Medicine Group . 2022 May 16;3(5):547-551. doi: 10.37871/jbres1477.
What we would like to do vs what we can truly Achieve as Surgeons. Treatment of the Humeral Ewing Sarcoma for the Young Pediatric Patient
Laura Saenz1* and Orestes Tamayo2
2Orthopedia Resident, National Children Hospital, San Jose, Costa Rica
Abstract
Ewing Sarcoma (ES) is mainly diagnosed during the second decade of life. It often compromises the appendicular skeleton but also can affect other bones. A surgical procedure is necessary despite its good response to chemotherapeutics and radiotherapy. When planning limb-sparing procedures for the young patient, the main challenges are bone size, growth remaining, and physical demands.
Several techniques have been proposed. However, there is no gold standard procedure because many factors must be considered, and each case should be individualized. Also, the resources available change from one medical center to another and every surgeon has to deal with a different reality.
This article presents one case of a 5-year-old male patient diagnosed with ES in the diaphyseal region of the humerus. It reviews the possible surgical options for humeral reconstruction in young children with ES and discusses the challenges that surgeons might have overcome.
Introduction
Ewing Sarcoma (ES) is the second most common bone sarcoma during the first and second decades of life. Although, it is more frequent than osteosarcoma between the ages of one to ten. It accounts for 5% of childhood neoplasms, and more than 50% of cases appear in the second decade. Usually shows a preference for diaphyseal or meta-diaphyseal regions but also compromises the axial skeleton and the short bones [1].
ES is not hereditary or associated with malformation syndromes. Chromosomal abnormalities have been identified, and some studies suggest that ES could arise from parasympathetic postganglionic primordial cells or neural crest stem cells. However, a clear origin for this aggressive malignant tumor is still to be identified [2].
Before chemotherapeutics, life expectancy for bone sarcoma patients was not promising, even for those who received an amputation [3,4].
With new medical treatments, including radiotherapy, the possibility of performing salvage procedures has increased, yet choosing the appropriate surgical technique can be complicated, especially with young children.
When planning limb-sparing procedures for the young patient, the main challenges are bone size, growth remaining, and physical demands. Additionally, socioeconomics, religious and cultural preferences could impact the decision-making process. What might be the right choice for one child with Ewing sarcoma on the humeral diaphysis might not be desirable for another. Different backgrounds and family scenarios prove true the phrase: "treat individuals, not x-ray images."
We present one case of a 5-year-old male patient diagnosed with Ewing's sarcoma in the diaphyseal region of the humerus. He was referred to our hospital, which is the only pediatric hospital in Costa Rica. This article reviews the possible surgical options for humeral reconstruction in young children with ES. Then, it discusses the challenges or difficulties that surgeons might have adapting those surgical techniques to different socioeconomic and cultural environments in addition to each patient's particular needs.
Localization Pitfalls
Proximal
Reconstruction after sarcoma resection of the proximal segment of the humerus in any growing child can be anticipated to be challenging. The proximal physis provides about 80% of the humeral growth; thus, any damage to the physis can significantly impact limb length [5]. The growth plate is considered a barrier that protects the epiphysis from tumor invasion. However, it is worth mentioning that the shoulder is one of the joints where the physis is intracapsular. This fact must be considered while planning surgery due to possible intracapsular contamination.
Also, the glenohumeral joint relies on static and dynamic stabilizers to maintain its stability [6]. Contrary to other prosthetic reconstructions secondary to arthrosis, bone sarcoma surgery also includes muscle resection to obtain a satisfactory free-of-tumor margin. Some nerves might also be impossible to preserve, which negatively impacts the possibility of achieving joint stability and maintaining a complete range of motion.
Diaphyseal
If Ewing sarcoma is mostly diaphyseal, reconstruction is often easier to achieve. Intercalary allograft and vascularized autograft (solely or combined) are the standard options for reconstruction. Ewing sarcoma usually has a good chemotherapy response. The mass size can decrease, allowing resection and reconstruction with fewer complications than when a physis or articular surface is involved. The radial nerve is the most critical structure at risk because it directly contacts the bone.
Distal
Finally, the distal portion of the humerus can be affected, albeit it is relatively rare. On average, Ewing sarcoma has an incidence of 1-3 cases per million/year. The humerus is the fourth bone localization preferred by Ewing sarcoma (10% of cases), and the proximal metaphysis with diaphyseal extension is the most frequent segment [7,8]. Hence, in a hundred million population, 100-300 cases of Ewing sarcoma could be diagnosed. From those cases, 1-3 would probably affect the humerus, and less than half will be near the elbow.
Limb discrepancy is expected when the distal humerus is compromised if the physis cannot be preserved. However, this inequity in length is usually well tolerated due to the lesser involvement of the distal physis in the global limb growth. Elbow stability is the main goal after achieving free margins.
Case Report
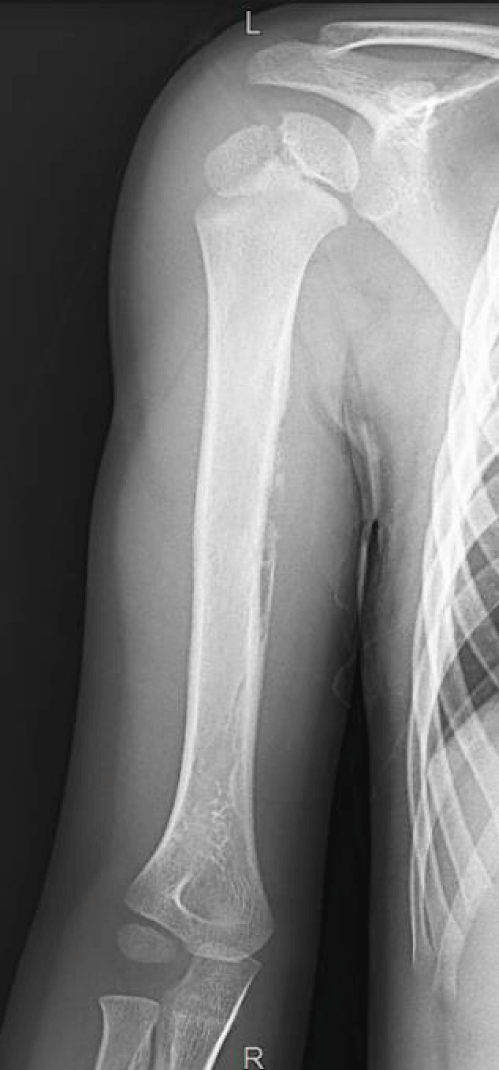
One 5-year-old male patient was referred to the orthopedics emergency department due to 3 weeks of mild pain around the shoulder and a slight increase in his mid-arm diameter with no constitutional symptoms. Initial X-rays were requested, and the images revealed periosteal reaction over the humeral diaphysis highly suspicious of malignancy (Figure 1). Therefore, the child was admitted immediately, and extension studies were requested. Because MRI was also strongly suggestive of bone sarcoma and CT scan revealed left lung metastases, a port-a-cath was implanted at the same surgical time as the biopsy.
Biopsy confirmed a Primitive Neuroectodermic Tumor (PNET), with EWS-fil 1 fusion t (11;22)(q24;q12), and Ewing CEOP chemotherapy protocol was initiated. After neoadjuvant chemotherapy, a re-evaluation was done to assess tumoral response and prepare surgical planning.
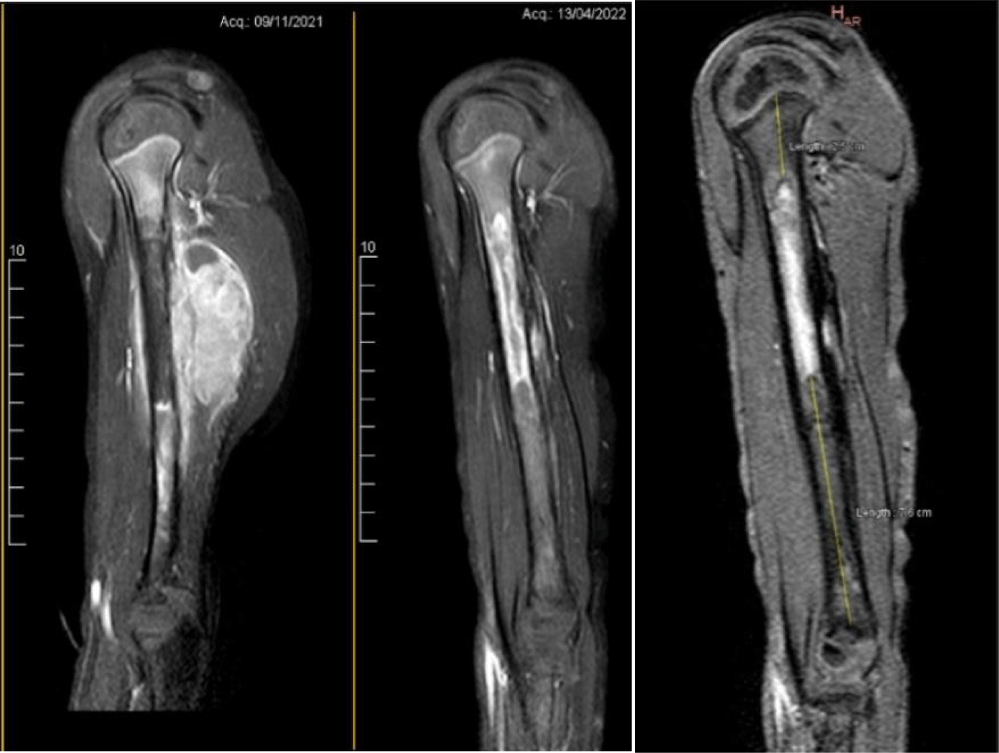
The metastases were no longer visible when evaluating the chest CT scan, and the MRI of the limb demonstrated important soft tissue mass involution. However, the axillar and radial nerves were still in direct contact with the tumoral mass. Salvage limb was offered to the parents, thoroughly discussing possible outcomes and sequelae.
Enhanced gadolinium MRI clearly showed a high-intensity area beginning 25 mm below the proximal physis and continued distally for 71mm. The distal third segment of the humerus appears to be free of tumor (Figure 2).
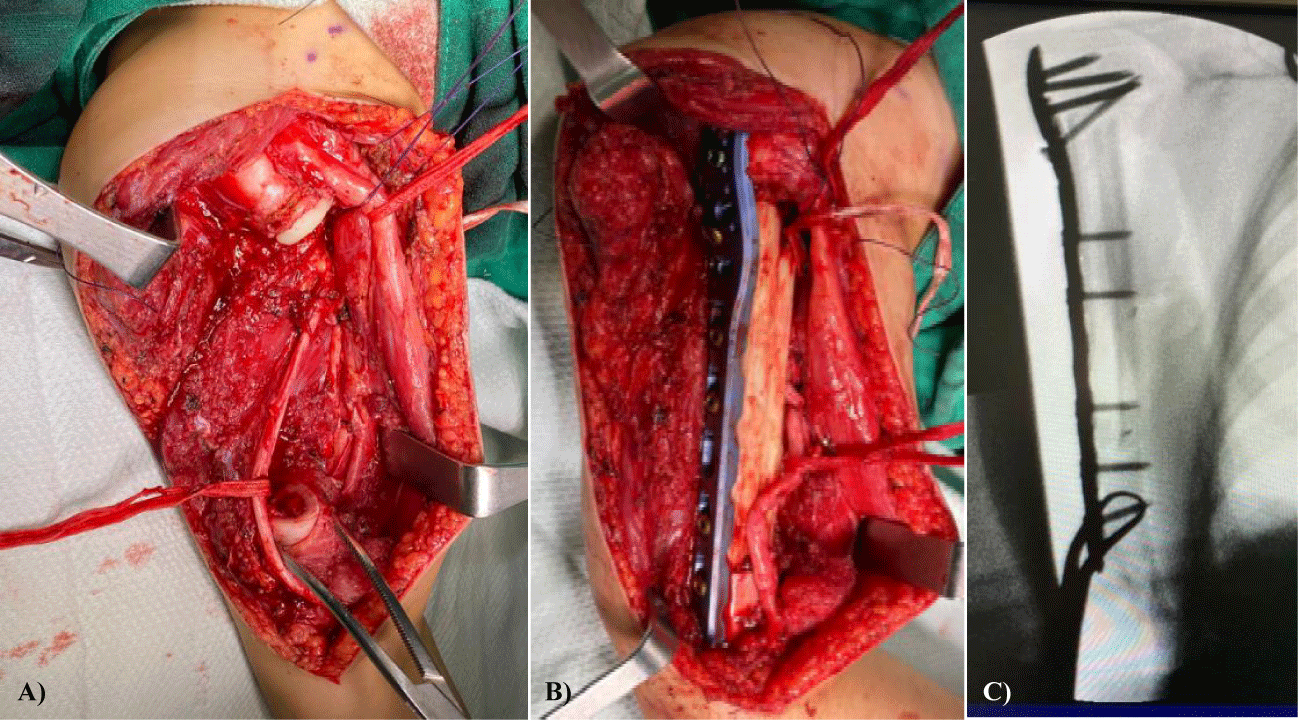
On the day of the surgery, after resecting the mass, soft tissue and medullar canal biopsy were sent to pathology for study. The proximal end of the resection was positive at 1 cm below the physis needing to extend the cut level. Initially, we intended to keep the osteosynthesis distally to the proximal physis, but it was not possible because of this. Perineural biopsy of the radial nerve was negative; thus, the radial nerve was preserved.
For the reconstruction, a fibular allograft and a Phylos long plate (Synthes) were used (Figure 3). Then, the muscles were re-attached, the suction drainage was placed, and the wound was finally closed. After 24hours of the surgery, the physical exam showed that all nerves but the axillar, which was impossible to preserve, were intact.
Reconstructive Options for Humeral ES
Autografts
Vascularized autografts: Vascularized bone grafts are technically demanding but represent an ideal tool for tumor reconstruction. Among their proposed advantages are good bone incorporation, increased resistance due to bone cell preservation, and the possibility of physis preservation for transfer. They can be used as intercalary grafts and to promote allograft consolidation [9-11].
However, there are some disadvantages as recipient site complications, including nonunion or malunion, infection, and mechanical failure. The incidence of complications ranges from 25 to 50%, having been reported Volkmann's ischemic contracture, resorption, and graft fractures, among others. Likewise, morbidity in the donor site has been described [12,13].
Non-vascularized autografts: They can be used to reconstruct some small to medium bone defects. Autografts have properties of osteo induction, osteo conduction, and osteogenesis. Given these qualities, they heal and incorporate faster than allografts which only have osteo conductive and osteo inductive characteristics [14]. Their main drawbacks are donor site morbidity and mechanical failure. It is worth mentioning that some studies have presented recycled bone as an option for reconstruction after tumoral resection if bone quality is still acceptable. Sterilization of the graft is necessary, which can be obtained by liquid nitrogen, high temperature, and irradiation. However, there are still some concerns about bone structural strength after sterilization and the possibility of local recurrence.
Physical transfer autografts: Reconstruction using a vascularized fibular autograft with preservation of the epiphysis and proximal fibular physis allows some growth at the reconstruction site. However, it is an extremely demanding technique. Results can vary widely between patients, and stability around the reconstructed joint is not easy to achieve. In the same way as the other autografts, the donor site is a concern, and transient or permanent common peroneal paralysis might result in drop foot [15].
Allografts
Allografts can be osteo articular, which gives the possibility of arthroplasty in joints where good prosthetic options are not available. They allow the possibility of tendon reinsertion and capsular reconstruction. However, it is difficult to find the right size for a pediatric patient, articular congruence is hard to achieve, and cartilage degeneration and subchondral bone fatigue are likely to occur early.
Intercalary allografts are frequently used for diaphyseal o metaphyseal reconstruction. These grafts allow the reconstruction of bigger defects as there is no donor site to be concerned about. They can be combined with vascularized autografts, especially when working with massive allografts or defects of more than 12 cm are to be addressed. They can also be used for composite reconstructions. Risks are those associated with the graft's lack of vascularity and acelullarity. Disadvantages in general related to allografts are the risk of fractures (19%), pseudoarthrosis (17%), infection (11%), and instability (6%) [16-18].
Endoprosthesis
When accounting for the future growth potential of the pediatric patient, the options are somehow limited. Prosthetic reconstruction of the limb can be done with essentially two different modalities:
Modular prosthetic components
Growing or expandable components: Modular components are thought to be stronger than expandable components. Disadvantages are that each lengthening requires surgery that involves a relatively large exposure. In the patient requiring multiple lengthenings, this results in excessive scar formation and a higher risk of infection; thus, expandable components might be desirable. Both systems are expensive, sometimes need to be custom-made, and mechanical failure is not negligible [19-21].
Modular/ 3D/ Custom made spacers
Systems are being developed that adapt and substitute a specific segment, giving bone-implant stability after resectioning the tumor in the humerus. Custom-made diaphyseal implants provide the advantage of immediate weight-bearing and ambulation but are expensive and issues regarding loosening, wear, and breakage remain [22,23].
Discussion
Each bone sarcoma patient must be individualized to get the best treatment possible based on his particular needs. Soft tissue involvement, physeal and neurovascular compromise must be carefully assessed. With children, the small size of structures, bone growth expectancy, and high biomechanical demands are factors that challenge our practice. Many implants and anatomical osteosynthesis materials are designed for skeletally mature patients, and most of the bone allografts are harvested from adult donors. Furthermore, even though osteosynthesis manufacturers do produce small pieces of equipment, they are mostly doomed to fail over time due to increased load and stress. The same fact is true for allografts.
Regarding bone grafts, if integration is not fully achieved when the patient grows and weight increases, the graft will eventually fail. Accumulative stress over any material that cannot heal itself will end in failure. This is one of the reasons why so many times, vascularized autografts are preferred or used together with other types of reconstructions in very young patients. However, vascularized allografts require microsurgery, and they may not be available in every medical center.
When we think about reconstruction with allografts, some other situations must be considered. For example, there is a bone bank in our country but without bone. There are still religious and administrative factors that limit its harvest. People do not feel comfortable donning bone despite the many others who might benefit from it. This lack of donors leads to the need to buy bone from other countries, which is not always possible and is expensive.
Endoprosthesis theoretically offers many advantages as they are meant to preserve joint function and allow lengthening and early physical activity. However, there are pricey, have a high rate of complications, and are not available to all. Something similar occurs with custom-made spacers or endoprosthesis, which require a multidisciplinary group including engineers and providers who might offer those types of implants.
Conclusion
To conclude, all physicians who perform reconstructive surgery in the pediatric population would have to face difficult decisions. There are several options for reconstruction after tumor resection of the humerus. However, adapting those options to a young child is challenging. Furthermore, health centers with limited budgets and weak health systems are unlikely to be capable of reproducing all the published techniques. Finally, even though all surgeons look to provide the best outcome for their patients, sociocultural, religious, and budget issues can stand in their way.
References
- Christopher DM. Diagnostic Histopathology of Tumors. 2021. https://tinyurl.com/5yd38hhr
- Proctor A, Brownhill SC, Burchill SA. The promise of telomere length, telomerase activity and its regulation in the translocation-dependent cancer ESFT; clinical challenges and utility. Biochim Biophys Acta. 2009 Apr;1792(4):260-274. doi: 10.1016/j.bbadis.2009.02.011. Epub 2009 Mar 2. PMID: 19264125.
- Durer S, Shaikh H. Ewing Sarcoma. StatPearls. Treasure Island (FL): StatPearls Publishing. 2022. PMID: 32644609.
- Balamuth NJ, Womer RB. Ewing's sarcoma. Lancet Oncol. 2010 Feb;11(2):184-192. doi: 10.1016/S1470-2045(09)70286-4. PMID: 20152770.
- Hannonen J, Hyvönen H, Korhonen L, Serlo W, Sinikumpu JJ. The incidence and treatment trends of pediatric proximal humerus fractures. BMC Musculoskelet Disord. 2019 Nov 27;20(1):571. doi: 10.1186/s12891-019-2948-7. PMID: 31775692; PMCID: PMC6882178.
- Curl LA, Warren RF. Glenohumeral joint stability. Selective cutting studies on the static capsular restraints. Clin Orthop Relat Res. 1996 Sep;(330):54-65. PMID: 8804275.
- Murphey MD, Senchak LT, Mambalam PK, Logie CI, Klassen-Fischer MK, Kransdorf MJ. From the radiologic pathology archives: ewing sarcoma family of tumors: radiologic-pathologic correlation. Radiographics. 2013 May;33(3):803-831. doi: 10.1148/rg.333135005. PMID: 23674776.
- Ebrahimpour A, Chehrassan M, Sadighi M, Azizmohammad Looha M, Karimi A, Akbari A, Raeisi A, Akbari ME. The survival and incidence rate of ewing sarcoma; a national population-based study in iran (2008-2015). Arch Bone Jt Surg. 2020 May;8(3):391-399. doi: 10.22038/abjs.2020.44095.2206. PMID: 32766398; PMCID: PMC7358229.
- Levin LS. Vascularized fibula graft for the traumatically induced long-bone defect. J Am Acad Orthop Surg. 2006;14(10 Spec No.):S175-176. doi: 10.5435/00124635-200600001-00038. PMID: 17003193.
- Migliorini F, La Padula G, Torsiello E, Spiezia F, Oliva F, Maffulli N. Strategies for large bone defect reconstruction after trauma, infections or tumour excision: A comprehensive review of the literature. Eur J Med Res. 2021;26(1):118. https://tinyurl.com/2twpc2mj
- Biazzo A, Romantini M, De Paolis M, Manfrini M, Donati D. Vascularized fibular autograft as salvage technique in failure of allograft intercalary reconstructions after tumor resections. Acta Orthop Belg. 2018 Mar;84(1):38-46. PMID: 30457498.
- Wodajo FM, Bickels J, Wittig J, Malawer M. Complex reconstruction in the management of extremity sarcomas. Curr Opin Oncol. 2003 Jul;15(4):304-312. doi: 10.1097/00001622-200307000-00005. PMID: 12874509.
- Asada N, Tsuchiya H, Kitaoka K, Mori Y, Tomita K. Massive autoclaved allografts and autografts for limb salvage surgery. A 1-8 year follow-up of 23 patients. Acta Orthop Scand. 1997 Aug;68(4):392-5. doi: 10.3109/17453679708996184. PMID: 9310047.
- Lawal YZ, Garba ES, Ogirima MO, Dahiru IL, Maitama MI, Abubakar K, Ejagwulu FS. Use of non-vascularized autologous fibula strut graft in the treatment of segmental bone loss. Ann Afr Med. 2011 Jan-Mar;10(1):25-28. doi: 10.4103/1596-3519.76571. PMID: 21311151.
- Taylor GI, Miller GD, Ham FJ. The free vascularized bone graft. A clinical extension of microvascular techniques. Plast Reconstr Surg. 1975 May;55(5):533-544. doi: 10.1097/00006534-197505000-00002. PMID: 1096183.
- O'Connor MI, Sim FH, Chao EY. Limb salvage for neoplasms of the shoulder girdle. Intermediate reconstructive and functional results. J Bone Joint Surg Am. 1996 Dec;78(12):1872-1888. doi: 10.2106/00004623-199612000-00011. PMID: 8986665.
- Panagopoulos GN, Mavrogenis AF, Mauffrey C, Lesenský J, Angelini A, Megaloikonomos PD, Igoumenou VG, Papanastassiou J, Savvidou O, Ruggieri P, Papagelopoulos PJ. Intercalary reconstructions after bone tumor resections: A review of treatments. Eur J Orthop Surg Traumatol. 2017 Aug;27(6):737-746. doi: 10.1007/s00590-017-1985-x. Epub 2017 Jun 5. PMID: 28585185.
- Kohler R, Lorge F, Brunat-Mentigny M, Noyer D, Patricot L. Massive bone allografts in children. Int Orthop. 1990;14(3):249-253. doi: 10.1007/BF00178754. PMID: 2279831.
- Sharma S, Turcotte RE, Isler MH, Wong C. Experience with cemented large segment endoprostheses for tumors. Clin Orthop Relat Res. 2007 Jun;459:54-59. doi: 10.1097/BLO.0b013e3180514c8e. PMID: 17545759.
- Wittig JC, Bickels J, Kellar-Graney KL, Kim FH, Malawer MM. Osteosarcoma of the proximal humerus: Long-term results with limb-sparing surgery. Clin Orthop Relat Res. 2002 Apr;(397):156-176. doi: 10.1097/00003086-200204000-00021. PMID: 11953608.
- Balke M, Ahrens H, Streitbürger A, Gosheger G, Hardes J. Modular endoprosthetic reconstruction in malignant bone tumors: Indications and limits. Recent Results Cancer Res. 2009;179:39-50. doi: 10.1007/978-3-540-77960-5_4. PMID: 19230533.
- Frassica FJ, Chao EY, Shives TC, Sim FH. Resection of malignant bone tumors about the shoulder. A preliminary report of reconstruction with a new modular spacer. Clin Orthop Relat Res. 1991 Jun;(267):57-64. PMID: 2044293.
- Tang X, Guo W, Yang R, Tang S, Ji T. Synthetic mesh improves shoulder function after intra articular resection and prosthetic replacement of proximal humerus. Clin Orthop Relat Res. 2015 Apr;473(4):1464-1471. doi: 10.1007/s11999-015-4139-7. Epub 2015 Jan 21. PMID: 25604875; PMCID: PMC4353552.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.