Environmental Sciences . 2022 May 05;3(5):488-500. doi: 10.37871/jbres1470.
Morchella esculenta Polysaccharide Attenuates Obesity through Ameliorating Adipose and Liver Tissue Inflammation in Obese Mouse Model
Ata Ur Rehman1, Asif Iqbal Khan1, Tian Zhiying1, Xiaoxiao Zhang1, Wen Yuqi1, Hazrat Bilal1, Duaa Mohammad Abdallah Alsholi1, Yi Xin1* and Liang Wang2*
2Stem Cell Clinical Research Center, National Joint Engineering Laboratory, Regenerative Medicine Center, The First Affiliated Hospital of Dalian Medical University, Liaoning Province, China
- Obesity
- Morchella esculenta
- Polysaccharide
- Adipose tissue
- Liver tissue inflammatio
Abstract
Morchella esculenta is an edible mushroom known for its high nutritional value and delicious taste. Morchella esculenta polysaccharide has many biological properties, including neuroprotective, nephroprotective, anti-proliferating, tumor-suppressive, and immunostimulatory functions. In this study, Morchella esculenta Polysaccharide (MEP) was evaluated for its anti-inflammatory effects in adipose and liver tissue and for effects on obesity and obesity-associated conditions in High-Fat Diet (HFD)-induced obese mice. After MEP administration, HFD mice exhibited lower body weight, epididymal and subcutaneous fat deposition, and adipocyte sizes, as well as lower Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), Triglycerides (TG), Total Cholesterol (TC) and Fasting Blood Glucose (FBG) than HFD control mice. Furthermore, MEP supplementation attenuates inflammation in the adipose and liver tissue via regulation of Toll-like Receptor 4 (TLR4) and inactivation of nuclear factor kappa B (NF-κB). It also regulates the expression of transforming growth factor beta (TGF-β) and pro-inflammatory cytokines. Moreover, MEP administration restores insulin resistance via inhibiting insulin receptor substrate-1 (IRS-1) in the adipose and liver tissue. In conclusion, MEP may be a promising pharmaceutic approach for body weight reduction, attenuating obesity, and inflammation.
Introduction
Obesity is the world's biggest health issue, which has been related to various health ailments, shortened life expectancy, and has reached epidemic levels in developing nations [1]. In recent years, the incidence of obesity has risen dramatically, and it has been projected that the obesity rate will reach up to 20% of the adult population by 2030 [2]. About 500 million people are obese, and 1.4 billion people are overweight worldwide [3]. Obesity predisposes people to develop and provoke a wide variety of diseases, including chronic low-grade inflammation, fatty liver disease, type 2 diabetes, insulin tolerance, respiratory problems, gastrointestinal issues, coronary disease, and multiple forms of cancer [4].
Adipose tissue hyperplasia and hypertrophy occur in obese people, resulting in increased production of adipokines, Non-Esterified Fatty Acids (NEFA), and other proinflammatory chemokines and cytokines, all of which cause lipotoxicity [5,6]. Adipocytes make up the majority of adipose tissue, which is essential for energy and metabolic homeostasis [7]. Energy and metabolic homeostasis and fat accumulation are associated with adipocyte tissue [8]. Adipose tissue also secretes adipokines (a wide range of cytokines) [9], that have a role in a range of pathophysiological functions such as appetite, taste control, inflammation, immunity, adipogenesis and insulin sensitivity [10,11]. Over 600 adipokines have been reported, several of which are involved in chronic inflammation (e.g., 1L-1β, IL-10, IL-6 and TNF-α [10]. Obesity-associated adipokine secretion dysregulation leads to enhanced proinflammatory activity both systemically and locally [12,13].
The liver is among the organ which sustains the most damage as a result of obesity. Steatosis of liver is a characteristic of histopathology alterations caused by an increase in Fatty Acid release (FA) and Fatty Acid Oxidation (FAO) [14,15]. Hepatocytes accumulate Triglycerides (TAG) within lipid droplets due to enhanced fatty acid absorption and lipid production [15]. Overabundance of FFAs and hyperinsulinemia promote lipotoxicity, hepatic inflammation, and apoptosis, all of which help in the development of steatohepatitis [16,17]. Increased dietary fats leads to obesity and the metabolic disorders by promoting increased fat accumulation in non-adipose tissues that aren't designed for fat storage, such as the liver. Although hepatic steatosis is typically considered a benign condition, it can progress to steatohepatitis [18].
In recent years, numerous medications have been produced to treat obesity and health problems linked to obesity. However, long-term use of anti-obesity medications causes serious side effects, including regaining weight after stopping treatment [19,20]. Researchers are now focused on seeking alternative therapeutic strategies to overcome this economic and social problem and resolve the challenges of obesity. Edible mushrooms have now been suggested as promising sources of biological functional ingredients and are the subject of the most recent nutrition research, in addition to expanded uses in innovative functional foods [21]. A wide range of nutraceutical substances, including polysaccharides, exhibit impressive biological effects, notably against obesity [22].
Morchella esculenta is an edible mushroom known for its high nutritional value and delicious taste [23]. It contains a wide range of bioactive substances that have traditionally been used in medicine [24]. A plethora of studies has revealed Morchella esculenta numerous biological properties, including neuroprotective and nephroprotective [23]. The anti-proliferating ability of M. esculenta polysaccharides against human colon cancer (HT-29 cells) has been identified [25]. In addition, M. esculenta demonstrated immunostimulatory function by stimulating T cells and proliferating splenocytes [26]. The anti-obesity impact of MEP by regulating HFD-induced obesity and inflammation and whether MEP exhibits any effect on obesity-related conditions is yet to be investigated. In the present research, considering the beneficial role of Morchella esculenta mushroom in preventing multiple health issues, we also studied the impact of MEP on obesity and inflammation induced by HFD.
Materials and Methods
The fruiting body of M. esculenta mushroom was obtained from Shandong Tai’an Ynsheng Food Co., Ltd Shandong, China. The high-fat diet of 60% was purchased from Jiangsu Medison Biomedical Co., Ltd., (Yangzhou, China). The Bicinchoninic Acid (BCA) protein assay kit and all Enzyme-Linked Immunosorbent Assay (ELISA) kits were purchased from TransGene Biotech (Beijing, China) and Shanghai Longton Biotechnology (Shanghai, China) respectively. The PrimeScript RT-PCR Kit was from the USA. All antibodies goat anti-rabbit CD68, NF-κB and IRS-1 were supplied by Proteintech, Wuhan, China, while TLR4 and TGF-β were from CUSABIO, Wuhan Donghu Hi-Tech Development Area, Wuhan, Hubei Province, China.
Extraction and characterization of polysaccharides from the M. esculenta mushroom
The crude polysaccharide from M. esculenta mushroom fruiting bodies was extracted according to the protocol previously reported [27]. Briefly, the dried fruiting bodies of M. esculenta were crushed into a fine powder and mixed with distilled water at a ratio of 1:50 g/ml, then heated at 80°C for 3 hours. The mixture was deproteinized with trichloroacetic acid at 1.5% (v/v), the pH was adjusted to 7.0 by 2 M (NaOH), and the supernatant was centrifuged for 10 minutes at "10,000 g". The supernatant was collected and concentrated by rotary evaporation at 65°C. The concentration of protein was measured by the BCA quantification method. The concentrated solution was precipitated by 3 volumes of ethanol at 4°C for 12 hours and then freeze-dried under freeze-drying vacuum systems. The carbohydrate content of M. esculenta crude Polysaccharide (MEP) was determined through the phenol-H2SO4 method [28]. For the determination of the monosaccharide composition, a High-Performance Liquid Chromatography (HPLC) technique was used shown in figure S1 [29].
Animals and study design
Forty male BALB/c mice weighing 15 ± 2 g were obtained from the animal care center Specific-Pathogen-Free (SPF) with the approval of Dalian Medical University (Animal Care and Research Ethics Committee, Dalian, China) (Approval Number: ARYX 2019-2021). All mice were randomly assigned to groups and housed in environmentally friendly conditions (22 ± 2°C temperature, 50 ± 10% relative humidity, and a 12 h light-dark cycle), with free access to their normal diet and water.
After one week of acclimation, mice were distributed into four groups (each group containing ten mice). One group was fed a normal chow diet, and the remaining three groups of mice were fed a 60% High-Fat Diet (HFD) for four weeks. From the 5th week, the normal diet (control) and High-Fat Diet (HFD) groups were administered with saline (200 μl). M. esculenta Polysaccharide (MEP) was gavaged (200 mg/kg) daily at a low dose [MEPL group] and a high dose [MEPH group] of 400 mg/kg of body weight orally till the 12th week. The study design and schematic presentation are shown in figure S2. The treatment and dosage were performed according to the previous study protocol [30]. The body weights of all animals were weighed daily during MEP treatment. After eight weeks of MEP treatment, all the mice were sacrificed and blood samples, organs, and tissues were collected in Eppendorf (EP) tubes and stored immediately at -80°C for further analysis.
Morphometry assessment of adipose and liver tissues
For adiposity assessment, freshly dissected adipose and liver tissues (epididymal) from all groups of mice were fixed in 10% formalin for 12 hrs. The 4 mm sections were prepared by deparaffinizing, rehydrating, and staining with hematoxylin and eosin. The morphological examination was performed under the microscope (Leica Microsystems, Wetzlar, Germany). The number of adipocytes from each sample of different study groups was counted, and the adipocyte size was evaluated via Image J (NIH, USA) as reported previously
Measurement of serum biochemical analysis
The mice were humanly sacrificed by cervical dislocation after 8 weeks of experimental duration. By extirpating the eyeball, blood was collected, and serum was separated by centrifuging at 1000 g for 10 minutes. The serum of all experimental groups was subjected to the HITACHI 7600 automatic biochemistry analyzer (Hitachi High-Technologies Co., Tokyo, Japan) to determine the concentration of Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), Triglycerides (TG), and Total Cholesterol (TC). A Fasting Blood Glucose (FBG) level was measured by a glucometer via puncturing the tail vein of mice.
Measurement of serum NEFA, LPS, and cytokines level
The serum insulin levels and Non-Esterified Fatty Acids (NEFA) were measured by an ELISA kit according to the protocol of the manufacturer. The serum concentration of endotoxin Lipopolysaccharide (LPS), anti-inflammatory cytokine interleukin 10 (IL-10), and pro-inflammatory cytokines [interleukin-1β (IL-1β), IL-6, and TNF-α] were determined by using the mouse ELISA kit (enzyme-linked immunosorbent assay) (Shanghai Longton Biotechnology Co., Ltd., Shanghai, China), following the manufacturer’s guidelines.
Determination of adipose and liver mRNA
The RNA from adipose tissue was isolated using the TRIzol reagent (Invitrogen Life Technology, Gaithersburg, MD, USA) by following the manufacturer's guidelines. The Real-Time PCR thermocycler (Applied Biosystems StepOnePlusTM) was used while employing SYBR Green (Kapa SYBR Fast Master Mix) in triplicate to perform qRT-PCR. A reaction mixture of a total volume of 10 μl was used for each tube containing 1 μl of target primers, 1.5 μl of cDNA, 5 μl of SYBR Green Master Mix, and 2.5 μl of nuclease-free water. For 50 cycles, RT-PCR was employed, and PCR conditions were set as: pre-incubation for 10 min at 95°C, denaturation at 94°C for 15 s, annealing at 60°C for 30 s, followed by elongation at 72°C for 30 s. The relative expression of the gene was quantified using method 2–DDCt [31] while using β-Actin as an internal control. The sequence details of the primers are shown in (Supplementary table S1).
Histopathological examination of adipose and liver tissue
Epididymal adipose and liver tissues (5 mm) were fixed in 10% formalin overnight, followed by dehydration, xylene vitrification and embedded in paraffin. The sections were prepared via microtome (Thermo, Waltham, MA, USA), deparaffinized, rehydrated and stained with Hematoxylin and Eosin (H&E). The histological study was performed by a microscope (Leica Microsystems, Wetzlar, Germany) as a blinded independent investigator.
To see the expression of TLR4 and NF-κB protein, Immunohistochemistry (IHC) staining was performed. The adipose and liver tissue sections were deparaffinized, rehydrated and incubated with 3% H2O2 for 10 minutes. In addition, the tissue slide is heated in antigen retrieval reagent (Na+2 EDTA, pH 8.0) for antigen retrieval. Then, it was incubated overnight with TLR4 and NF-κB primary antibodies at 4°C followed by (HRP) horseradish peroxidase-conjugated secondary antibody at room temperature for 1 h. The (DAB) 3,3-diaminobenzidine was used as substrate and hematoxylin was applied as counterstaining. The slides were then mounted and examined at a magnification of 20x under a light microscope. Under the light microscope at 20x magnification.
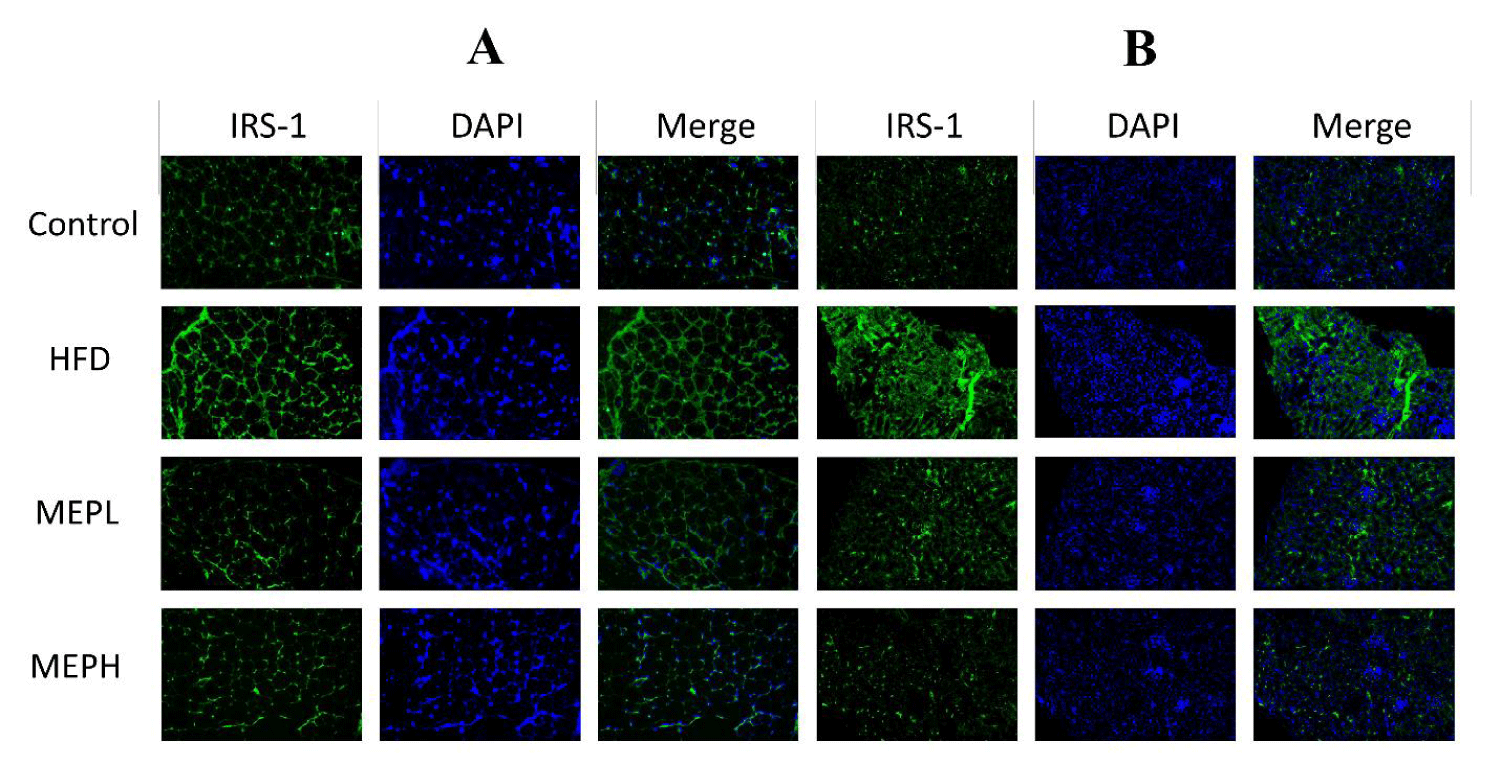
The levels of expression of CD68, TGF-β and IRS1 in the adipose and liver were examined using immunofluorescent staining. The deparaffinized and rehydrated tissue sections were allowed to treat for antigen retrieval in citrate buffer for 30 minutes at 100 watts in the microwave, then allowed to cool for 1 hour. After that, the tissue slides were immersed in a 3% BSA solution for 1 hour. CD68, TGF-β and IRS1 antibodies (1:100) were incubated on tissues overnight at 4°C. After washing, tissue sections were incubated for 60 minutes with Alexa 488-conjugated secondary antibodies, and DAPI was employed for nucleus staining. A confocal scanning microscope was used to capture the images.
Statistical analysis
Statistical analysis was performed by the software GraphPad Prism 8.01 (La Jolla, CA, USA). One-Way Analysis Of Variance (ANOVA) was performed with Tukey’s multiple comparison post hoc test to determine the significance of differences, and a value of 0.05 was considered statistically significant.
Results
Characterization and chemical analysis of MEP
The polysaccharide of M. esculenta (MEP) was extracted and the concentration was found to be 11.96 mg/mL by using the phenol-sulphuric acid method. The percentage yield was 13.5%, the total polysaccharide and protein content were 96% and 2.26%, respectively. The major functional components of the monosaccharide were mannose 5.77%, glucose 81.35%, arabinose 8.99% and galactose 3.54%.
Effect of MEP treatments on HFD-induced obesity parameters
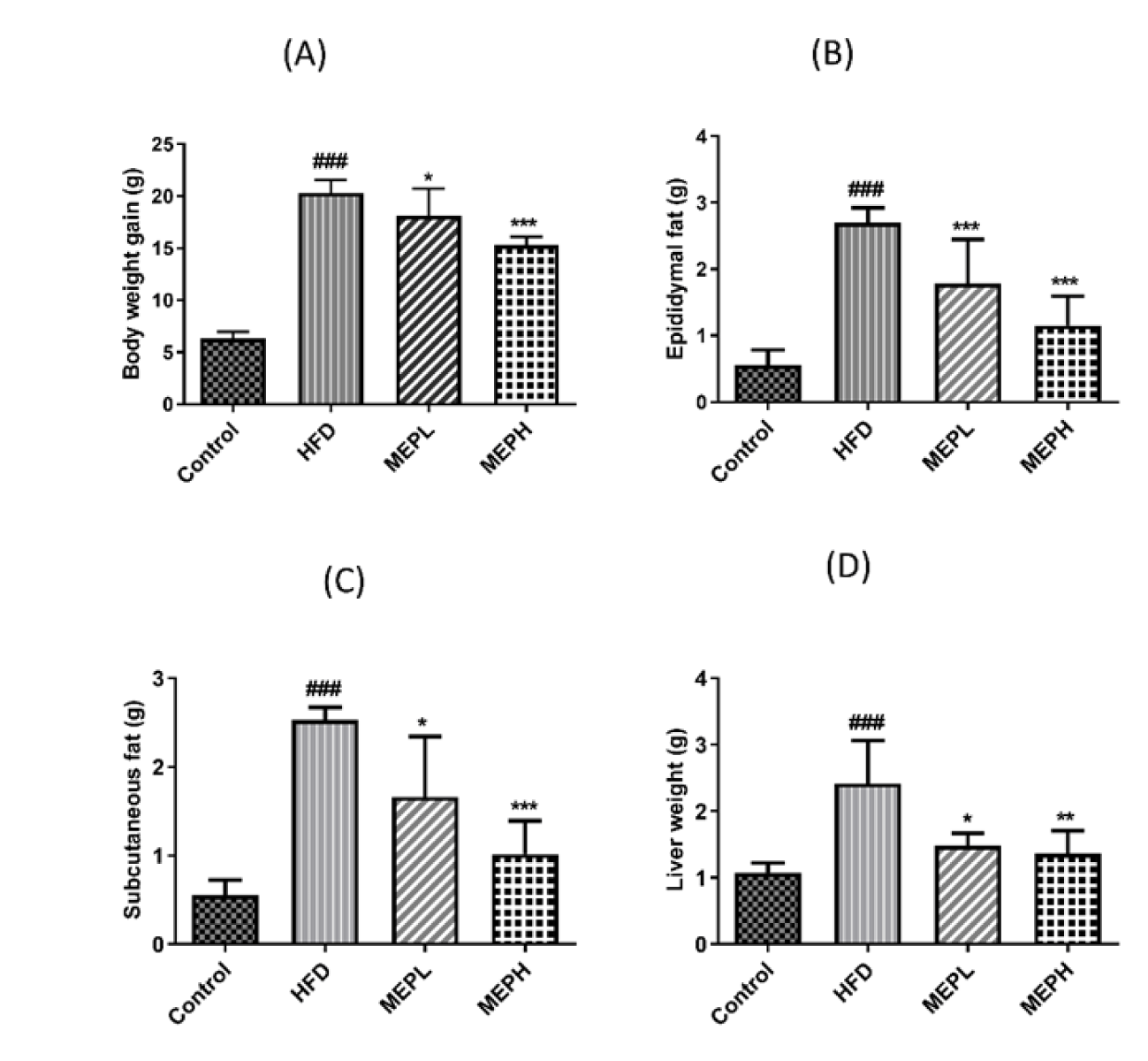
Upon HFD feeding, the obesity parameters (body and liver weight, subcutaneous and epididymal fat deposition) in the HFD group showed significant increments as associated to the control group (Figure 1). MEP administration significantly decreased body and liver weight, as well as epididymal and subcutaneous fat deposition in the MEP treatment groups as associated to the HFD group in a dose-dependent manner (Figures 1A-D). A significant reduction in weight gain has been observed upon eight weeks of MEP supplementation in the MEPH group (p < 0.001), the MEPL group (p < 0.05) (Figure 1A). And for subcutaneous and epididymal fat deposition, the MEPH group (p < 0.001), the MEPL group (p < 0.05) (Figures 1B & C). However, the weight of the liver was significantly increased in the HFD group (p < 0.001) as compared to the control group. Morover, the liver weight was significantly reduced in the MEPH group (p < 0.01) and the MEPL group (p < 0.05) respectively (Figure 1D).
Effect of MEP on the histology of adipose and liver tissue and expression of lipogenic and adipogenic genes
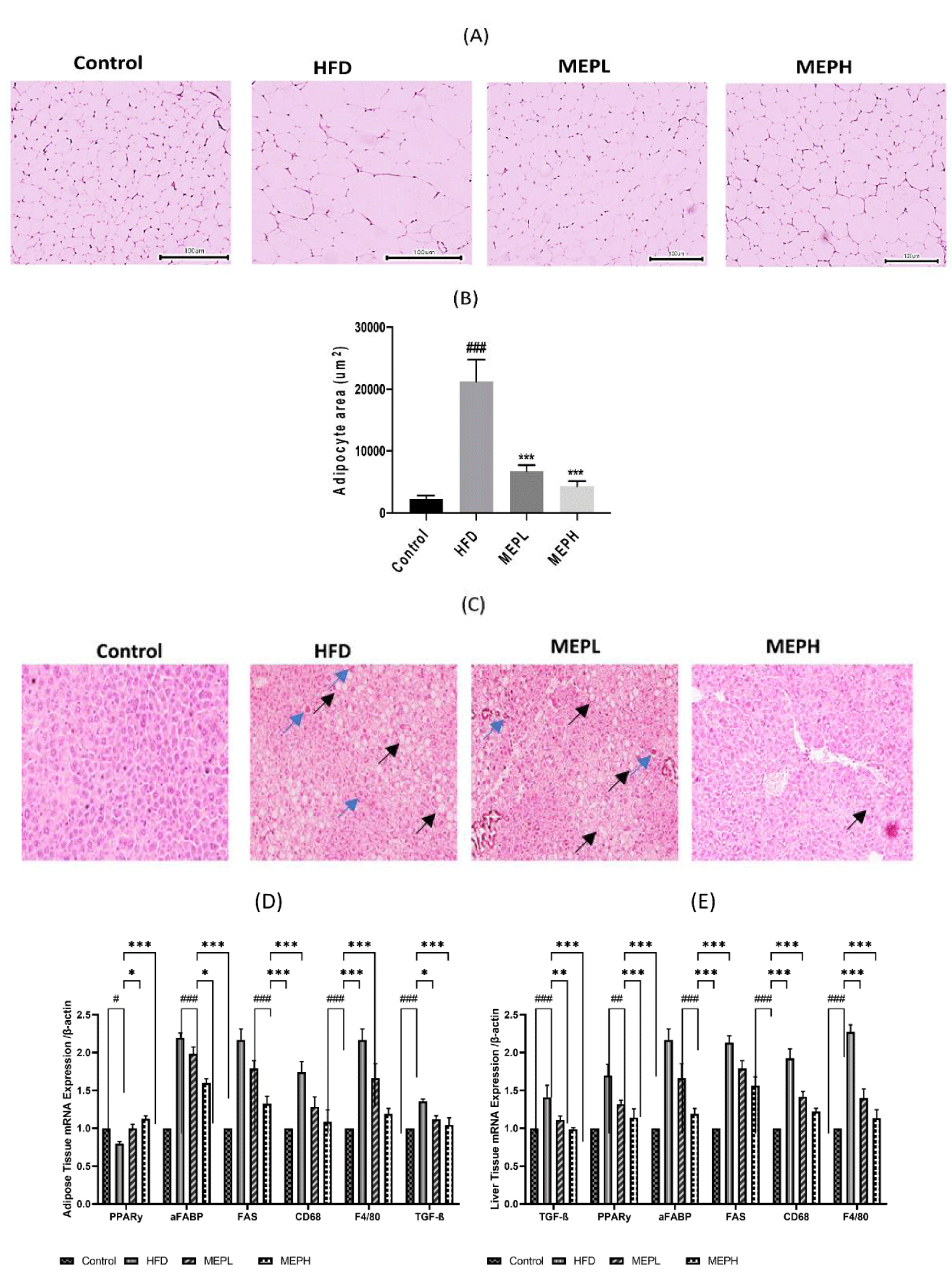
We further examined the adipocyte size in the HFD-fed and MEP-treated groups. The histological examination showed the level of lipid deposition in the adipose tissue, which would have been proportionate to the size of the tissue. The adipocyte size in the HFD group (p < 0.001) was significantly larger as compared to the control group. A significant reduction in adipocyte size occurred in the MEPL and MEPH groups (p < 0.001) (Figures 2A & B). Our finding suggests the regulatory effect of MEP on fat deposition in HFD-fed mice.
The consumption of HFD was provided a link to liver damage caused by local inflammation and lipid accumulation. Histopathological analyses were conducted on frozen fixed liver tissues from mice to test the impacts of MEP on HFD-induced hepatic steatosis and fibrosis. Mice were fed an HFD had significant fat and collagen incursion, as well as reduced glycogen condensation (Figure 2C). A comparable identity was found in the livers of HFD-fed MEP-treated mice, albeit to a lesser extent (Figure 2C). The above research results suggest that long-term use of MEP keeps hepatic homeostasis by trying to prevent diet-induced liver loss of function and injury.
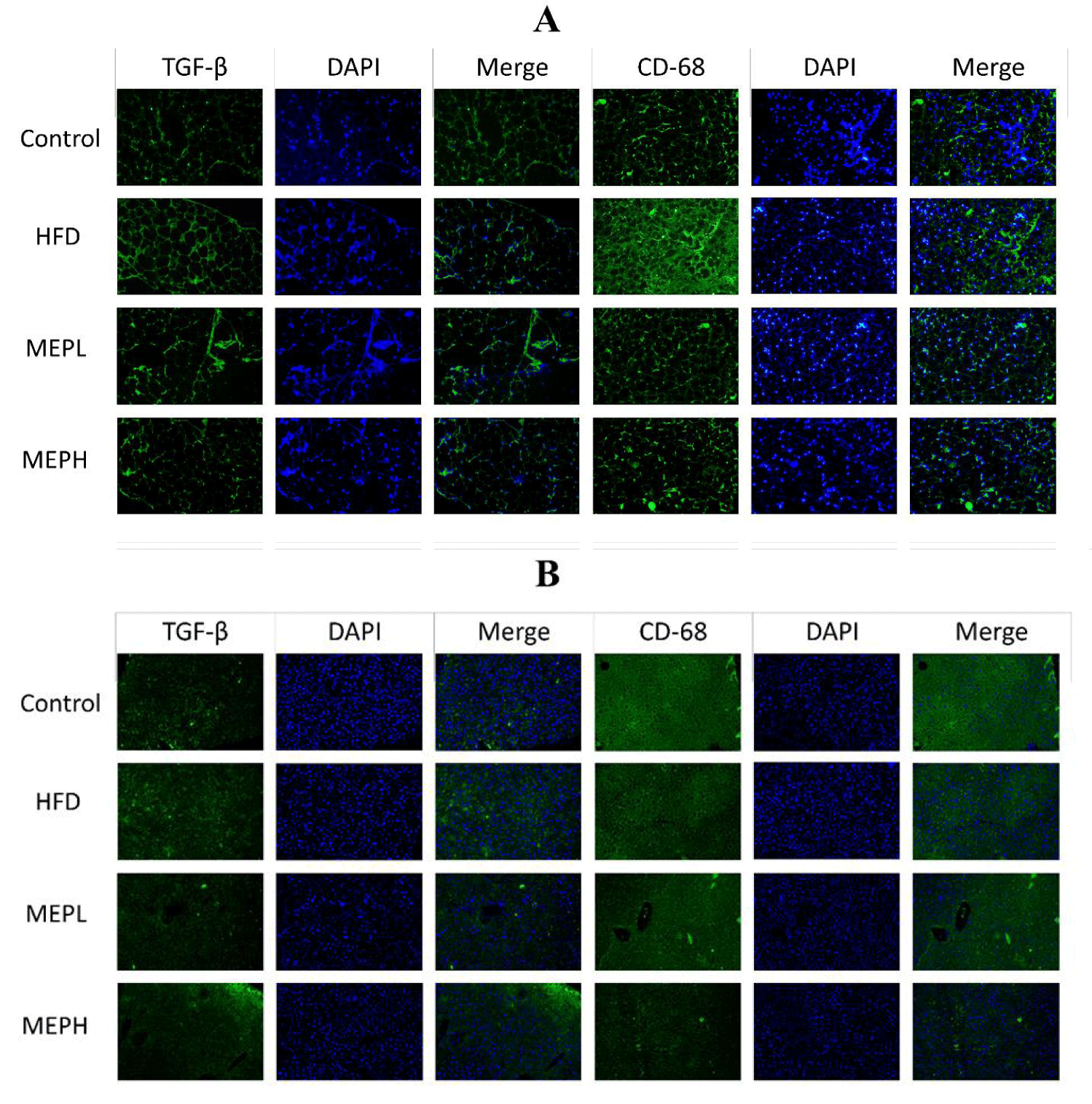
The overexpression of adipogenic genes in adipose and liver tissues (PPAR-γ, aFABP, and FAS) was significantly increased in the HFD group as compared to the control group. The levels of mRNA expression of CD68 and F4/80 markers of macrophages and transforming growth factor-beta (TGF-β) were also significantly increased in the adipose and liver tissues of HFD mice as compared with the control group (Figures 2D & E). However, the MEP-treated group, specifically the MEPH group, significantly decreased the expression level of adipogenic genes. In addition, immunofluorescence staining was used to determine the expression of CD68 and TGF-β protein in adipose and liver tissue. In the HFD group, we found higher levels of CD68 and TGF-β. While MEP treatment reduced the expression of these proteins in the liver and adipose tissues (Figure 3).
Effect of MEP on glucose, insulin levels, lipid profile, and liver markers in obese mice
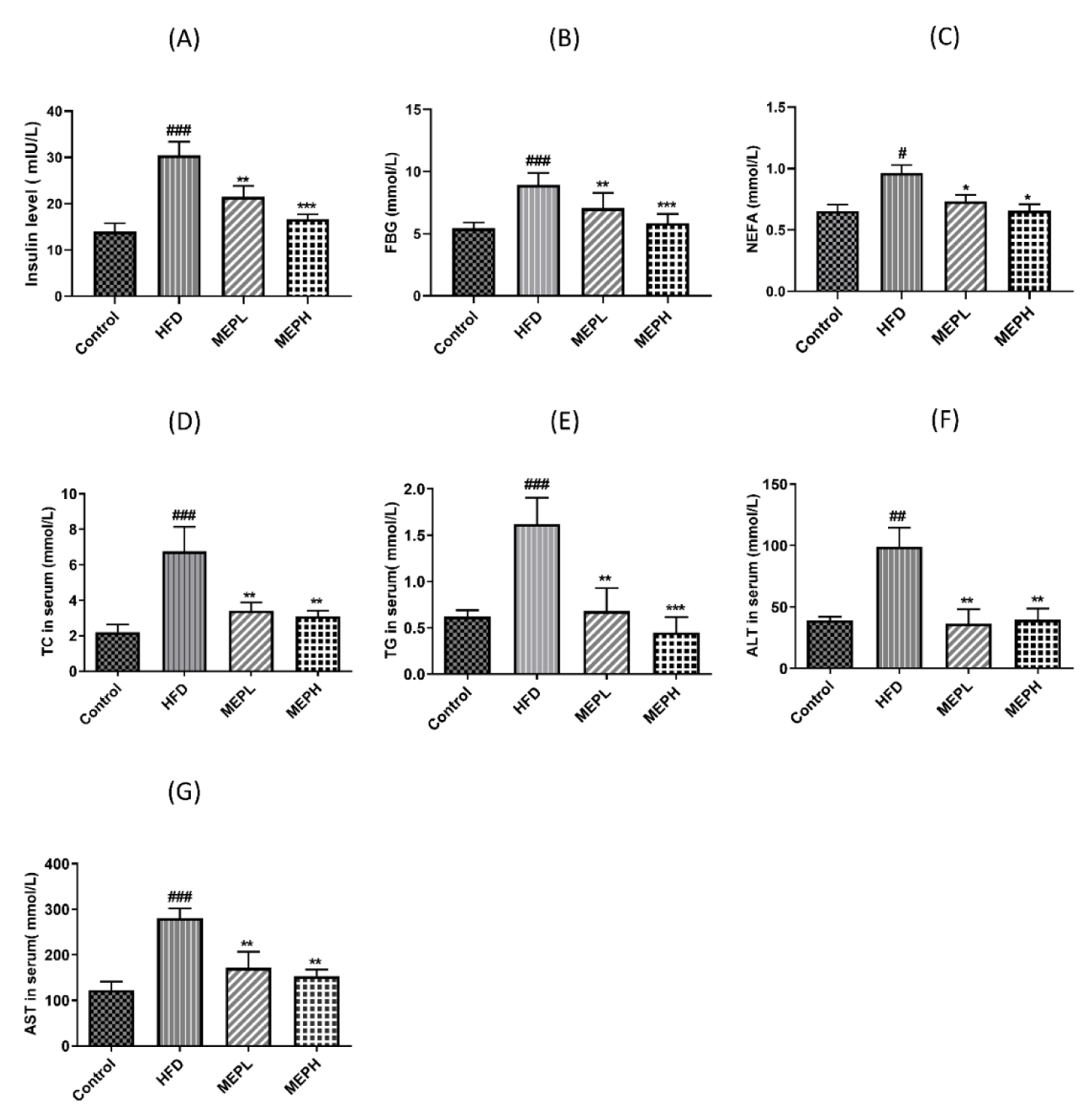
We studied the effects of MEP treatment on glucose, insulin, TC, TG, ALT, AST, and NEFA (Figures 4A-F). To investigate the MEP effect on the homeostasis of glucose, we determined the levels of fasting blood glucose and fasting insulin. Our results revealed that the HFD group showed a significant increment in fasting blood glucose and insulin levels as compared to the control group, However, MEP treatment significantly controlled the level of fasting glucose and fasting insulin (Figures 4A & B). Our findings further demonstrated that the HFD group exhibited a significant increment of serum TC, TG, AST, ALT, and NEFA (p < 0.01) as compared with the control group (Figures 4C-G). However, MEP treatment reduced the level of TC in MEPH (p < 0.01), MEPL (p < 0.01), TG level in MEPH (p < 0.001) and MEPL (p < 0.01). Moreover, liver markers such as ALT in MEPH (p < 0.01), MEPL (p < 0.01), and TG in MEPH (p < 0.01), MEPL (p < 0.01). While NEFA levels in MEPH (p < 0.05) was also significantly reduced.
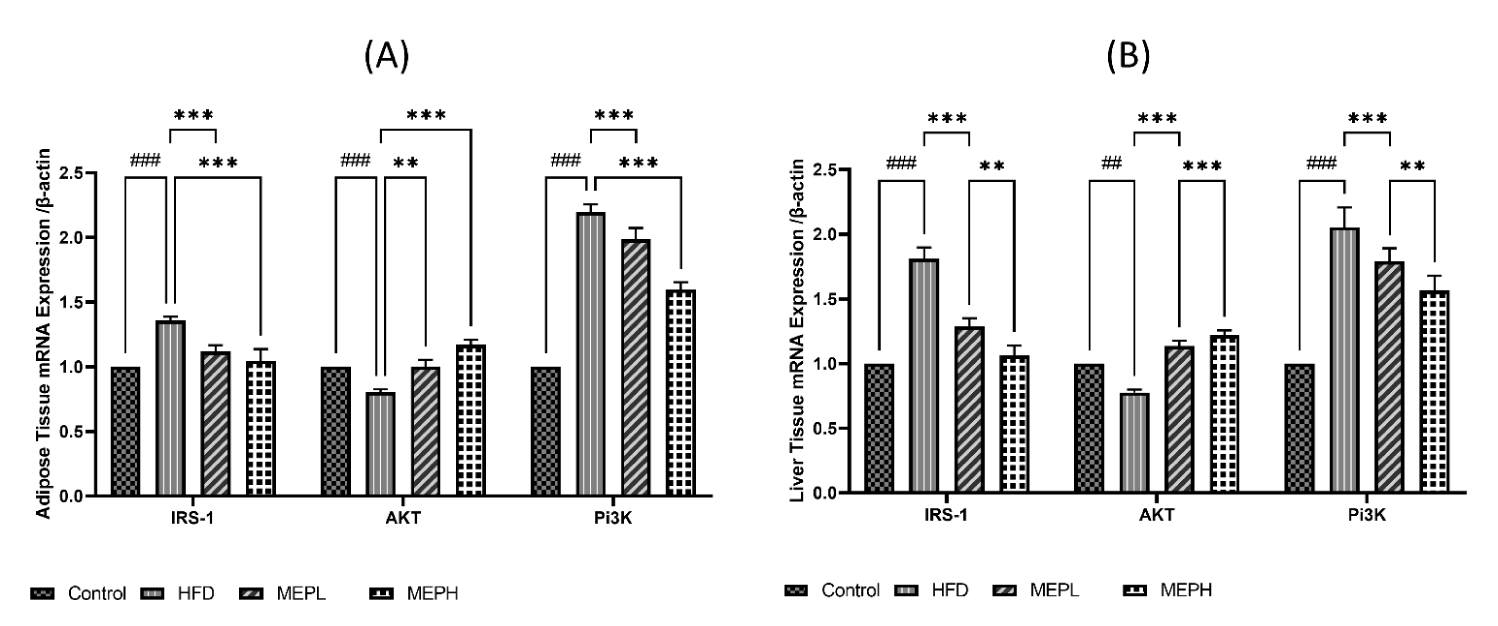
Effect of MEP on insulin resistance-related protein IRS-1
To investigate the MEP effect on the insulin resistance-related protein IRS-1, Protein kinase (Akt) and Phosphoinositide 3-kinase (PI3K) proteins. We have observed overexpression of IRS-1, PI3K and under expression of Akt at the mRNA level in the liver and adipose tissues of HFD-induced obese mice. Our findings revealed an increase in IRS-1 (p < 0.05), PI3K (p < 0.01), and decreased Akt (p < 0.05) expression in the HFD group when compared to the control group (Figures 5A & B). When compared to the HFD group, MEP administration significantly reduced IRS-1 in the MEPH group (p < 0.01) and the MEPL group (p < 0.05), PI3K in the MEPH group (p < 0.01) and the MEPL group (p < 0.05) and increased Akt expression in the MEPH group (p < 0.01) and the MEPL group (p < 0.05) groups. Furthermore, we observed the expression of IRS-1 through immunofluorescence in adipose and liver tissue. We observed overexpression of IRS-1 in the HFD group. While MEP administration reduced the expression of IRS-1 in the liver and adipose tissues (Figure 6).
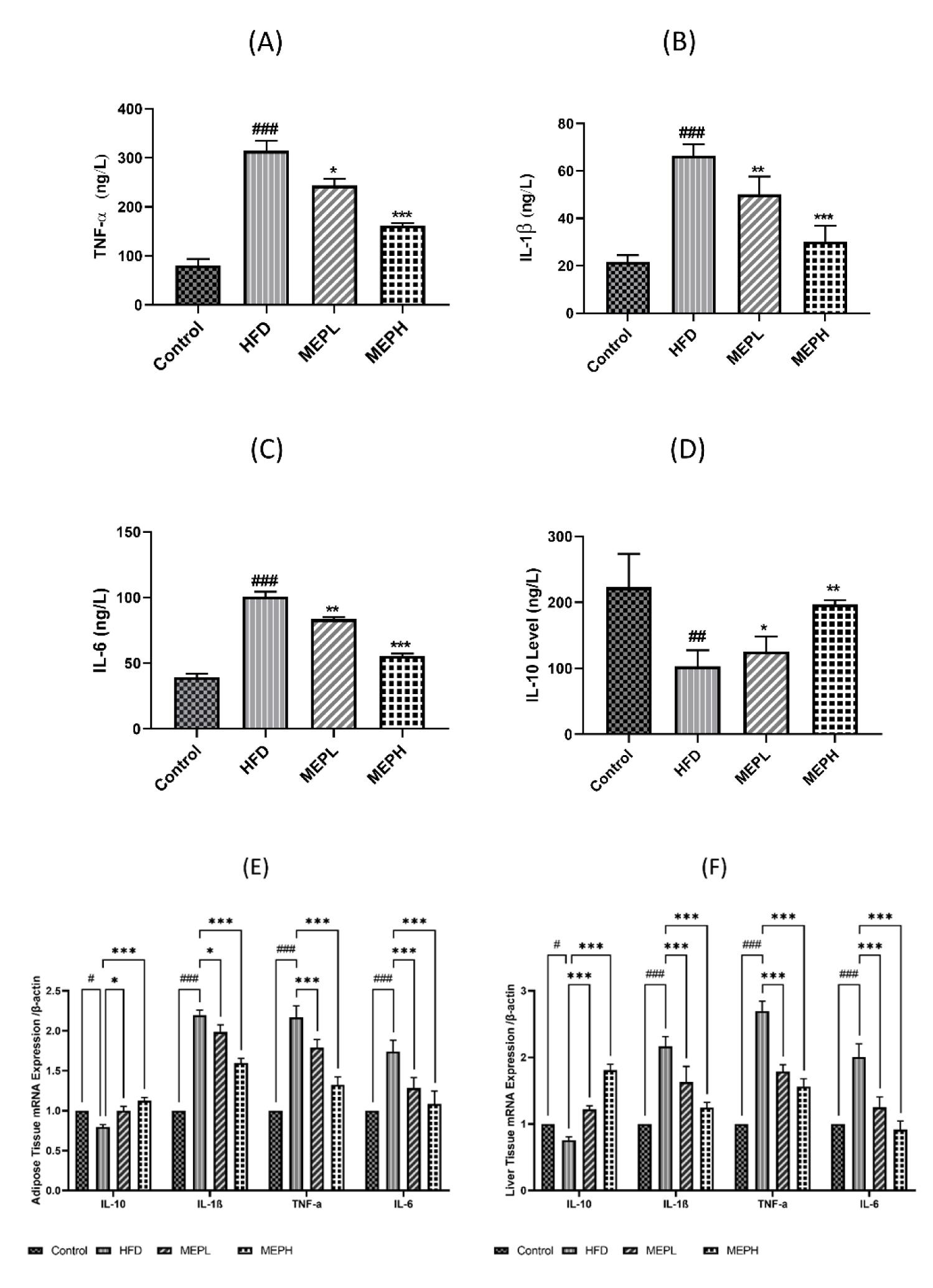
Effect of MEP supplementation on inflammatory cytokines in HFD-induced obese mice
The effects of MEP on pro-inflammatory circulatory cytokines including IL-1β, TNF-α, and IL-6 as well as anti-inflammatory cytokines such as IL-10 were determined in different treated groups (Figures 7A-C). We also determined messenger RNA (mRNA) levels of these cytokines in the liver and adipose tissues (Figures 7E,F). We have found a significantly higher level of pro-inflammatory cytokines (IL-1β, TNF-α, and IL-6) in the serum, liver and adipose tissues of the HFD group versus normal mice. The anti-inflammatory cytokine (IL-10) level was significantly lower in the HFD group (Figure 7D). Consequently, MEP supplementation significantly decreased the level of pro-inflammatory cytokines and increased anti-inflammatory cytokines in MEP-treated groups in a dose-dependent manner. These results indicate that MEP treatment regulates inflammatory markers in HFD-induced obese mice.
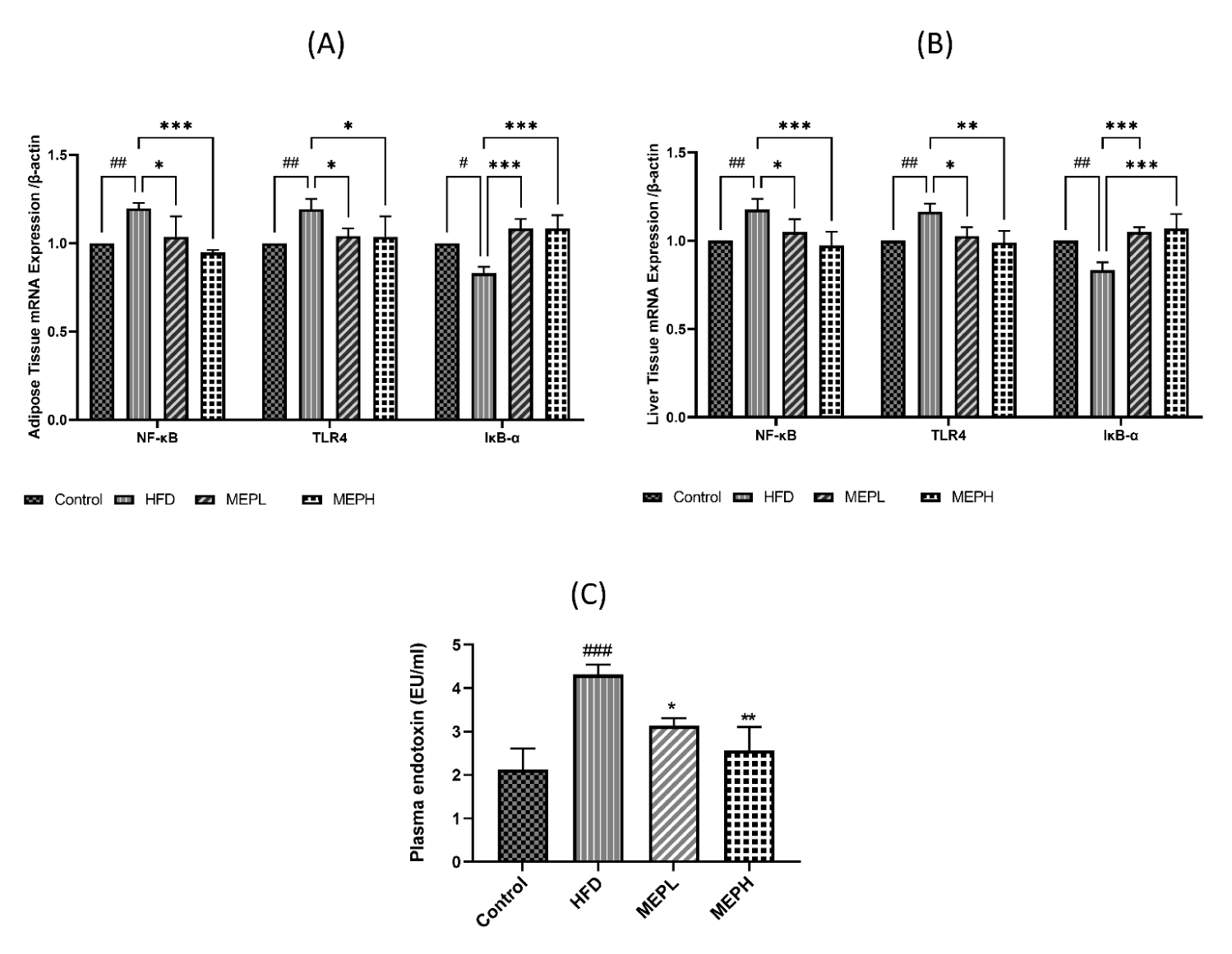
Modulatory effects of MEP on inflammatory-related (TLR4) signaling pathway protein
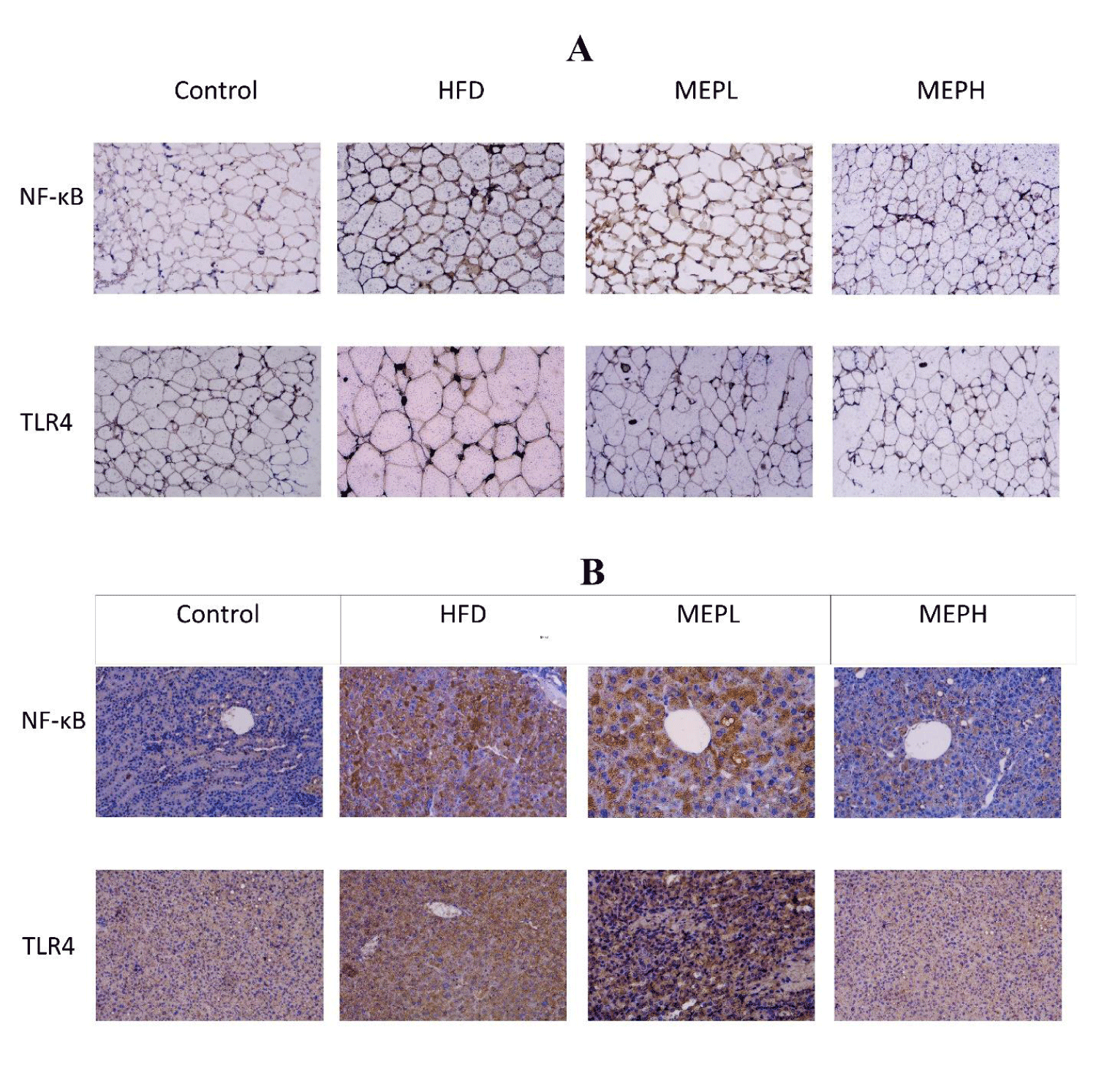
The effects of MEP on inflammatory-related TLR4 signaling pathway protein in adipose and liver tissues were also examined. We measured the mRNA expression of some of these proteins. Our results demonstrated that the TLR4 expression of the HFD group significantly increased compared with the control group (Figures 8A & B). However, MEP treatment significantly decreased TLR4 expression in the MEPH group (p < 0.01), MEPL group (p < 0.01) as compared to the HFD group. The NF-κB p65 phosphorylation of the HFD group was also increased significantly (p < 0.05) as compared with the control group, showing the activation of NF-κB. The p65 phosphorylation was significantly decreased in the MEPH group (p < 0.01) and the MEPL group (p < 0.05) upon MEP treatment. IkB-α expression decreased in the HFD group, whereas MEP treatment increased it in the MEPH (p < 0.01) and MEPL (p < 0.05) (Figures 8A & B).
Moreover, the impact of the MEP on the level of serum endotoxin was measured. Our results demonstrated that serum endotoxin levels in the HFD group (p < 0.001) elevated significantly compared to the control group (Figure 8C). While MEP treatments significantly reduced LPS levels in the MEPH group (p < 0.01) and MEPL (p < 0.05). Furthermore, the expression of TLR4 and NF-κB were confirmed through immunostaining in the adipose and liver tissue. We observed a higher expression in the HFD group. While MEP treatment reduced the expression of these proteins in liver and adipose tissues (Figure 9).
Discussion
The potential role of medicinal and edible mushroom polysaccharides in the treatment of various diseases is well known [32]. Among those, M. esculenta is an edible mushroom with many biological properties [23,33]. The potential of the mushroom M. esculenta polysaccharides on obesity, inflammation and obesity-related complications has not been studied. Our study demonstrates that M. esculenta polysaccharides attenuate HFD-induced obesity and inflammation via regulating inflammatory factors and inflammatory-associated signaling pathways.
Excessive fat deposition is known to be the key factor contributing to the progression of obesity [34]. In our work, HFD consumption resulted in a significant increase in liver weight, body weight gain, and subcutaneous and epididymal fat. Moreover, the body and liver weight, epididymal, and subcutaneous fat deposition in the HFD group show significant increments as compared to the control group. On the other hand, MEP administration significantly reduces liver and bodyweight, as well as subcutaneous and epididymal fat deposition, when compared to the HFD alone group. These results demonstrate that MEP produces anti-obesity effects against HFD-induced obesity in mice.
In several studies, the increase in the size of epididymal adipose tissue has been reported, and the number of adipocytes decreased in obese people and in-vivo [35]. Furthermore, changes in transcriptional factor cascades, such as increased expression of adipogenic genes (PPAR-γ, aFABP, lipogenic genes (FAS), and macrophage-specific markers (CD68, F4/80), have been reported in obesity-related studies [35,36]. Consistent with these findings, our results also show a larger size of adipocytes and over-expression of lipogenic, adipogenic genes, and macrophage-specific markers in the liver and adipose tissue of the HFD alone group. While MEP treatment resulted in decreasing adipocyte size and also reducing the expression levels of lipogenic, adipogenic, and macrophage-specific markers. In addition, TGF-β activation has been shown to cause liver and other organ fibrosis [37]. TGF-β augmentation was also observed in HFD-induced obese mice, and MEP treatment reduced TGF-β mRNA expression in obese mouse adipose and liver tissues.
Moreover, the development of obesity is attributed to the rise in adipocyte size owing to the secretion of pro-inflammatory cytokines [38]. Previous research has shown that excessive production of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) causes chronic inflammation and insulin resistance [39,40]. Therefore, it is necessary to regulate inflammatory cytokine secretion to prevent chronic inflammation and insulin resistance. In our study, we also observed the overproduction of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6,) and the under-production of anti-inflammatory cytokines (IL-10) in HFD-induced obese mice. Notably, MEP treatment attenuated the inflammatory markers of HFD-induced obesogenic mice, which indicates their possible effects on inflammation. HFD consumption increases adiposity, which causes liver damage by increasing liver biomarkers (AST and ALT) as well as TG [26]. NEFA comes from adipocytes that are related to metabolic disorders such as obesity. Adipocytes generate NEFA that affects the action of insulin and is also associated with metabolic disorders such as obesity [41]. In line with these results, we have also observed increased levels of (AST, ALT, TG, and NEFA) in HFD-induced obese mice. However, we found that MEP decreased these markers, indicating the effectiveness of MEP in the improvement of liver function and adiposity.
Obesity generated by the HFD is associated with hyperglycemia and hyperinsulinemia, according to previous research findings [42]. TLR4 pathways enhance the synthesis of pro-inflammatory cytokines via altering the activation of NF-κB. We investigated the effects of MEP on insulin resistance since increased activation of the NF-κB pathways can cause insulin resistance by phosphorylating Insulin Receptor Substrate-1 (IRS-1) and dephosphorylating Akt [39,43,44]. Phosphorylated IRS-1 activates PI3K, which regulates Akt downstream, resulting in glucose utilization and a lower blood glucose level [45]. MEP reduced NF-κB phosphorylation in HFD-fed mice liver and adipose tissues. Furthermore, MEP treatment increased the synthesis of IkB-α, whose association with NF-κB limits NF-κB translocation and activation. In addition, we also found an elevated level of fasting insulin and glucose levels in our study. Interestingly, MEP treatment attenuated the increased levels of insulin and glucose at different doses in HFD-induced obese mice.
Higher levels of plasma LPS and increased endotoxemia caused by the HFD were previously associated with increased expression of Toll-Like Receptor (TLR) 4 and NF-κB in mononuclear cells [46]. NF-κB activation alleviates inflammatory reactions by up-regulating macrophage infiltration [47]. Adipose tissue produces higher levels of inflammatory markers such as TNF-α and IL-6 in dietary-manipulated obesity models. Currently, macrophages of adipose tissue have been focused on, which are involved in adipose tissue by chemo-attractants and work as mediators to produce inflammatory reactions to obesity [48]. Macrophages invade the adipose tissue, which leads to the expression of TNF-α and IL-6 [49]. Our study confirms this series of pathways, as we observe increased expression of inflammatory cytokines and TLR4. As a result, this study supports the previous finding that HFD induces inflammation via TLR4 induction and NF-κB activation. Our finding demonstrated the anti-inflammatory activity of MEP by NF-κB dephosphorylation, TLR4 inhibition IL-6, and TNF-α reduction respectively in MEP-treated HFD-induced obese group.
Conclusion
In conclusion, MEP supplementation could be beneficial for its potential health benefits on obesity-related parameters, inflammation, and weight loss. Furthermore, MEP administration reduced inflammation in adipose and liver tissues via regulation of TLR4 and NF-κB expression and controlled dysregulation of inflammatory cytokines both systemically and locally.
Acknowledgment
We would like to thank Xiaoxiao Zhang and Wang Zexu for their technical assistance.
Supplementary materials
The following supporting information can be downloaded at: Figure S1: Analysis of crude polysaccharide; Table S1: qRT-PCR primer sequence.
Author contributions
Conceptualization, AUR, and YX. Methodology: AUR and AIK. Software: AUR and AIK. Formal analysis: AUR. Investigation: AUR and AIK. Resources: YX: Manuscript writing: AUR. Review and Editing: AIK, YX and WL. Validation: YX and WL. Supervision: YX. Visualization: YX and WL. Project: Administration: YX. Funding Acquisition: YX and WL.
Funding
This research was supported by the National Nature Science Foundation of China (Grant No. 31600614, 82072953); Top young talents of Liaoning Provincial Government (Grant No. XLYC1907009); Dalian outstanding young scientific and technological talents (Grant No. 2021RJ12).
Institutional review board statement
The animal investigations were permitted by the experimental and Animal Ethics Committee of Dalian Medical University, All applicable international, national, and/or institutional guidelines for the care and use of animals were followed under the supervision of the principal investigator. (Protocol number: AEE17015). “This article does not contain any studies with human participants performed by any of the authors.”
Data availability statement
The original data for this work are available upon email request to the corresponding author.
References
- Bray GA, Frühbeck G, Ryan DH, Wilding JP. Management of obesity. Lancet. 2016 May 7;387(10031):1947-56. doi: 10.1016/S0140-6736(16)00271-3. Epub 2016 Feb 10. PMID: 26868660.
- Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond). 2008 Sep;32(9):1431-7. doi: 10.1038/ijo.2008.102. Epub 2008 Jul 8. PMID: 18607383.
- El-Sayed Moustafa JS, Froguel P. From obesity genetics to the future of personalized obesity therapy. Nat Rev Endocrinol. 2013 Jul;9(7):402-13. doi: 10.1038/nrendo.2013.57. Epub 2013 Mar 26. Erratum in: Nat Rev Endocrinol. 2014 Jan;10(1):4. PMID: 23529041.
- Segula D. Complications of obesity in adults: a short review of the literature. Malawi Med J. 2014 Mar;26(1):20-4. PMID: 24959321; PMCID: PMC4062780.
- Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, Cushman SW, Periwal V. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput Biol. 2009 Mar;5(3):e1000324. doi: 10.1371/journal.pcbi.1000324. Epub 2009 Mar 27. PMID: 19325873; PMCID: PMC2653640.
- Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes. 2014 Dec 4;7:587-91. doi: 10.2147/DMSO.S67400. PMID: 25506234; PMCID: PMC4259868.
- Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011 Sep 28;12(11):722-34. doi: 10.1038/nrm3198. PMID: 21952300; PMCID: PMC7171550.
- Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006 Oct;4(4):263-73. doi: 10.1016/j.cmet.2006.07.001. PMID: 17011499; PMCID: PMC1958996.
- Francisco V, Pino J, Gonzalez-Gay MA, Mera A, Lago F, Gómez R, Mobasheri A, Gualillo O. Adipokines and inflammation: is it a question of weight? Br J Pharmacol. 2018 May;175(10):1569-1579. doi: 10.1111/bph.14181. Epub 2018 Apr 10. PMID: 29486050; PMCID: PMC5913397.
- Blüher M. Adipokines - removing road blocks to obesity and diabetes therapy. Mol Metab. 2014 Jan 21;3(3):230-40. doi: 10.1016/j.molmet.2014.01.005. PMID: 24749053; PMCID: PMC3986498.
- Yang J, Leng J, Li JJ, Tang JF, Li Y, Liu BL, Wen XD. Corosolic acid inhibits adipose tissue inflammation and ameliorates insulin resistance via AMPK activation in high-fat fed mice. Phytomedicine. 2016 Feb 15;23(2):181-90. doi: 10.1016/j.phymed.2015.12.018. Epub 2016 Jan 21. PMID: 26926180.
- Blüher M. Adipose tissue dysfunction in obesity. Exp Clin Endocrinol Diabetes. 2009 Jun;117(6):241-50. doi: 10.1055/s-0029-1192044. Epub 2009 Apr 8. PMID: 19358089.
- Catalán V, Gómez-Ambrosi J, Rodríguez A, Salvador J, Frühbeck G. Adipokines in the treatment of diabetes mellitus and obesity. Expert Opin Pharmacother. 2009 Feb;10(2):239-54. doi: 10.1517/14656560802618811. PMID: 19236196.
- Nakamura T, Mizuno S. The discovery of Hepatocyte Growth Factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proceedings of the Japan Academy, Series B. 2010;86:588-610.
- Setyaningsih WAW, Sari DCR, Romi MM, Arfian N. Liver fibrosis associated with adipose tissue and liver inflammation in an obesity model. Med J Malaysia. 2021 May;76(3):304-310. PMID: 34031327.
- Doege H, Grimm D, Falcon A, Tsang B, Storm TA, Xu H, Ortegon AM, Kazantzis M, Kay MA, Stahl A. Silencing of hepatic fatty acid transporter protein 5 in vivo reverses diet-induced non-alcoholic fatty liver disease and improves hyperglycemia. J Biol Chem. 2008 Aug 8;283(32):22186-92. doi: 10.1074/jbc.M803510200. Epub 2008 Jun 3. PMID: 18524776; PMCID: PMC2494916.
- Pohl J, Ring A, Korkmaz U, Ehehalt R, Stremmel W. FAT/CD36-mediated long-chain fatty acid uptake in adipocytes requires plasma membrane rafts. Mol Biol Cell. 2005 Jan;16(1):24-31. doi: 10.1091/mbc.e04-07-0616. Epub 2004 Oct 20. PMID: 15496455; PMCID: PMC539148.
- Lian CY, Zhai ZZ, Li ZF, Wang L. High fat diet-triggered non-alcoholic fatty liver disease: A review of proposed mechanisms. Chem Biol Interact. 2020 Oct 1;330:109199. doi: 10.1016/j.cbi.2020.109199. Epub 2020 Aug 15. PMID: 32805210.
- Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007 Nov 17;370(9600):1706-13. doi: 10.1016/S0140-6736(07)61721-8. Erratum in: Lancet. 2008 Feb 16;371(9612):558. PMID: 18022033.
- Kang JG, Park CY. Anti-Obesity Drugs: A Review about Their Effects and Safety. Diabetes Metab J. 2012 Feb;36(1):13-25. doi: 10.4093/dmj.2012.36.1.13. Epub 2012 Feb 17. PMID: 22363917; PMCID: PMC3283822.
- Blumfield M, Abbott K, Duve E, Cassettari T, Marshall S, Fayet-Moore F. Examining the health effects and bioactive components in Agaricus bisporus mushrooms: a scoping review. J Nutr Biochem. 2020 Oct;84:108453. doi: 10.1016/j.jnutbio.2020.108453. Epub 2020 Jun 13. PMID: 32653808.
- Mingyi Y, Belwal T, Devkota HP, Li L, Luo Z. Trends of utilizing Mushroom Polysaccharides (MPs) as potent nutraceutical components in food and medicine: A comprehensive review. Trends in Food Science & Technology. 2019;92:94-110. doi: 10.1016/j.tifs.2019.08.009.
- Tietel Z, Masaphy S. True morels (Morchella)-nutritional and phytochemical composition, health benefits and flavor: A review. Crit Rev Food Sci Nutr. 2018 Jul 24;58(11):1888-1901. doi: 10.1080/10408398.2017.1285269. Epub 2017 Jul 21. PMID: 28350213.
- Ferreira IC, Vaz JA, Vasconcelos MH, Martins A. Compounds from wild mushrooms with antitumor potential. Anticancer Agents Med Chem. 2010 Jun;10(5):424-36. doi: 10.2174/1871520611009050424. PMID: 20545620.
- Hu M, Chen Y, Wang C, Cui H, Duan P, Zhai T, Yang Y, Li S. Induction of apoptosis in HepG2 cells by polysaccharide MEP-II from the fermentation broth of Morchella esculenta . Biotechnol Lett. 2013 Jan;35(1):1-10. doi: 10.1007/s10529-012-0917-4. Epub 2012 Jul 14. PMID: 22798042.
- Cui HL, Chen Y, Wang SS, Kai GQ, Fang YM. Isolation, partial characterisation and immunomodulatory activities of polysaccharide from Morchella esculenta . J Sci Food Agric. 2011 Sep;91(12):2180-5. doi: 10.1002/jsfa.4436. Epub 2011 May 5. PMID: 21567413.
- Kanwal S, Joseph TP, Owusu L, Xiaomeng R, Meiqi L, Yi X. A Polysaccharide Isolated from Dictyophora indusiata Promotes Recovery from Antibiotic-Driven Intestinal Dysbiosis and Improves Gut Epithelial Barrier Function in a Mouse Model. Nutrients. 2018 Jul 31;10(8):1003. doi: 10.3390/nu10081003. PMID: 30065236; PMCID: PMC6115818.
- Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F. Colorimetric method for determination of sugars and related substances. Analytical chemistry. 1956;28:350-356. https://bit.ly/39Eproq
- Hao L, Sheng Z, Lu J, Tao R, Jia S. Characterization and antioxidant activities of extracellular and intracellular polysaccharides from Fomitopsis pinicola. Carbohydr Polym. 2016 May 5;141:54-9. doi: 10.1016/j.carbpol.2015.11.048. Epub 2015 Nov 19. PMID: 26876995.
- Liang Z, Yuan Z, Li G, Fu F, Shan Y. Hypolipidemic, Antioxidant, and Antiapoptotic Effects of Polysaccharides Extracted from Reishi Mushroom, Ganoderma lucidum (Leysser: Fr) Karst, in Mice Fed a High-Fat Diet. J Med Food. 2018 Dec;21(12):1218-1227. doi: 10.1089/jmf.2018.4182. Epub 2018 Sep 5. PMID: 30183494.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001 Dec;25(4):402-8. doi: 10.1006/meth.2001.1262. PMID: 11846609.
- Chang CJ, Lu CC, Lin CS, Martel J, Ko YF, Ojcius DM, Wu TR, Tsai YH, Yeh TS, Lu JJ, Lai HC, Young JD. Antrodia cinnamomea reduces obesity and modulates the gut microbiota in high-fat diet-fed mice. Int J Obes (Lond). 2018 Feb;42(2):231-243. doi: 10.1038/ijo.2017.149. Epub 2017 Jun 20. PMID: 28630461; PMCID: PMC5803574.
- Liu C, Peng L, Qian M, Hao J. Antihyperlipidemic effect of endo-polysaccharide of Morchella esculenta and chemical structure analysis. Oxidation Communications. 2016;39:968-976. https://bit.ly/3scnJRs
- van Herpen NA, Schrauwen-Hinderling VB. Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol Behav. 2008 May 23;94(2):231-41. doi: 10.1016/j.physbeh.2007.11.049. Epub 2007 Dec 5. PMID: 18222498.
- Chang CJ, Lin CS, Lu CC, Martel J, Ko YF, Ojcius DM, Tseng SF, Wu TR, Chen YY, Young JD, Lai HC. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015 Jun 23;6:7489. doi: 10.1038/ncomms8489. Erratum in: Nat Commun. 2017 Jul 11;8:16130. PMID: 26102296; PMCID: PMC4557287.
- Kim KA, Gu W, Lee IA, Joh EH, Kim DH. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One. 2012;7(10):e47713. doi: 10.1371/journal.pone.0047713. Epub 2012 Oct 16. PMID: 23091640; PMCID: PMC3473013.
- Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Invest. 2017 Jan 3;127(1):55-64. doi: 10.1172/JCI88881. Epub 2017 Jan 3. PMID: 28045404; PMCID: PMC5199698.
- Kim MH, Park SJ, Kim JH, Seong JB, Kim KM, Woo HA, Lee DS. Peroxiredoxin 5 regulates adipogenesis-attenuating oxidative stress in obese mouse models induced by a high-fat diet. Free Radic Biol Med. 2018 Aug 1;123:27-38. doi: 10.1016/j.freeradbiomed.2018.05.061. Epub 2018 May 16. PMID: 29777756.
- Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007 Jun 15;21(12):1443-55. doi: 10.1101/gad.1550907. PMID: 17575046.
- Khan AI, Rehman AU, Farooqui NA, Siddiqui NZ, Ayub Q, Ramzan MN, Wang L, Xin Y. Effects of Shrimp Peptide Hydrolysate on Intestinal Microbiota Restoration and Immune Modulation in Cyclophosphamide-Treated Mice. Molecules. 2022 Mar 6;27(5):1720. doi: 10.3390/molecules27051720. PMID: 35268821; PMCID: PMC8911659.
- Delarue J, Magnan C. Free fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care. 2007 Mar;10(2):142-8. doi: 10.1097/MCO.0b013e328042ba90. PMID: 17285001.
- Lu Y , Zhao A , Wu Y , Zhao Y , Yang X . Soybean soluble polysaccharides enhance bioavailability of genistein and its prevention against obesity and metabolic syndrome of mice with chronic high fat consumption. Food Funct. 2019 Jul 17;10(7):4153-4165. doi: 10.1039/c8fo02379d. PMID: 31241065.
- Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, Davis RJ. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013 Jan 11;339(6116):218-22. doi: 10.1126/science.1227568. Epub 2012 Dec 6. PMID: 23223452; PMCID: PMC3835653.
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005 Feb;11(2):183-90. doi: 10.1038/nm1166. Epub 2005 Jan 30. PMID: 15685173; PMCID: PMC1440292.
- Haeusler RA, McGraw TE, Accili D. Biochemical and cellular properties of insulin receptor signalling. Nat Rev Mol Cell Biol. 2018 Jan;19(1):31-44. doi: 10.1038/nrm.2017.89. Epub 2017 Oct 4. PMID: 28974775; PMCID: PMC5894887.
- Ghanim H, Abuaysheh S, Sia CL, Korzeniewski K, Chaudhuri A, Fernandez-Real JM, Dandona P. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care. 2009 Dec;32(12):2281-7. doi: 10.2337/dc09-0979. Epub 2009 Sep 15. PMID: 19755625; PMCID: PMC2782991.
- Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998 Apr;42(4):477-84. doi: 10.1136/gut.42.4.477. PMID: 9616307; PMCID: PMC1727068.
- Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010 Jan 15;314(1):1-16. doi: 10.1016/j.mce.2009.07.031. Epub 2009 Aug 12. PMID: 19682539.
- Cancello R, Tordjman J, Poitou C, Guilhem G, Bouillot JL, Hugol D, Coussieu C, Basdevant A, Bar Hen A, Bedossa P, Guerre-Millo M, Clément K. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes. 2006 Jun;55(6):1554-61. doi: 10.2337/db06-0133. PMID: 16731817.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.