations of markers of TNFalpha and Fas-mediated pathways and renal function in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol. 2009 Jan;4(1):62-70. doi: 10.2215/CJN.03010608. Epub 2008 Dec 10. PMID: 19073786; PMCID: PMC2615709.
- Coca SG, Nadkarni GN, Huang Y, Moledina DG, Rao V, Zhang J, Ferket B, Crowley ST, Fried LF, Parikh CR. Plasma Biomarkers and Kidney Function Decline in Early and Established Diabetic Kidney Disease. J Am Soc Nephrol. 2017 Sep;28(9):2786-2793. doi: 10.1681/ASN.2016101101. Epub 2017 May 5. PMID: 28476763; PMCID: PMC5576932.
- Ruster C, Wolf G. The role of chemokines and chemokine receptors in diabetic nephropathy. Front Biosci. 2008 Jan 1;13:944-55. doi: 10.2741/2734. PMID: 17981602.
- Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2008 Apr;294(4):F697-701. doi: 10.1152/ajprenal.00016.2008. Epub 2008 Feb 13. PMID: 18272603.
- Herder C, Peltonen M, Koenig W, Kräft I, Müller-Scholze S, Martin S, Lakka T, Ilanne-Parikka P, Eriksson JG, Hämäläinen H, Keinänen-Kiukaanniemi S, Valle TT, Uusitupa M, Lindström J, Kolb H, Tuomilehto J. Systemic immune mediators and lifestyle changes in the prevention of type 2 diabetes: results from the Finnish Diabetes Prevention Study. Diabetes. 2006 Aug;55(8):2340-6. doi: 10.2337/db05-1320. PMID: 16873699.
- Flyvbjerg A. The role of the complement system in diabetic nephropathy. Nat Rev Nephrol. 2017 May;13(5):311-318. doi: 10.1038/nrneph.2017.31. Epub 2017 Mar 6. PMID: 28262777.
- Hajishengallis G, Reis ES, Mastellos DC, Ricklin D, Lambris JD. Novel mechanisms and functions of complement. Nat Immunol. 2017 Nov 16;18(12):1288-1298. doi: 10.1038/ni.3858. PMID: 29144501; PMCID: PMC5706779.
- Tang S, Zhou W, Sheerin NS, Vaughan RW, Sacks SH. Contribution of renal secreted complement C3 to the circulating pool in humans. J Immunol. 1999 Apr 1;162(7):4336-41. PMID: 10201966.
- Rasmussen KL, Nordestgaard BG, Nielsen SF. Complement C3 and Risk of Diabetic Microvascular Disease: A Cohort Study of 95202 Individuals from the General Population. Clin Chem. 2018 Jul;64(7):1113-1124. doi: 10.1373/clinchem.2018.287581. Epub 2018 Mar 9. PMID: 29523638.
- Hansen TK, Gall MA, Tarnow L, Thiel S, Stehouwer CD, Schalkwijk CG, Parving HH, Flyvbjerg A. Mannose-binding lectin and mortality in type 2 diabetes. Arch Intern Med. 2006 Oct 9;166(18):2007-13. doi: 10.1001/archinte.166.18.2007. PMID: 17030835.
- Yiu WH, Li RX, Wong DWL, Wu HJ, Chan KW, Chan LYY, Leung JCK, Lai KN, Sacks SH, Zhou W, Tang SCW. Complement C5a inhibition moderates lipid metabolism and reduces tubulointerstitial fibrosis in diabetic nephropathy. Nephrol Dial Transplant. 2018 Aug 1;33(8):1323-1332. doi: 10.1093/ndt/gfx336. PMID: 29294056.
- Anand G, Vasanthakumar R, Mohan V, Babu S, Aravindhan V. Increased IL-12 and decreased IL-33 serum levels are associated with increased Th1 and suppressed Th2 cytokine profile in patients with diabetic nephropathy (CURES-134). Int J Clin Exp Pathol. 2014 Oct 15;7(11):8008-15. PMID: 25550844; PMCID: PMC4270517.
- Zeng C, Shi X, Zhang B, Liu H, Zhang L, Ding W, Zhao Y. The imbalance of Th17/Th1/Tregs in patients with type 2 diabetes: relationship with metabolic factors and complications. J Mol Med (Berl). 2012 Feb;90(2):175-86. doi: 10.1007/s00109-011-0816-5. Epub 2011 Oct 1. PMID: 21964948.
- Moon JY, Jeong KH, Lee TW, Ihm CG, Lim SJ, Lee SH. Aberrant recruitment and activation of T cells in diabetic nephropathy. Am J Nephrol. 2012;35(2):164-74. doi: 10.1159/000334928. Epub 2012 Jan 25. PMID: 22286547.
- Selye H. Production of Nephrosclerosis by Overdosage with Desoxycorticosterone Acetate. Can Med Assoc J. 1942 Dec;47(6):515-9. PMID: 20322632; PMCID: PMC1827573.
- Arnedo RD, Fontalvo JER, Salcedo NA, Alfaro M, Torrejano DN, Blanco MC, Gulfo UI, Sarabia RM, Franco AE. Finerenone y su papel en la enfermedad renal diabética. Estado del arte. Arch Med. 2022;18(1):5.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.