Medicine Group . 2022 March 25;3(3):287-293. doi: 10.37871/jbres1435.
Simple Review of Environmental Analytic Methods for the Determination of Pesticide Metabolites
Alphonse Mendy*, Jean Pierre Bakhoum, Diène Diégane Thiaré, Mame Diabou Gaye-Seye and Atanasse Coly
- Pesticide metabolites
Abstract
The findings of research proceedings on pesticide metabolites detection are both an environmental inventory and prevention of potential foodstuffs contaminants. Since, the requirement of a quality environment is a condition for ensuring food security, the newly designed methods with validated sensitivities and efficiencies should be highlighted as solutions for reducing pesticide metabolites. In this respect, this review provides information about pesticides metabolism subject to various analytical conditions applied to standard methods, in addition to the pesticide/related metabolites ratio (R) developed for the intended purpose.
Introduction
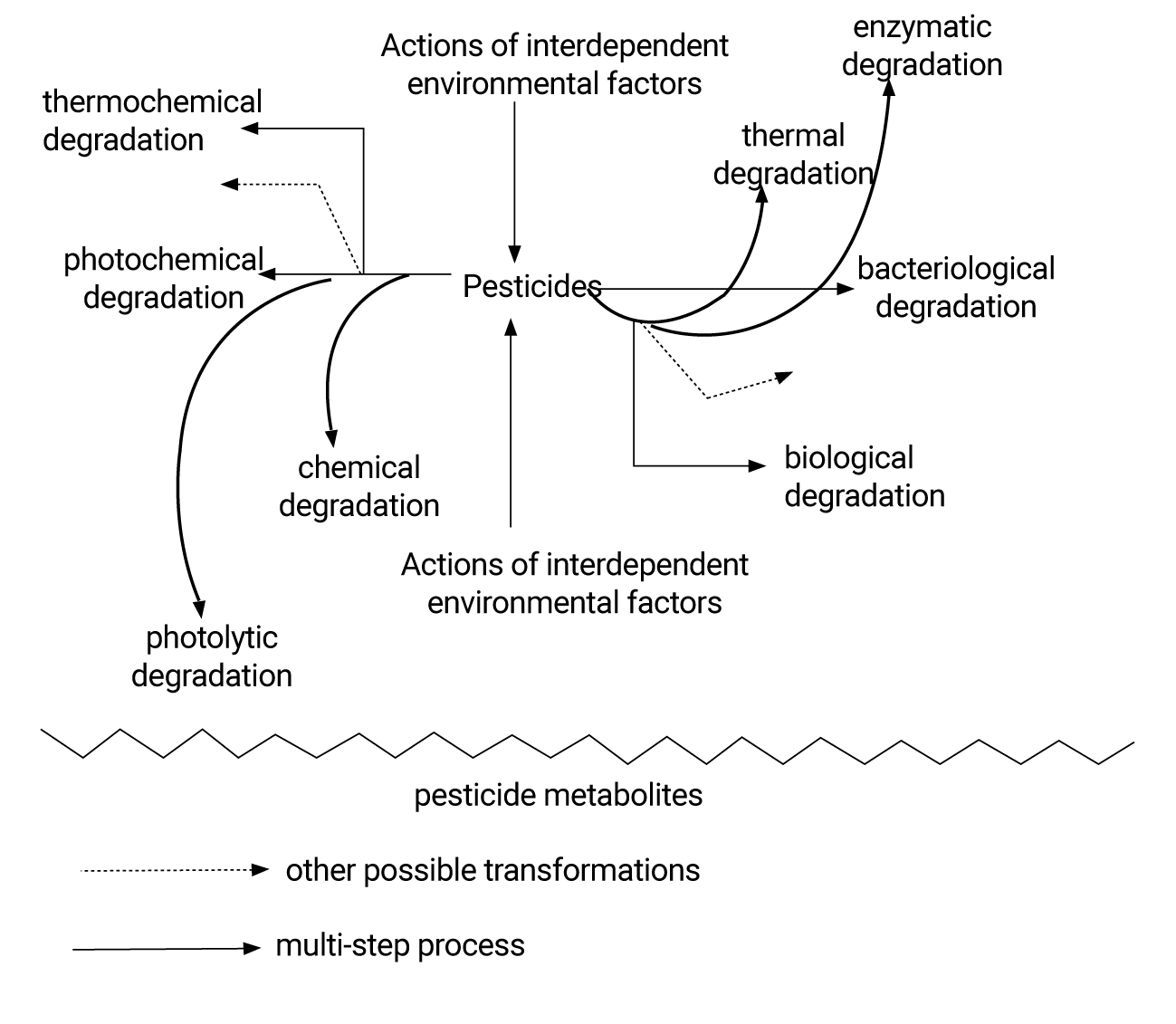
With reference to the European Directives [1], rationalization of the pesticide’s usefulness can contribute to environmental protection. Although the development of the pesticides use requires both constant management and control of their impact on the environment−taking over these measures will unavoidably allow agricultural resources use in a sustainable way. Indeed, it comes to setting a balance between food needs, the environment quality requirements and the disturbances that pesticide metabolites could bring about in some agricultural and food [2]. These risks are all the more concerning that most pesticide residues develop multiple-origin instability, usually by degradation pathway, and their metabolites with high lifetime in matrices. Pesticide residues for agricultural use subjected to an array of biotic and/or abiotic transformations generate potentially hazardous compounds. Therefore, the proposed diagram refers to the main known degradation types that pesticide residues are subjected, as illustrated in the figure 1. In fact, it is difficult to assess the consequences of the pesticides use, including their toxicological profile inherent in their nature, their chemical, biological, bacteriological degradations, as well as the various complex and interdependent internal and external factors of the environment components. To that end, research devoted to the determination of pesticide metabolites in the environment has well proven their presence in several biosphere points. For instance, the United States Environmental Protection Agency (USEPA) reports that as a result of agricultural pesticide use, 46 other compounds had been detected in surface water and 76 other in surface water bodies [3]. Also, these pesticide residues metabolites lead to serious environmental problems, in particular through contamination of the human food supply chain since according to a World Health Organization (WHO) report, one to five million pesticide poisoning cases, including several thousand fatal cases, occur annually worldwide [4]. For these reasons, Maximum Residue Limits (MRLs) for pesticides have been established by the Food and Agriculture Organization (FAO) of the United Nations and the World Health Organization (WHO) [5], as well as their metabolites. In fact, the MRL reflects the maximum amount of pesticide residues and its toxic metabolites that would be expected on a product if good agricultural practices were followed while using the pesticide. Therefore, pesticide concentrations and their metabolites levels in the environment should be monitored on an ongoing basis. In contrast, with the multi-sectoral development of analytical research, correlations between biological and chemical effects of pesticide metabolites measured in the environment have nevertheless been established. However, those correlations remain insufficient since the high systematic dissemination of metabolites tributary to environmental contours and unresolved properties remain problematic. In this context, the search for effective dosing methods is inevitable. It represents a huge health and economic stake. Also, the improvement of the environmental analytical chemistry continues with the innovative use of generally combined standard techniques including chromatography and mass spectrometry. These multi-residue methods have detected important pesticide metabolites for a wide range of samples, including water, soil, plants and animals [6-11]. However, due to the inherent properties of sensitivity, selectivity and versatility, the applicability of the newfound methods to environmental analysis should be an alternative suitable to the climatic conditions for better participation in the fields of environmental control and management. This literature research is to collect data from environmental analytical applications relating to that pollution and toxicity problems posed by pesticide metabolites. Through this channel, this document describes the current state of the significant contribution of pesticide metabolites pollution from various environmental samples without forgetting the food chain.
Analytical Methods Established
Several techniques implemented have made tremendous progress−including Gas Chromatography (GC), liquid chromatography (GC), Thin-Layer Chromatography (TLC), High-Performance Liquid Chromatography (HPLC), Mass Spectrometry (MS) and Nuclear Magnetic Resonance (NMR). In addition to their possible combinations, these standard methods can be combined with Liquid Scintillation Counter (LSC), Radiolabel (RAD), Solid-Supported Liquid–Liquid Extraction (SS-LLE), Ion Trap (IT), Electrospray Ionization (ESI) and/or Diode Array Detection (DAD) for the analysis of pesticide metabolites, as the findings set out (Table 1) highlighting their problematic in the environment. These approaches, with their high resolution power and selectivity in full scan detection mode, have the ability to characterize pesticide metabolites as well as their degree of toxicity for a significant contribution to environmental control.
| Table 1: Overview of multi-residue methods for the analysis of pesticide metabolites in various environmental matrices. | ||||
| Analytes | Pathway/condition/matrix | R | Methods | References |
| Atrazine (A) metabolites deethyl-atrazine (DEA); deisopropylatrazine (DIA); diaminochlorotriazine (DACT) |
Atrazine can be degraded in surface water by photolysis and microorganisms via N-dealkylation and hydrolysis of the chloro substituent / soil | 1/3 | SPE-GC/MS | [12] |
| Metolachlor (MET) metabolites metolachlor-oxanilic acid (MET-OA); metolachlor-ethanesulfonic acid (MET-ESA) |
Dealkylated metabolites/ hydrological environments. | 1/ 2 | HPLC/DAD LC/MS | [12] |
| Diuron metabolites (3-(3,4-dichlorophenyl)-1-methylurea; 3-(3,4-dichlorophenyl)-urea; 3,4-dichloroaniline; 4-chloroaniline; 4-chloroacetanilide; 3,4-dichloroacetanilide, aniline |
Successive N-demethylation and amide bond cleavage involving demethylase and amidase reactions / bacteria from soil / soil of sugar cane. | 1/7 | HPLC LC-MS GC-MS |
[13-15] |
| Amitraz metabolites N-(2,4-dimethylphenyl)-Nmethylformamidine; 2,4-dimethylformamidine; 2,4-dimethyl aniline; N,N′-bisdimethylphenylformamidine |
Degradation / human blood Decomposition/ pears |
1/4 | UHPLC-QTOF SLE-MS/MS QqTOF-MS MS/MS |
[16] [17] |
| Fenthion metabolites Sulfoxide; sulfone |
Degradation by sunlight photolysis / oranges | 1/2 | IT-MS QqTOF-MS |
[18] |
| Simazine (SIM) metabolites 2-hydroxy-simazine (OH-SIM); desethyl-simazine (DE-SIM) |
Formation, degradation, transformation, biotransformation and biotic and abiotic processes its main metabolites / presence - absence of urea, microbiologically active / sterilized soil | 1/ 2 | LC-ESI/MS | [19] |
| Fluometuron metabolites desmethyl fluometuron; trifluoromethyl phenylurea; trifluoro-methylaniline |
Series of oxidative and hydrolytic transformations during / microbial metabolism / soil or aquatic environments/ Tillage soils | 1/3 | HPLC, LSC RAD-TLC, NMR |
[20] |
| Carbaryl metabolites 4-hydroxycarbaryl; 5-hydroxycarbaryl; 7-hydroxycarbaryl; Hydroxymethylcarbaryl; 1-naphtol. 2-hydroxy-1,4-naphthoquinone; 1-naphtol and three other photoproducts |
Rapid. Proceeds via hydrolysis, alkyl oxidation, arene oxide formation, epoxide hydrolysis and glutathione conjugation/ Pathways similar in humans, rodents and other species investigated / chicken urine. Photo-oxidation process together with products from photo-Fries, photo ejection and methyl carbamate hydrolysis /excitation with a Suntest/silica-kaolin-sand. |
1/5 1/5 |
TLC LC-DAD LC-MS HPLC/ESI/MS |
[21] [22] |
| Penconazole (PEN) metabolites 4-(2,4-dichloro-phenyl)-5-[1,2,4] triazol-1-yl-pentane-2,3-diol ; 4-(2,4-dichloro-phenyl)-5-[1,2,4] triazol-1-yl-pentanol ; glucuronide conjugate of PEN-OH; 4-(2,4-dichloro-phenyl)-3-hydroxy-5-[1,2,4] triazol-1-yl-pentan-2-one, 4-(2,4-dichloro-phenyl)-5-[1,2,4] triazol-1-yl-pentanoic acid; glucuronide conjugate of iso-PEN-OH, 3-(2,4-dichloro-phenyl)-4-[1,2,4] triazol-1-methyl-butanol |
multiple reaction following the protonated molecular ions different oxidized metabolites were found, both in the free and glucuronide conjugate forms / Farmers exposed to penconazole / human urine samples | 1/7 | LC/Q-TOF-MS, ESI | [23] |
| Carbosulfan metabolites carbosulfan sulfinamide ; carbofuran ; dibutylamine ; 3-hydroxycarbofuran ; 7-phenolcarbofuran; 3-ketocarbofuran; 3-hydroxy-7-phenolcarbofuran; 3-keto-7-phenolcarbofuran |
oxidation of sulfur to carbosulfan sulfinamide and the cleavage of the nitrogen sulfur bond (N–S) to give carbofuran and dibutylamine / in vitro biotransformation by mammalian hepatic microsomes / hepatic microsomes from human, rat, mouse, dog, rabbit, minipig, and monkey. | 1/8 | LC–MS | [24] |
| Dimethoate metabolites desmethyl dimethoate ; dimethoate carboxylic acid ; dimethyl dithiophosphoric acid ; dimethyl thiophosphoric acid ; dimethyl phosphoric acid ; phosphoric acid |
Degradation affected by hydrolysis followed by oxidation or oxidation followed by hydrolysis/ soil-alkaline solution | 1/6 | TLC | [25] |
| Bifenthrin metabolites cyclopropanecarboxylic acid; 2-methyl-3-biphenylyl methanol; 4-trifluoromethoxy phenol; 2-chloro-6-fluoro benzylalcohol; 3,5-dimethoxy phenol. |
Hydrolysis of ester linkage and cleavage of biphenyl/microorganism/potential contaminated soils treatment. | 1/5 | GC-MS | [26] |
| Chlorpyrifos metabolites diethyl thiophosphate; diethyl phosphate; 3,5,6-trichloro-2-pyridinol |
biotransformation products / urine samples / human urine | 1/3 | LC-ESI-MS/MS | [27] |
| LC: Liquid Chromatography; GC: Gas Chromatography; MS: Mass Spectrometry; TLC: Thin-Layer Chromatography; NMR: Nuclear Magnetic Resonance; HPLC: High-Performance Liquid Chromatography; DAD: Diode-Array Detection, LSC: Liquid Scintillation Counter; RAD: Radiolabel; ESI: Electrospray Ionization; Qqtof or QTOF: Quadrupole Time-of-Flight; SLE: Solid-Support Liquid–Liquid Extracts; UHPLC: Ultra-High Performance Liquid Chromatography; SPE: Solid Phase Extraction; MS/MS: Mass Spectrometry Tandem; IT: Ion Trap; R: Pesticide/Related Metabolites Ratio. |
||||
Hereby, two metabolites of the chloroacetanilide Herbicides-Ethane Sulfonic Acid (ESA) and Oxanilic Acid (OXA)−are detected by HPLC/DAD and LC/MS for metolachlor [12]. Seven and three phenylurea herbicides metabolites of diuron [13-15] and fluometuron [20] are, respectively, analyzed by HPLC, LC/MS and GC/MS on the one hand and HPLC/LSC, RAD/TLC and NMR on the other. Phenylurea herbicide analysis also includes the metabolites of simazine (SIM)-2-hydroxy-simazine (OH-SIM) and Desethyl-Simazine (DE-SIM) detected by LC/ESI /MS [19]. Phenylurea herbicides are widely used to selectively control weeds in a variety of crops. However, these compounds exhibit chronic toxicity, and their residues and degradation products are potential risks to human health through their accumulation in the environment and the food chain [28]. Elsewhere, metabolites, although tributaries of conditions and methods used, five carbamates insecticides metabolites of carbaryl are detected as well by TLC and combining HPLC/DAD, LC/MS and HPLC/ESI/MS techniques [21,22]. Carbosulfan toxicological evaluation was compiled by the Joint FAO/WHO Meeting on Pesticide Residues (JMPR) [29]. As such, eight carbamates insecticides metabolites of carbosulfan are only analyzed by LC/MS [24]. Other insecticide metabolites such as dimethoate [25], bifenthrin [26] and chloropyrifos [27] are analyzed and detected under various conditions by TLC, GC/MS and LC/ESI/MS/MS, respectively, as well as amitraz and fenthion [16-18]. For both pesticides, their applications in conjunction with Time-of-Flight (TOF) or Quadrupole Time-of-Flight (QTOF) analyzers (TOF-MS or QTOF-MS) in detecting amitraz and fenthion metabolites in human blood and food samples such as UHPLC/QTOF, SLE/MS/MS, QTOF/MS, MS/MS and IT/MS, QqTOF/MS have been applied, respectively.
Critical analysis of the findings
Pesticide metabolites in samples: The environment is a complex setting where the pesticide residues transition processes through metabolites accumulation in various environmental matrices follow degradation and/or transformation steps. That can be carried out in soil by hydrolysis and N-dealkylation. Indeed, fungi and soil bacteria can transform atrazine into hydroxy-atrazine, sometimes quickly [30]. The main product of acid or basic hydrolysis [31,32] is 3, 4-dichloroaniline, a highly toxic aromatic amine. Likewise, studies [33-35] have shown that measured concentrations in hydrological media environments from normal agricultural use reveal the presence of metolachlor metabolites. In diuron-contaminated environments, the main degradation products detected are methyl (3,4-dichlorophenyl methylurea) and dimethyl (3,4-dichlorophenyl-methylurea) [13]. Amitraz intermediate and its final products are more toxic, especially 2,4-dimethylaniline, characterized by mutagenicity, carcinogenicity and genotoxicity [36-38]. Elsewhere, photoproducts are derived from N-demethylation, hydroxylation and rearrangement reactions [39]. Photoproducts metabolites [12,40] of diflubenzuron, including p-chlorophenylurea, 2,6-difluorobenzamide and 2,6-difluorobenzoic acid, have been detected in groundwater, surface water, soil and sediment [40] on the one hand, and sulfoxide and sulfone, metabolites of fenthion have been detected in oranges [12] on the other hand. Typically, the main degradation products obtained and less sensitive to photodegradation are more persistent and more toxic than the parent compounds. Consequently, contamination of waters, soils, crops [14-20,23-27], and biological matrices by simazine metabolites (Table 1), among other things, resulting from biotic and / or abiotic reactions of pesticides residues is a public health issue. Also, other processes such as oxidation followed by hydrolytic transformation of fluometuron, alkyl-oxidation and/or photo-oxidation of carbaryl penconazole, carbosulfan and dimethoate [20-25] have also been recorded. Through this, these compounds produced metabolites under the conditions of the applied method (Table 1). And, given the quality of the analytical methods applied, the validity and relevance of the experimental data collected in this document (Table 1) are as much among others illustrations. For a pesticide used, at least two related metabolites are detected (Table 1). In other words, the metabolites growth rate, although dependent on matrices conditions and analytical methods, doubles at least in each medium. Moreover, the lower the pesticide / related metabolites ratio, the more contaminated the matrix identified, since new contaminants are constantly being added to crop varieties. In this regard, a taxonomic study of contaminated matrix based on the identification, quantification and study of the pesticide metabolites toxicity would be an important information source. It would measure the level of danger of contamination. However, recommendations based on this classification will make it possible to operate on pesticides with lesser ecotoxicological consequences. From a holistic view point, it is necessary to move from a static analysis of the research achievements developed in the samples to a dynamic analysis which, in relation to real matrices, will unavoidably raise awareness, given the rates of accumulation and/or bioaccumulation of metabolites related to the pesticides used. Moreover, ecotoxicological studies [41-45] show that pesticides contribute to the development of various pathologies including carcinogenic, neurological, respiratory and reproductive diseases since thousands of deaths occur every year worldwide according to the World Health Organization (WHO) with one to five million cases of pesticide poisoning [46]. At this point, in addition to the complexity of the environmental conditions favorable to the transition from chemically and / or biologically unstable pesticides, the foodstuffs genuine contaminants relate to the toxic metabolites bioaccumulation.
On the other hand, we must affirm the absolute need to combat the pesticide metabolites which accumulate continuously in highly complex matrix such as surface and groundwater, soil, food and food products. With the development of suitable methods to assess the toxicity of pesticide metabolites in order to establish the Maximum Residue Limits (MRLs) allowed, improving crop yields remains a feasible measure. It requires monitoring the rational and reasoned use of pesticides to ensure the regulation of authorized levels. In this context, newly designed, fast and reliable methods for improvements adapted to environmental conditions for the reduction of pesticide metabolites are needed. Thus, the transfer phenomena affecting highly complex plant protection products and the possible ecosystem reactions in their largely unknown presence will be reduced. For this purpose, chromatography (LC-HPLC-UPLC) and / or Mass Spectrometry (MS) are effective tools for a large-scale use analysis. Their assessment depends on the quality of the performance, and reasons our investigation. Their applications in conjunction with powerful tools in extraction to investigate the presence of pesticide metabolites in varied samples are the most appropriate, given the high sensitivity, the mass analysis precision, the spectra high resolution, the improvement selectivity and characterization of metabolites, including their degree of toxicity for both environmental quality control and management.
Pesticide metabolites in foodstuffs
Despite the varied and rich agricultural potential, the critical nature and problematic of the pesticide metabolites in the environment have motivated the increasing use of several newfound, rapid and accurate techniques. Therefore, numerous analytical methods of multi-residue types developed have validated the determination of 83 pesticide residues in wine samples, 51 in grapes, 126 and 144 in cereals and dry foods for animals respectively, 129 in leafy vegetables and 260 in agricultural products [45-50]. Furthermore, with the network of biotic and/or abiotic reactions, one of the main food chain contamination remains related to the toxic effects of pesticide residue metabolites to which involving are subjected the food matrices. In fact, with food conservation and storage conditions, the pesticide residues detected are highly subjected to metabolic decompositions due to the biological, bacteriological and enzymatic factors, including several known mechanisms of actions. Besides, biomonitoring studies have often detected pesticide metabolites in consumer urine samples [23,27]. Such investigations achievements require an objective and thorough introspection in order to site the potential and hazardous contaminant. Indeed, it has been shown that between the pesticide residue and their metabolites, the latter are typically more toxic. At this level, the stable presence of the metabolites can be threatening. The irreversibility of such chemical and / or biological processes should guide the choice on pesticides with minor toxicological and ecotoxicological consequences. So the pesticide residues metabolites, often overshadowed, are those truly responsible. Therefore, is not it fair to mention the Maximum Residue Limits (MRLs) before any transformation and after, so that the problematic of food-borne poisoning pesticides-related could really be apprehended? Also, with the impressive number of pesticide metabolites more toxic [51-57] and more persistent than their parent, detected along the food chain, the use of these foodstuffs can generate toxicological consequences often unknown and/or overlooked.
Pesticide usefulness guidelines
It is difficult to assess the consequences of pesticide use, including the various complex and interdependent environmental factors. Originally, it should be stated at the outset that pesticides undergo the same storage mode followed by a lack of control and evaluation before use for possible denaturing. Thus, the pesticides risks losing their intrinsic values are tremendous. In addition, several pesticides are often used for selective weeding crops such as metolachlor (corn, sunflower, sorghum, peanut, bean, soybean), fluometuron (wheat, barley, corn, rye, oats, rice, sugar cane, cotton), diuron (pear, apple, quince, vegetables) and so on. However, we must be realistic and assert that each harvest type has its specific insect pest targets. And, the pesticide ineffectiveness on some of these crops may deplete their agricultural yield and contribute to the product release into the environment. Moreover, taking into account detection limits, and for a more targeted application, analytical research of pesticide metabolites on a variety of crops has proven the efficacy of the compound’s convenience on a one-dimensional monoculture. In sum, we must develop a policy of rational measures relating to the usefulness of pesticides «a pesticide a one-dimensional monoculture» motivated by multidisciplinary lessons drawn from environmental research carried out on pesticide metabolites in the context of the globalization of strategies. Thus, there are still recommendations necessary to be adopted as benchmarks for sustainable agriculture:
- Integration of European directives [1];
- Analytical methods findings accessibility;
- Certification of the effectiveness of the pesticide's usefulness in monocultures;
- Improvement of storage conditions for pesticides;
- Optimization treatment techniques into minimizing the content of pesticide metabolites;
- Utilization of pesticides in adquation with the local cultures according to the climatic conditions.
With the strong integration of pesticides into modern agriculture, European pesticide legislation places more emphasis on risk assessment, source control measures and substitution. However, the substitution principle also obeys the above measures.
Conclusion
This paper has examined some publications related to the analysis of pesticide metabolites in various environmental samples. Somewhat, this review quantifies the metabolites of each pesticide in its investigative matrix, without forgetting their complexity. It is therefore sustainable that pesticide metabolites are potential environmental contaminants, particularly in the agri-food sector. This document also proposes a policy on the usefulness of pesticides with particular emphasis on some requests made in terms of comments and/or guidelines for improving food safety.
Acknowledgment
Service of Cooperation and Cultural Action of the Embassy of France in Senegal (763818C) who has funded this work.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- European Parliament and Council, “Directive 2013/39/EU of 12 August 2013 amending Directives 2000/60/EC and 2008/ 105/EC as regards priority substances in the field of water policy,” Official Journal of the European Union. 2013.
- Mendy A, Thiaré DD, Bodian ET, Sambou S, Sarr I, Gaye-Seye MD, Coly A. Micellar-enhanced thermochemically induced fluorescence derivatization (ME-TIFD) method for the determination of metolachlor herbicide residues in water. Chemical Thermodynamics and Thermal Analysis. 2021;3-4: 100009. doi: 10.1016/j.ctta.2021.100009.
- USEPA. United States Environmental Protection Agency. Research program description-Ground water research. EPA/600/9-88/005. USEPA: Washington DC, 1998.
- WHO/FAO/UNEP, Children are facing high risks from pesticide poisoning. Geneva, 24 September 2004. http://www.who.int
- Pesticide Residues in Food and Feeds. Codex Pesticides Residues in Food Online Database. https://tinyurl.com/3yskkktd
- Liu Y, Zhao E, Zhu W, Gao H, Zhou Z. Determination of four heterocyclic insecticides by ionic liquid dispersive liquid–liquid microextraction in water samples. J Chromatogr A. 2009;1216:885-891. doi: 10.1016/j.chroma.2008.11.076.
- Xu X, Yang H, Wang L, Han B, Wang X, Lee FSC. Analysis of chloroacetanilide herbicides in water samples by solid phase microextraction coupled with gas chromatography-mass spectrometry. Analytica Chimica Acta. 2007;591:87-96. doi: 10.1016/j.aca.2007.03.044.
- Gonçalves C, Carvalho JJ, Azenha MA, Alpendurada MF. Optimization of supercritical fluid extraction of pesticide residues in soil by means of central composite design and analysis by gas chromatography-tandem mass spectrometry. J Chromatogr A. 2006 Mar 31;1110(1-2):6-14. doi: 10.1016/j.chroma.2006.01.089. Epub 2006 Feb 14. PMID: 16480994.
- Mai H, Gonzalez P, Pardon P, Tapie N, Budzinski H, Cachot J, Morin B. Comparative responses of sperm cells and embryos of Pacific oyster (Crassostrea gigas) to exposure to metolachlor and its degradation products. Aquat Toxicol. 2014 Feb;147:48-56. doi: 10.1016/j.aquatox.2013.11.024. Epub 2013 Dec 7. PMID: 24378469.
- Schummer C, Salquèbre G, Briand O, Millet M, Appenzeller BM. Determination of farm workers' exposure to pesticides by hair analysis. Toxicol Lett. 2012 Apr 25;210(2):203-10. doi: 10.1016/j.toxlet.2011.11.019. Epub 2011 Nov 28. PMID: 22154536.
- Yurchenko OV, Radashevsky VI, Hsieh HL, Reunov AA. Ultrastructural com-parison of the spermatozoa of the Pacific oyster Crassostrea gigas inhabitingpolluted and relatively clean areas in Taiwan. Aquat Ecol. 2009;43:513-519. doi: 10.1007/s10452-007-9161-8.
- Picó Y, Farré M, Soler C, Barceló D. Confirmation of fenthion metabolites in oranges by IT-MS and QqTOF-MS. Anal Chem. 2007 Dec 15;79(24):9350-63. doi: 10.1021/ac071559l. Epub 2007 Nov 17. PMID: 18020315.
- Tixier C, Sancelme M, Aït-Aïssa S, Widehem P, Bonnemoy F, Cuer A, Truffaut N, Veschambre H. Biotransformation of phenylurea herbicides by a soil bacterial strain, Arthrobacter sp. N2: structure, ecotoxicity and fate of diuron metabolite with soil fungi. Chemosphere. 2002 Jan;46(4):519-26. doi: 10.1016/s0045-6535(01)00193-x. PMID: 11838430.
- Caracciolo AB, Grenni P, Ciccoli R, Di Landa G, Cremisini C. Simazine biodegradation in soil: analysis of bacterial community structure by in situ hybridization. Pest Manag Sci. 2005 Sep;61(9):863-9. doi: 10.1002/ps.1096. PMID: 16015577.
- Sørensen SR, Albers CN, Aamand J. Rapid mineralization of the phenylurea herbicide diuron by Variovorax sp. strain SRS16 in pure culture and within a two-member consortium. Appl Environ Microbiol. 2008 Apr;74(8):2332-40. doi: 10.1128/AEM.02687-07. Epub 2008 Feb 22. PMID: 18296530; PMCID: PMC2293153.
- Gao X, Guo H, Zhang WW, Gu C. Simultaneous Determination of Amitraz and its Metabolites in Blood by Support Liquid Extraction Using UPHLC-QTOF. J Anal Toxicol. 2016 Jul;40(6):437-44. doi: 10.1093/jat/bkw044. Epub 2016 Jun 23. PMID: 27339482.
- Picó Y, Farré Ml, Tokman N, Barceló D. Rapid and sensitive ultra-high-pressure liquid chromatography-quadrupole time-of-flight mass spectrometry for the quantification of amitraz and identification of its degradation products in fruits. J Chromatogr A. 2008 Aug 29;1203(1):36-46. doi: 10.1016/j.chroma.2008.07.018. Epub 2008 Jul 11. PMID: 18656887.
- El Beit IOD, Wheelock JV, Cotton DE. Separation and characterization of dimethoate metabolites developing in soil and alkaline solution. International Journal of Environmental Studies. 1977;12:215-225. doi: 10.1080/00207237808709784.
- Scribner EA, Thurman EM, Zimmerman LR. Analysis of selected herbicide metabolites in surface and ground water of the United States. Sci Total Environ. 2000 Apr 5;248(2-3):157-67. doi: 10.1016/s0048-9697(99)00539-2. PMID: 10805236.
- Egea TC, da Silva R, Boscolo M, Rigonato J, Monteiro DA, Grünig D, da Silva H, van der Wielen F, Helmus R, Parsons JR, Gomes E. Diuron degradation by bacteria from soil of sugarcane crops. Heliyon. 2017 Dec 28;3(12):e00471. doi: 10.1016/j.heliyon.2017.e00471. PMID: 29322098; PMCID: PMC5753625.
- Review of carbaryl-Australian Pesticides and Veterinary Medicines Authority, Australia. https://tinyurl.com/3vt9yxtu
- Locke MA, Zablotowicz RM, Steinriede RW, Kingery WL. Degradation and sorption of fluometuron and metabolites in conservation tillage soils. J Agric Food Chem. 2007 Feb 7;55(3):844-51. doi: 10.1021/jf062070g. PMID: 17263484.
- Mercadante R, Polledri E, Scurati S, Moretto A, Fustinoni S. Identification of Metabolites of the Fungicide Penconazole in Human Urine. Chem Res Toxicol. 2016 Jul 18;29(7):1179-86. doi: 10.1021/acs.chemrestox.6b00149. Epub 2016 Jun 17. PMID: 27268969.
- Abass K, Reponen P, Mattila S, Pelkonen O. Metabolism of carbosulfan. I. Species differences in the in vitro biotransformation by mammalian hepatic microsomes including human. Chem Biol Interact. 2009 Oct 7;181(2):210-9. doi: 10.1016/j.cbi.2009.06.001. Epub 2009 Jun 11. PMID: 19523935.
- Siampiringue M, Chahboune R, Wong-Wah-Chung P, Sarakha M. Carbaryl photochemical degradation on soil model surfaces. Soil Syst. 2019;17:1-14. doi: 10.3390/soilsystems3010017.
- Chen S, Luo J, Hu M, Geng P, Zhang Y. Microbial detoxification of bifenthrin by a novel yeast and its potential for contaminated soils treatment. PLoS One. 2012;7(2):e30862. doi: 10.1371/journal.pone.0030862. Epub 2012 Feb 13. PMID: 22348025; PMCID: PMC3278408.
- Bicker W, Lämmerhofer M, Lindner W. Determination of chlorpyrifos metabolites in human urine by reversed-phase/weak anion exchange liquid chromatography-electrospray ionisation-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005 Aug 5;822(1-2):160-9. doi: 10.1016/j.jchromb.2005.06.003. PMID: 15994139.
- Chang PL, Hsieh MM, Chiu TC. Recent Advances in the Determination of Pesticides in Environmental Samples by Capillary Electrophoresis. Int J Environ Res Public Health. 2016 Apr 8;13(4):409. doi: 10.3390/ijerph13040409. PMID: 27070634; PMCID: PMC4847071.
- FAO/WHO Pesticide residues in food. Toxicological evaluations, World Health Organization, Geneva, 2003.
- Mandelbaum RT, Sadowsky MJ, Wackett LP. Microbial degradation of s-triazine herbicides in: Le Baron H, McFarland J, Burnside O. editors. The triazine herbicides 50 years revolutionizing agriculture. Elsevier; San Diego: California. 2008. p. 301-328.
- Laudien R, Mitzner R. Phenylureas. Part 2. Mechanism of the acid hydrolysis of phenylureas. J Chem Soc Perkin Trans 2. 2001;12230-2232. doi: 10.1039/B008535I
- Laudien R, Mitzner R. Phenylureas. Part 1. Mechanism of the basic hydrolysis of phenylureas. J. Chem Soc Perkin Trans 2. 2001;11:2226-2229. doi: 10.1039/B008532O.
- Aga DS, Thurman EM. Formation and transport of the sulfonic acid metabolites of alachlor and metolachlor in soil. Environ Sci Technol. 2001 Jun 15;35(12):2455-60. doi: 10.1021/es991264s. PMID: 11432548.
- Kolpin DW, Thurman EM, Linhart SM. Finding minimal herbicide concentrations in ground water? Try looking for their degradates. Sci Total Environ. 2000 Apr 5;248(2-3):115-22. doi: 10.1016/s0048-9697(99)00535-5. PMID: 10805232.
- Lewis SE, Schaffelke B, Shaw M, Bainbridge ZT, Rohde KW, Kennedy K, Davis AM, Masters BL, Devlin MJ, Mueller JF, Brodie JE. Assessing the additive risks of PSII herbicide exposure to the Great Barrier Reef. Mar Pollut Bull. 2012;65(4-9):280-91. doi: 10.1016/j.marpolbul.2011.11.009. Epub 2011 Dec 14. PMID: 22172236.
- Hugnet C, Buronrosse F, Pineau X, Cadoré JL, Lorgue G, Berny PJ. Toxicity and kinetics of amitraz in dogs. Am J Vet Res. 1996 Oct;57(10):1506-10. PMID: 8896693.
- Hsu WH, Kakuk TJ. Effect of amitraz and chlordimeform on heart rate and pupil diameter in rats: mediated by alpha 2-adrenoreceptors. Toxicol Appl Pharmacol. 1984 May;73(3):411-415. doi: 10.1016/0041-008x(84)90093-0. PMID: 6326347.
- Hsu WH, Lu ZX, Hembrough FB. Effect of amitraz on heart rate and aortic blood pressure in conscious dogs: influence of atropine, prazosin, tolazoline, and yohimbine. Toxicol Appl Pharmacol. 1986 Jun 30;84(2):418-22. doi: 10.1016/0041-008x(86)90150-x. PMID: 3012823.
- Amine-Khodja A, Boulkamh A, Boule P. Photochemical behaviour of phenylurea herbicides. Photochem Photobiol Sci. 2004 Feb;3(2):145-56. doi: 10.1039/b307968f. Epub 2003 Nov 21. PMID: 14872229.
- Chemtura Netherlands BV. Diflubenzuron product‐type 18 insecticide. Swedish Chemicals Agency. November 2007.
- Weselak M, Arbuckle TE, Wigle DT, Krewski D. In utero pesticide exposure and childhood morbidity. Environ Res. 2007 Jan;103(1):79-86. doi: 10.1016/j.envres.2006.09.001. Epub 2006 Nov 7. PMID: 17084836.
- Thiam A, Sagna MB. Monitoring des pesticides au niveau des communautés à la base. Rapport Régional Afrique. Pesticide Action Network Africa, Dakar, Sénégal. 2009. p. 57.
- Ding G, Bao Y. Revisiting pesticide exposure and children's health: focus on China. Sci Total Environ. 2014 Feb 15;472:289-95. doi: 10.1016/j.scitotenv.2013.11.067. Epub 2013 Nov 30. PMID: 24291629.
- WHO/FAO/UNEP, Children are facing high risks from pesticide poisoning. Geneva, 24 September 2004. http://www.who.int
- Patil SH, Banerjee K, Dasgupta S, Oulkar DP, Patil SB, Jadhav MR, Savant RH, Adsule PG, Deshmukh MB. Multiresidue analysis of 83 pesticides and 12 dioxin-like polychlorinated biphenyls in wine by gas chromatography-time-of-flight mass spectrometry. J Chromatogr A. 2009 Mar 20;1216(12):2307-19. doi: 10.1016/j.chroma.2009.01.091. Epub 2009 Jan 31. PMID: 19215926.
- Banerjee K, Patil SH, Dasgupta S, Oulkar DP, Patil SB, Savant R, Adsule PG. Optimization of separation and detection conditions for the multiresidue analysis of pesticides in grapes by comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. J Chromatogr A. 2008 May 9;1190(1-2):350-7. doi: 10.1016/j.chroma.2008.03.017. Epub 2008 Mar 12. PMID: 18371973.
- Walorczyk S. Development of a multi-residue screening method for the determination of pesticides in cereals and dry animal feed using gas chromatography-triple quadrupole tandem mass spectrometry. J Chromatogr A. 2007 Sep 21;1165(1-2):200-12. doi: 10.1016/j.chroma.2007.07.071. Epub 2007 Aug 1. PMID: 17707387.
- Walorczyk S. Development of a multi-residue method for the determination of pesticides in cereals and dry animal feed using gas chromatography-tandem quadrupole mass spectrometry II. Improvement and extension to new analytes. J Chromatogr A. 2008 Oct 24;1208(1-2):202-14. doi: 10.1016/j.chroma.2008.08.068. Epub 2008 Aug 23. PMID: 18778832.
- Walorczyk S. Application of gas chromatography/tandem quadrupole mass spectrometry to the multi-residue analysis of pesticides in green leafy vegetables. Rapid Commun Mass Spectrom. 2008 Dec;22(23):3791-801. doi: 10.1002/rcm.3800. PMID: 18973193.
- Okihashi M, Takatori S, Kitagawa Y, Tanaka Y. Simultaneous analysis of 260 pesticide residues in agricultural products by gas chromatography/triple quadrupole mass spectrometry. J AOAC Int. 2007 Jul-Aug;90(4):1165-79. PMID: 17760355.
- Alder L, Greulich K, Kempe G, Vieth B. Residue analysis of 500 high priority pesticides: better by GC-MS or LC-MS/MS? Mass Spectrom Rev. 2006 Nov-Dec;25(6):838-65. doi: 10.1002/mas.20091. PMID: 16755599.
- Kuster M, López de Alda M, Barceló D. Analysis of pesticides in water by liquid chromatography-tandem mass spectrometric techniques. Mass Spectrom Rev. 2006 Nov-Dec;25(6):900-16. doi: 10.1002/mas.20093. PMID: 16705628.
- Lacorte S, Fernandez-Alba AR. Time of flight mass spectrometry applied to the liquid chromatographic analysis of pesticides in water and food. Mass Spectrom Rev. 2006 Nov-Dec;25(6):866-80. doi: 10.1002/mas.20094. PMID: 16752429.
- Niessen WM, Manini P, Andreoli R. Matrix effects in quantitative pesticide analysis using liquid chromatography-mass spectrometry. Mass Spectrom Rev. 2006 Nov-Dec;25(6):881-99. doi: 10.1002/mas.20097. PMID: 16783795.
- Picó Y, Font G, Ruiz MJ, Fernández M. Control of pesticide residues by liquid chromatography-mass spectrometry to ensure food safety. Mass Spectrom Rev. 2006 Nov-Dec;25(6):917-60. doi: 10.1002/mas.20096. PMID: 16788925.
- García-Reyes JF, Molina-Díaz A, Fernandez-Alba AR. Identification of pesticide transformation products in food by liquid chromatography/time-of-flight mass spectrometry via "fragmentation-degradation" relationships. Anal Chem. 2007 Jan 1;79(1):307-21. doi: 10.1021/ac061402d. PMID: 17194155.
- Richardson SD. Environmental mass spectrometry: emerging contaminants and current issues. Anal Chem. 2008 Jun 15;80(12):4373-402. doi: 10.1021/ac800660d. Epub 2008 May 23. PMID: 18498180.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.