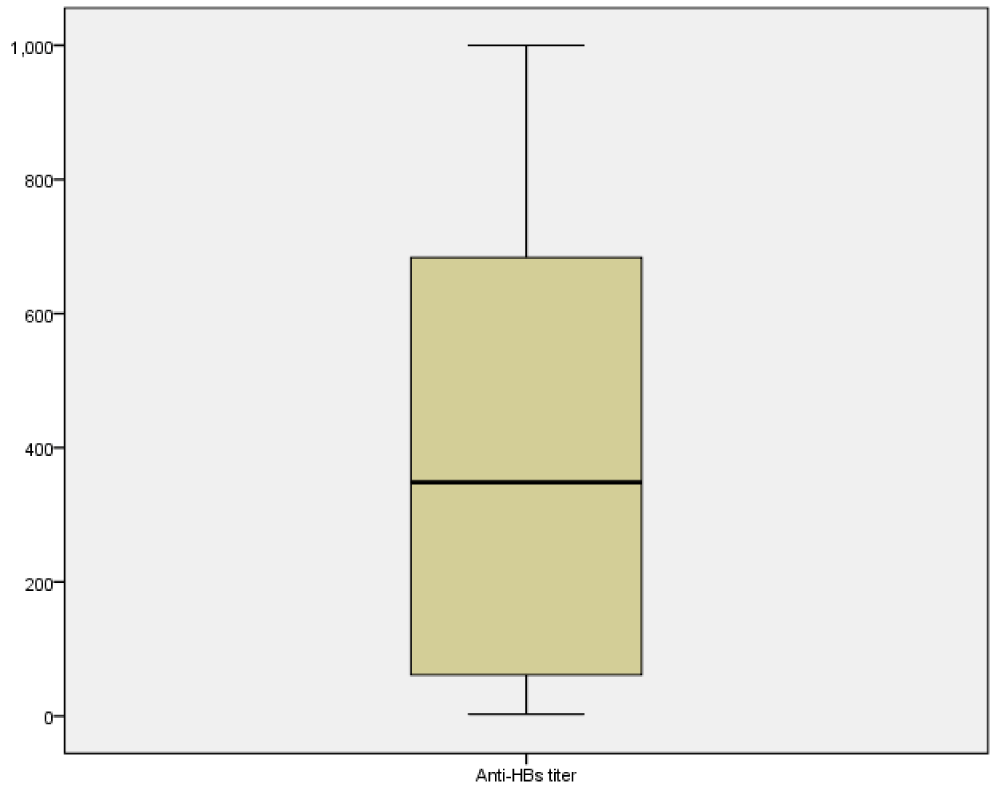Medicine Group . 2022 March 31;3(3):316-319. doi: 10.37871/jbres1440.
Non Inferiority Study to Prevent Hepatitis B Vertical Infection, Non Randomized Clinical Trial
Khaled Ali Abu Ali*
Abstract
Background: Hepatitis B is a vaccine-preventable liver infection caused by the Hepatitis B Virus (HBV). Hepatitis B (HB) is spread when blood, semen, or other body fluids from a person infected with the virus enters the body of someone who is not infected.
Aim of the study: To identify the efficacy of hepatitis B immunoglobulin with hepatitis B vaccine versus hepatitis B vaccine alone to prevent the HB vertical infection.
Method: An quasi-experimental clinical trial was used, 114 new born were included in the control group and 114 newborn were included in the intervention group, both groups were given HB vaccine but the control group HB vaccine combined with hepatitis B immunoglobulin, all of the subjects were born to mother positive HBV and negative HBeAg indicating inactive HBV cases.
Results: Revealed that the used regimens were effective to interrupt mother to child transmission of HBV, the geometric mean titration was higher (207.64 IU/L) among the infants were included in the control group versus (180.87 IU/L) to the infants were included in the intervention group, non-protective rate was 6.6%, relative risk 2.63.
Conclusion: Hepatitis B vaccine alone is not inferior to HB vaccine combined with HBIG to prevent the infection and both regimens were effective.
Background
Hepatitis B belongs to the hepadnaviridae class of viruses, it has a circular DNA genome with partially double stranded and is 42 nm in diameter. It is transmitted by direct percutaneous or per mucosal exposure to infected blood. The hepatitis B infection occurs in children, adolescents and adults and can lead to acute hepatitis, subclinical infection, or the development of chronic infection [1,2]. HBV is present in blood, saliva, semen, vaginal secretions, and menstrual blood of infected individuals. Because HBV is resistant to breakdown outside the body, it is easily transmitted through contact with infected bodily fluids. Perinatal vertical transmission is the most common mode of transmission worldwide [1,2]. Chronic Hepatitis B Virus (HBV) infection affects over 350 million people worldwide and over 1 million die annually of HBV-related chronic liver disease [3]. HBV is comprised of a number of clinically important viral proteins, including the envelope protein (HBsAg), Hepatitis B Core Antigen (HBcAg), and a soluble nucleocapsid protein [4-7]. An estimated 257 million people are living with HBV infection. In 2015, HB resulted in 887,000 deaths, mostly from HB complications “HCC and cirrhosis” [8,9]. In Palestine, the incidence rate of HBV infection among blood donors was 1.48% [10]. In Palestine, there are two adopted policies; in the first one both HB vaccine and HBIG is given to all newborns born to known reactive HBV infected mothers within 72 hours after delivery, but the inactive mothers only HB vaccine is given to their newborn. This policy is adopted by the Epidemiology departments in the GS. The other policy is adopted in the hospitals in which both HB vaccine combined with HBIG is given to all newborns of HBV infected mothers even active or inactive HBV infection.
Aim of the Study
The study aimed to identify the efficacy of hepatitis B immunoglobulin with hepatitis B vaccine versus hepatitis B vaccine alone to prevent HB vertical infection.
Methods
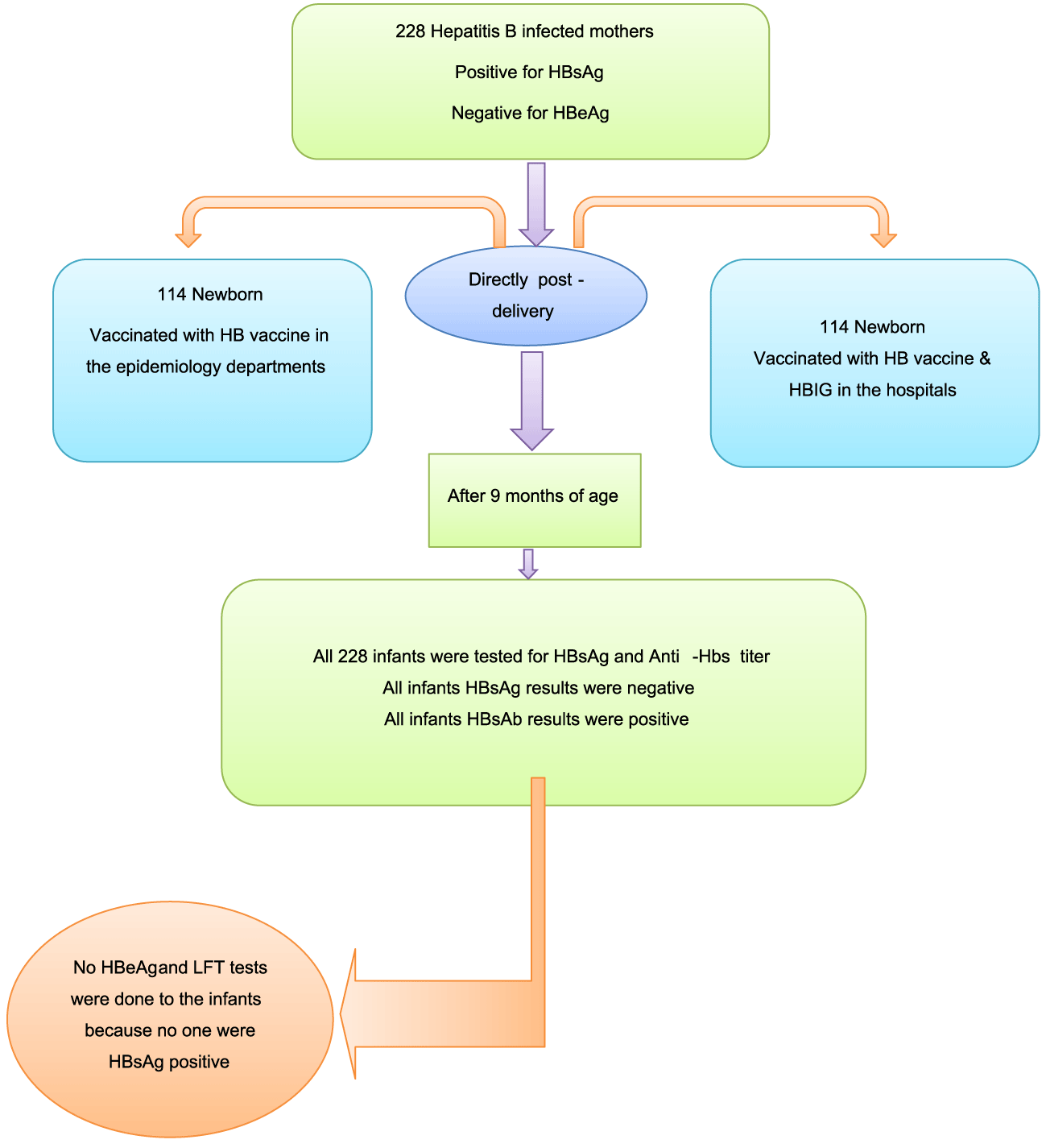
An quasi-experimental clinical trial (non-inferiority study) was used, 114 new born were included in the control group and 114 newborn were included in the intervention group, both groups were given HB vaccine but the control group HB vaccine combined with hepatitis B immunoglobulin, all of the subjects were born to mother positive HBV and negative HBeAg indicating inactive HBV cases, mother mean age 29 years. The study was conducted in the five Epidemiology departments and the delivery rooms in three governmental hospitals in GazaStripin Palestine.
The study sample composed of 228 subjects with control-intervention ratio 1:1.
Participants were subjected to the following activities (Figure 1):
1. Using predesigned interviewing questionnaire
The questionnaire included questions related to:
- Questions related to the mothers which included: demographic variables, birth history variables, risk factors related variables and laboratory related variables.
- Questions related to the newborn which included: birth related variables, breast feeding variables, immune-prophylaxis variables and laboratory related variables.
2. Testing for HBsAg, HBeAg and liver function tests from the mothers prior or during delivery.
- The results indicated inactive mothers` HBV infection included in the study "HBsAg positive and HBeAg negative.
3. HB vaccine "0.5 ml by intramuscular injection" were given to all newborns "228" and 114 newborns were given 100 IU hepatitis B immunoglobulin by intramuscular injection combined to HB vaccine "intervention group".
4. Blood sampling for serological testing to assess the infection and protection rates "HBsAg to assess infection state" and Anti-HBs titer to assess the protection rate" after 9 months of age.
Results
Detection of the difference of immunity among the infants at 9 months of age.
Results revealed that the used regimens were effective to interrupt mother to child transmission of HBV and the adopted regimens gave the infants the complete immune level (100 IU/L and more). The geometric mean titration was higher (207.64 IU/L) among the infants were included in the intervention group versus (180.87 IU/L) to the infants were included in the control group, this means that HB vaccine alone is not inferior to HB vaccine combined with HBIG to prevent HBV infection among the infants born to non-reactive HBV infected mothers, (Figure 2). The non-protective rate was 6.6% and 93.4% achieved a protective level against HBV infection by getting of HB antibodies more than 10 IU/L. Determination of the infection rate among infants at 9 months of age.
The immunization regimens succeeded to prevent HB infections, the study didn`t report any HBV infected cases.
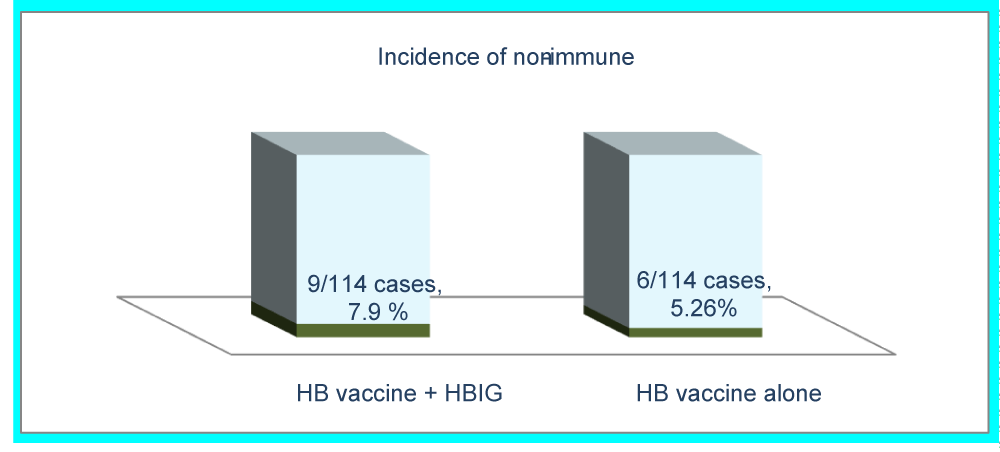
All the infants (228/228) 100 % were responsive to the immunization regimen, while 6.6 % didn`t get the protection level of Anti-HBs higher than 10 IU/L, figure 3. Incidence of non-protection rate was 6.6 % and it was 7.89 % among the intervention group "those infants given HB vaccine alone" while it was (5.26 %) among the infants were included in the control group "vaccinated with HB vaccine combined with HB immunoglobulin ", RR 2.63, (Table 1). The necessity to administer HB vaccine booster dose. The results revealed that no need for booster dose to 93.4 % infants because they got the protection level higher than 10 IU/L Anti-HBs titer, and revaccination to 6.6 % of non-protected infants was recommended and intervened. Anti-HBs titer may decrease over time after vaccination, after five years a booster dose could be needed, so further research may be needed to decide whether HB vaccine booster dose is needed or not. HBV infection and breastfeeding. In this study there is no evidence that breastfeeding increases the risk of Mother to Child Transmission (MTCT), so the researcher demonstrated that breastfeeding is not contraindicated to infants born to mothers who are HBsAg positive according to the study results. The results showed that 99.6 % of the mother’s breast fed their babies and no one case of HB infection was reported. So the researcher encourage HBV-infected mothers to breastfeed their infants.
| Table 1: Incidence rate of non-protection among infants. | ||||||
| Incidence of non-protection | 0-9 IU/L | RR* | ARR** | CI | ||
| No. | % | Upper | Lower | |||
| HB vaccine alone | 6 | 5.26 | 2.64 | 33.3 % | -3.491 | 2.158 |
| HB vaccine+ HBIG | 9 | 7.89 | ||||
| Total | 15 | 6.57 | ||||
| *Relative Risk (RR) **Attributable Risk Reduction (ARR). |
||||||
Conclusion
The used regimens were effective to interrupt mother to child transmission of HBV and the adopted regimens gave the infants the complete immune level. The used regimens succeeded to prevent HBV vertical infection.
No need for booster dose of HB vaccine at least for five years for the infants their Anti-HBs was not less than 10 IU/L. Breast feeding is not prohibited if the babies of the HBV infected mothers immunized directly after delivery. The immunization regimen which was used with the intervention group was more cost effective than the regimen used with the control group. The geometric mean titration was higher (207.64 IU/L) among the infants were included in the intervention group versus (180.87 IU/L) to the control group.
Recommendations
- HB vaccine is effective to prevent HB vertical infection.
- Assessing the protection rate to detect non-respondent rate, 2-3 months after the last dose of the vaccine.
- Vaccination of the infants must be given hastily post-delivery.
- Mass immunization of sall risky groups in order to control the infection.
- The researcher recommends that there is no need to delay breastfeeding until the infant is fully immunized.
- During pregnancy all pregnant women should be tested for HBsAg.
- All HB infected women should be tested for HBeAg and liver function tests in the last trimester of pregnancy in order to decide which prophylaxis regimen to be adopted.
- Ongoing monitoring and investigation of the Anti-HBs titer level in children and other ages to ensure protective level of immunity.
- Health education and public awareness programs that include information regarding the rationale for and importance of newborn HB vaccination and prevention strategies.
References
- Viral Hepatitis, Division of Viral Hepatitis. Atlanta-USA: Center for Disease Control and Prevention. 2014. https://tinyurl.com/5n7h3dje
- What is hepatitis?. Geneva, World Health Organization. 2016. https://tinyurl.com/3ez8dtyv
- Chelsea B, Jennifer M, Benjamin C. Addressing the increasing global burden of viral Hepatitis. Hepatobiliary Surgery and Nutrition. 2017;6(4):274-276. https://tinyurl.com/tvjywrev
- Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: Epidemiology and vaccination. Epidemiol Rev. 2006;28:112-25. doi: 10.1093/epirev/mxj009. Epub 2006 Jun 5. PMID: 16754644.
- Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J, Stephenson SL, Gray DF. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998 Jul 9;339(2):61-8. doi: 10.1056/NEJM199807093390201. PMID: 9654535.
- Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008 Oct 2;359(14):1486-500. doi: 10.1056/NEJMra0801644. Erratum in: N Engl J Med. 2010 Jul 15;363(3):298. PMID: 18832247.
- Kramvis A, Kew M, François G. Hepatitis B virus genotypes. Vaccine. 2005 Mar 31;23(19):2409-23. doi: 10.1016/j.vaccine.2004.10.045. PMID: 15752827.
- Hepatitis B, Fact sheet N° 204. Geneva, World Health Organization. 2015. https://tinyurl.com/yfnnzp66
- World Hepatitis Day. Geneva, World Health Organization. 2018. https://tinyurl.com/2p8mcuny
- Dheir M, Ghuneim N, Abu-Ali K. Annual epidemiological report Gaza strip, Ministry of Health. Palestine. 2012. https://tinyurl.com/2p8r8mhr
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.