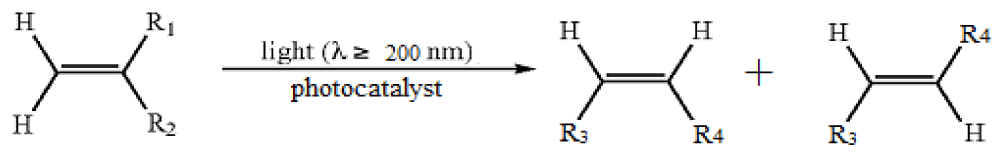Biology Group. 2021 November 30;2(11):1170-1175. doi: 10.37871/jbres1367.
12-Tungstophosphate Acids: An Efficient, Green and Recyclable Photocatalyst in Carbon-Carbon Double Bond Isomerization on Linear Alpha Olefins
Wangjing Ma1*, Xiao TC2, Liu BN2, Xu ZC1, Jin ZQ1 and Gong QT1
Abstract
The accelerated UV visible photocatalytic carbon-carbon double bond isomerization of Linear Alpha Olefins (LAO) with 12-Tungstophosphate Acids (12-TPA) as an efficient, environmentally-friendly and recyclable catalyst was described, which produced the corresponding Linear Internal Olefins (LIO) in general high selectivity and high yields.
Introduction
Carbon-carbon double bond isomerization of alkenes is of essential importance for producing high quality transportation fuels because it can lead to an increase of the octane number to improve the combustion efficiency of fuel oil. And alkenes possess higher octane number as compared to the corresponding alkanes. In addition, Linear Internal Alkenes (LIO) with internal carbon-carbon double bond has higher octane number than those Linear Alpha Olefins (LAO) with terminal carbon-carbon double bond [1]. And carbon-carbon double bond isomerisation of LAO to LIO can be used to not only obtain branched aldehydes via a consecutive isomerization/hydroformylation synthetic pathway, but also obtain branched copolymers of, e.g., 2-butene and ethylene [2]. A vast number of studies on alkene isomerisation have been carried out using both zeolites and mesoporous materials as catalysts or catalyst supports [3-6]. In several past years, more and more solid-acid were extensively used as catalyst in organic reaction [7]. Compared with conventional homogeneous catalyst, heterogeneous solid-acid catalyst was easier separated from the mixture by simple filtration, and may be recovered after activation or without activation to make the reaction more economically and practically. Amongst heterogeneous solid acid, Heteropoly Acids (HPAs) are most attractive as a catalyst in most of organic reactions due to its following properties such as not only redox property, strong Brønsted acidity, and high thermal stability, but also extremely low toxicity and environmental-friendly. What’s more, heteropoly acid possesses “pseudoliquid phase”, with a mobile ionic structure and any polar molecules absorbed in its bulk, so both the surface protons and the bulk ones were involved in the catalytic reaction. Among a various of HPAs, the best-known ones were Keggin ones, HnXn+M12O40, where X was Si, Ge; M was Mo, W when n = 4; and X was P, As, B; M was Mo, W when n = 3. n was the oxidation state of X. 12-Tungstophosphate acids of these Keggin type HPAs were widely used in all fields of organic chemistry. However, little attention was paid to 12-tungstophosphate acids as a heterogeneous photo catalyst, and therefore 12-tungstophosphate acids should be regarded as a heterogeneous photo catalyst in some organic synthesis due to that these cluster exhibit physico-chemistry properties in some fields of catalysis, materials science, analytic science, and surface and interface science Ruya RO & Wang, et al. [8,9].
In this paper, we studied that the 12-phosphotungstic (or tungstophosphate acid, 12-TPA) used as heterogeneous photo-catalyst on C=C isomerization of Linear Alpha Olefins (LAO) in the range of from 1-hexene (C6H10) to octadecene (C18H36) under ultraviolet light or visible light irradiation at not high temperature. In order to further study isomerization of olefins, we report a Keggin-type 12-tungstophosphate acids as a heterogeneous photo catalyst in C=C isomerization of terminal linear alkenes irradiated under ultraviolet-visible light or only visible light at mild temperature, shown as the following scheme 1.
Results and Discussion
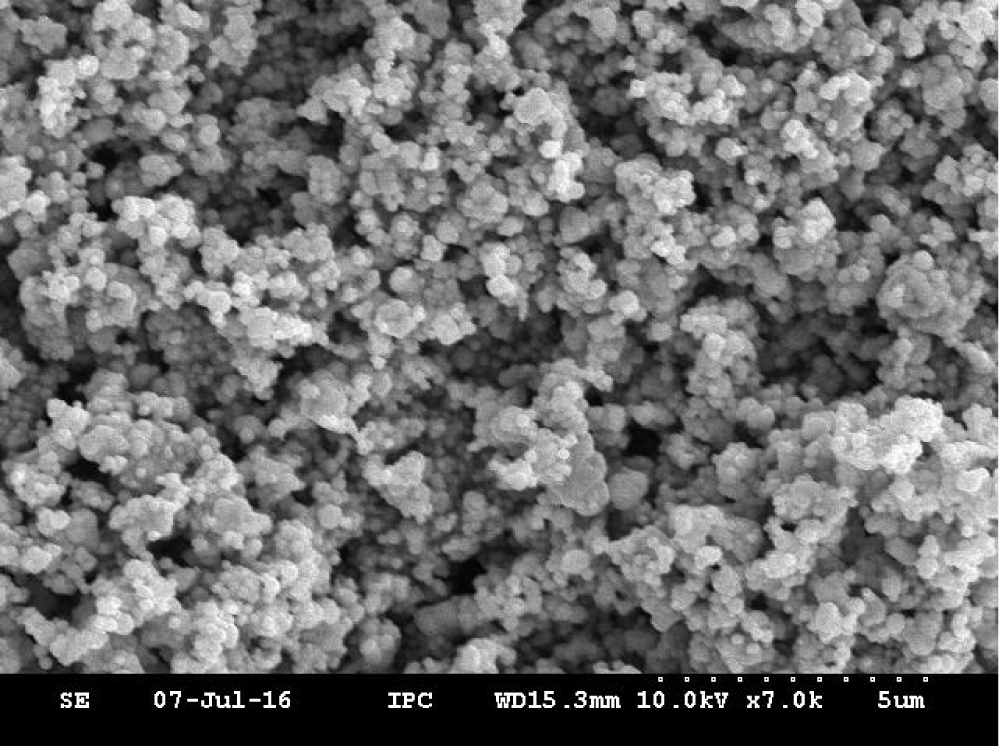
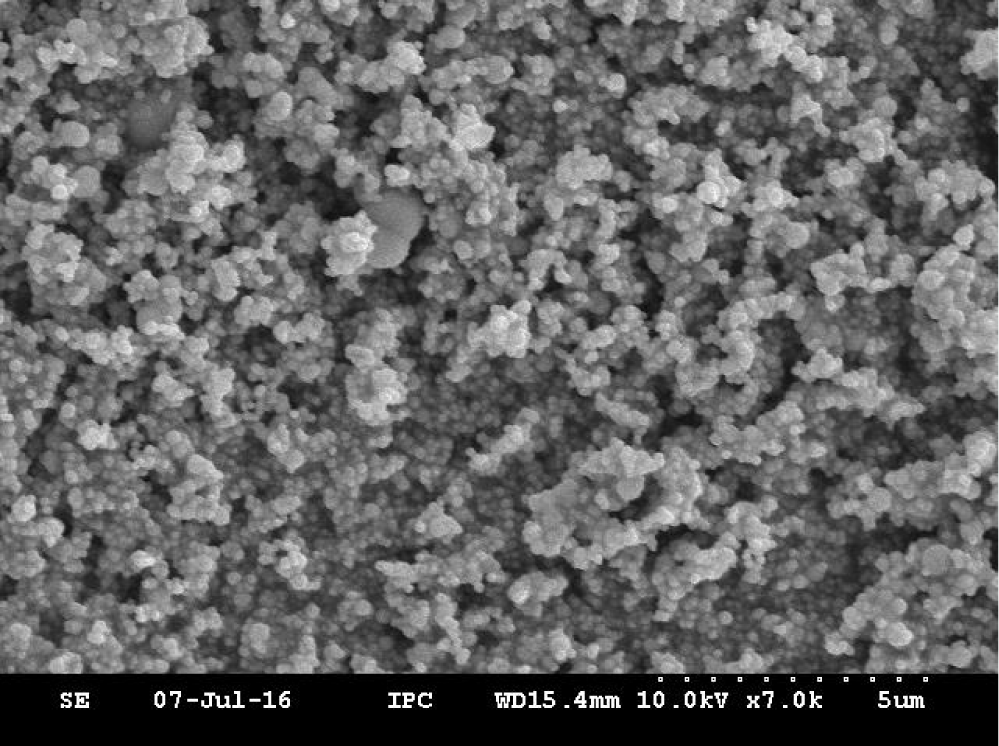
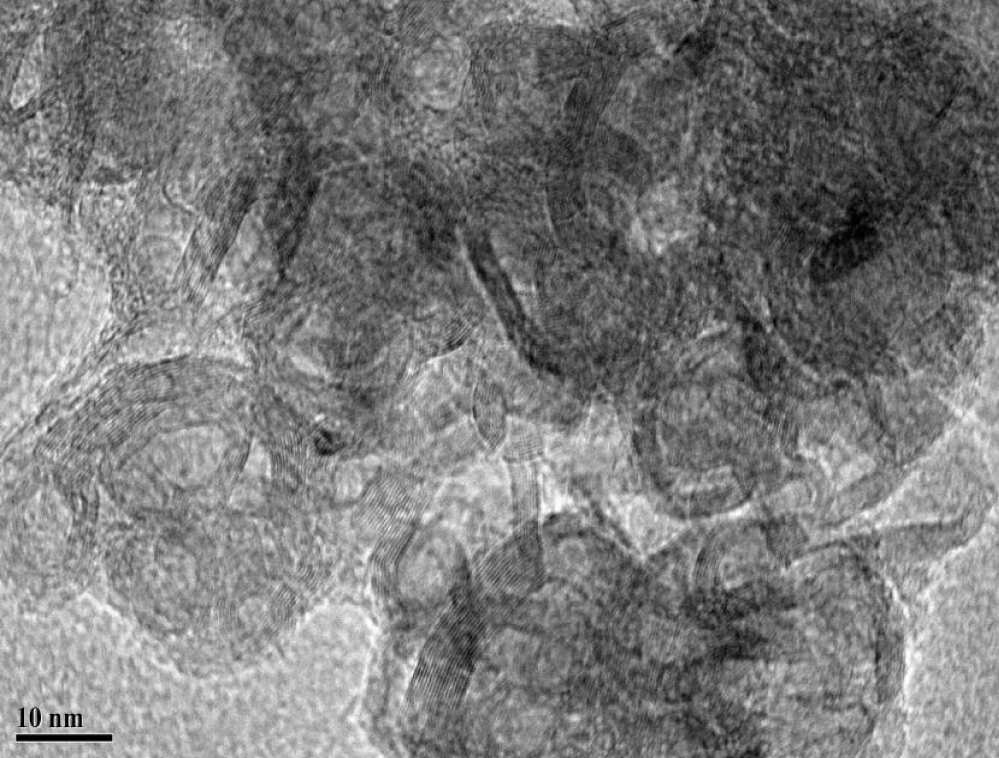
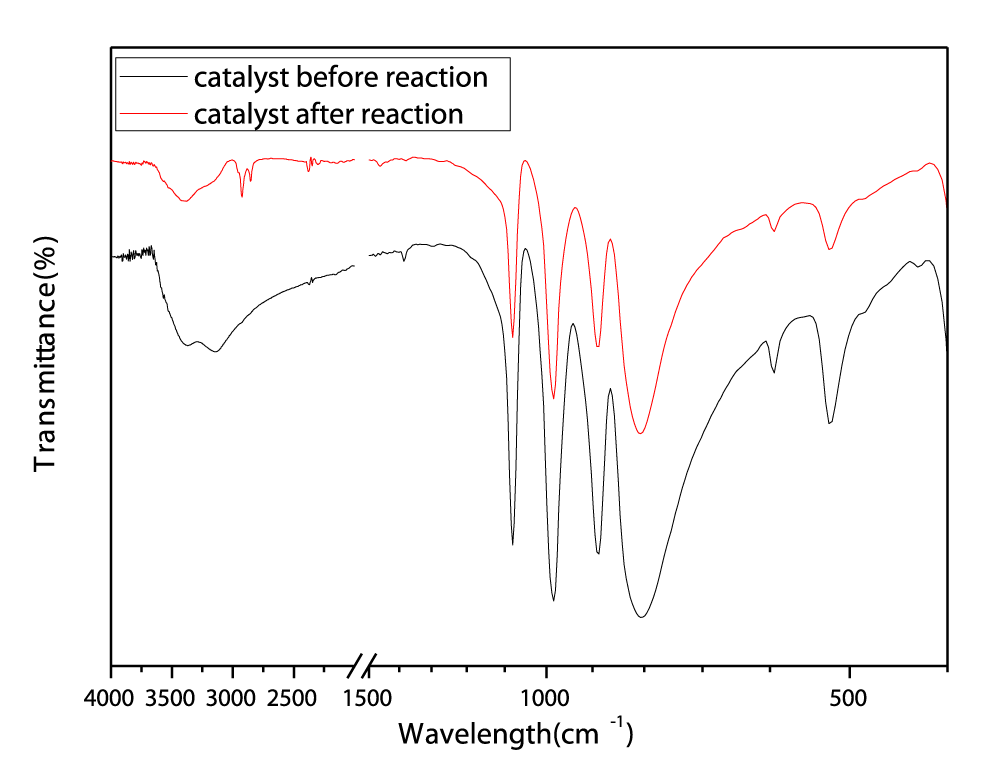
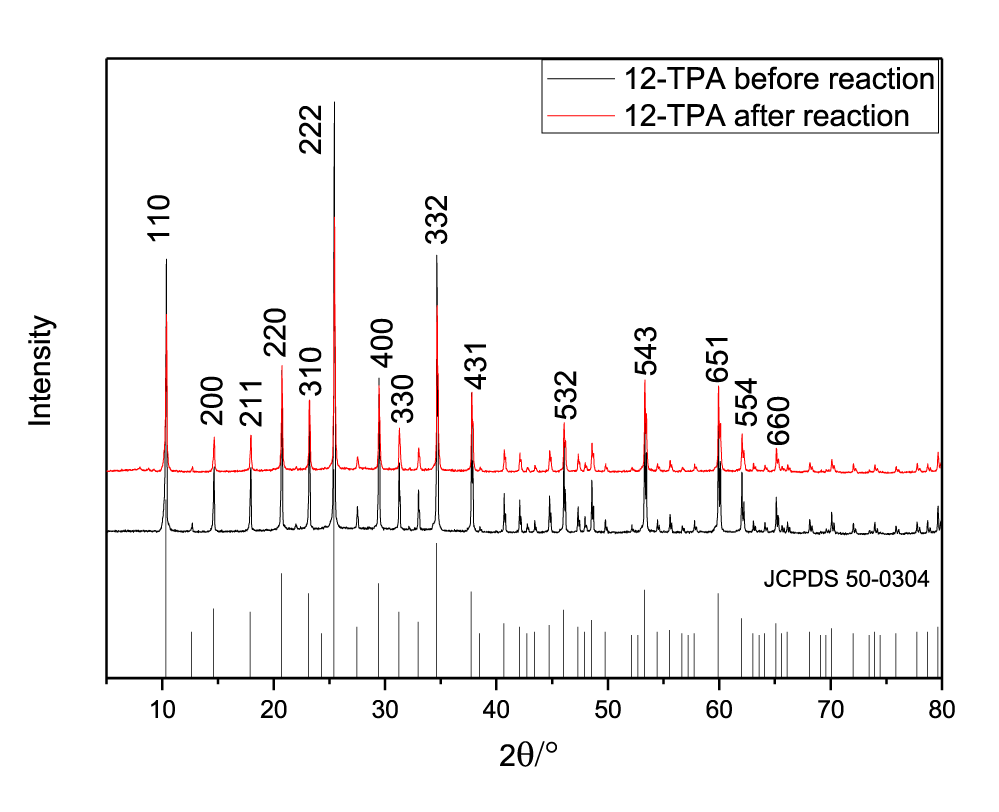
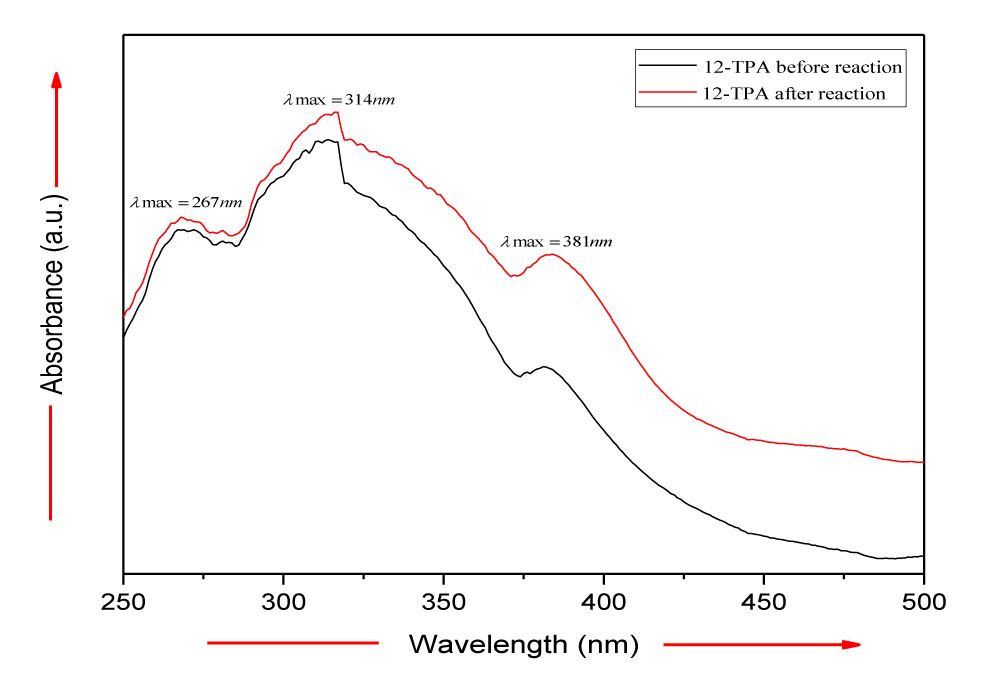
A series of photocatalysts with the Keggin structure were prepared by means of acidization-ether extraction method, which include 12-tungstophosphoric acid (12-TPA, H3PW12O40•xH2O), 12-molybdophosphoric acid (12-MPA, H3PMo12O40•xH2O) and 12-silicotungstic acid (12-SPA, H3SiW12O40•xH2O). And then samples were characterized via SEM (Figures 1,2), HR-TEM (Figure 3), FT-IR (Figure 4), XRD (Figures 5a,5b) and UV-vis-DRS (Figure 6). And the morphology of the particles of both before isomerisation reaction and after the reaction is mainly irregular and uniform-sized. From the infrared and XRD spectra of two samples, it can be seen that the samples synthesized are the same as commercial 12- phosphotungstic acid, all of which are Keggin type ones. In infrared spectrum, The four sharp peaks observed from 700cm-1 to 1100cm-1 serve as a signature of Keggin anion: 1081cm-1: P-Oa symmetric stretch in PO4 tetrahedron; 983cm-1: W=Ot stretch; 888cm-1: W-Ob-W(inter-bridges between corner sharing octahedral); 805cm-1: W-Oc-W(intra bridges between edge sharing octahedral).In XRD spectrum, The X-ray diffraction patterns were collected using German burker D8 Advance diffract meter. CuKα radiation (40 kV, 200 mA) was used. All patterns were recorded in the range 5°-80° of 2θ (increment = 0.02 and scan speed = 0.2). The measurements were performed at an ambient temperature. As far as the 12-TPA sample synthesized is concerned, the various peaks identified correspond to the main phases of keggin type 12-TPA, No major changes in the XRD patterns occurred after the carbon-carbon double bond isomerisation reaction of 1-hexene indicated that the crystal phase of 12-TPA species hardly changed in agreement with IR observations. And by using Debye-Scherrer equation (d=Bλ/βcosθ) to calculate crystalline size of a 12-TPA particle, it is known that particle size of catalysts was decreased by about 45 nm (176.3 nm-130.9nm=45.4 nm) coincide with observation of SEM images from figure 1. Three peaks at λ1 = 381 nm and λ2 = 314 nm, λ3 = 267 nm shown in figure 6. UV-Vis-DRS spectrum of 12-TPAs before and after C=C isomerisation reaction indicated that 12-tungstophosphate acids in the ground state may be excited by near-visible light, especially, ultraviolet light to excited state. And all of three peaks were assigned to the charge transfer from O2- to W6+, which lead to formation of an electron-trap center (W5+) and a hole center (O-) pairs as shown in the following equation:
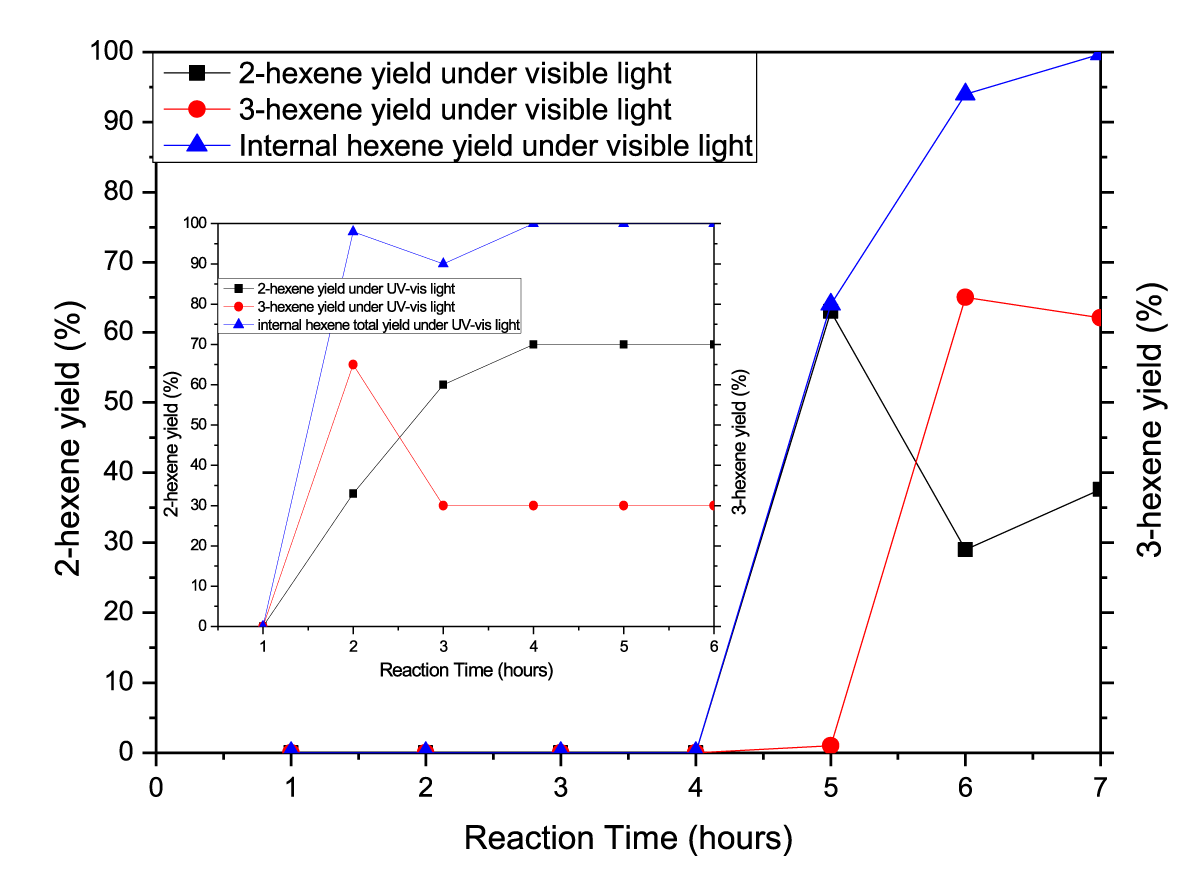
The band gap energy (Eg) of the samples prepared was calculated from the UV-Vis absorption spectra. The values of Eg obtained ranged from 2.83 to 2.87eV. There was no internal hexene produced from 1-hexene as reactant both when 12-TPA was used as catalyst in the dark at 30°C for 12 hours, and when irradiated by direct UV-vis or visible light in the absence of 12-TPA as catalyst at 30℃ for 12 hours. However, if 1-hexene as reactant was irradiated by UV-vis or visible light with 12-TPA as catalyst, a large amount of internal hexene as both 2-hexene and 3-hexene were generated at mild temperature, as illustrated in figure 7. As DBI reaction continued under visible or UV-vis light, the conversion rate of 1-hexene was gradually increased, eventually reached 100% in 7hours and 4 hours, The results showed that 12-TPA had no effect on Double Bond Isomerisation (DBI) of 1-hexene in the absence of light, while had effect on DBI of 1-hexene under visible light, and had more powerful effect on DBI of 1-hexene under UV-vis irradiation. Which suggested that the Double Bond Isomerization (DBI) of 1-hexene molecules interact with the surface tungstyl (-Wδ+-Oδ-) of 12-tungstophosphate acids in the excited state, which lead to double-bond shift (1-hexene → 2-hexene →3-hexene) reaction at 30°C; the yields of isomers increased with the extension of visible or UV-visible irradiation. Anpo, et al. [10] reported that an excited charge-transfer pairs of “M (n-1)-O-”, on the surface of some metal oxide such as TiO2, or supported V2O5 et al may react with hexene molecule to produce a free radical intermediate due to the open of C=C double bond of hexene molecule. Similarly, C=C double bond photocatalytic isomerization reaction from terminal olefins to internal olefins on 12-tungstophosphate acids crystals was carried on by the same reaction mechanism, which means that, the charge- transfer tungstyl (-Wδ+-Oδ-) interacts with 1-hexene to form the radical Intermediate (I) shown as following formula:
CH3-CH-CH2-CH2-CH2-CH3 (I)
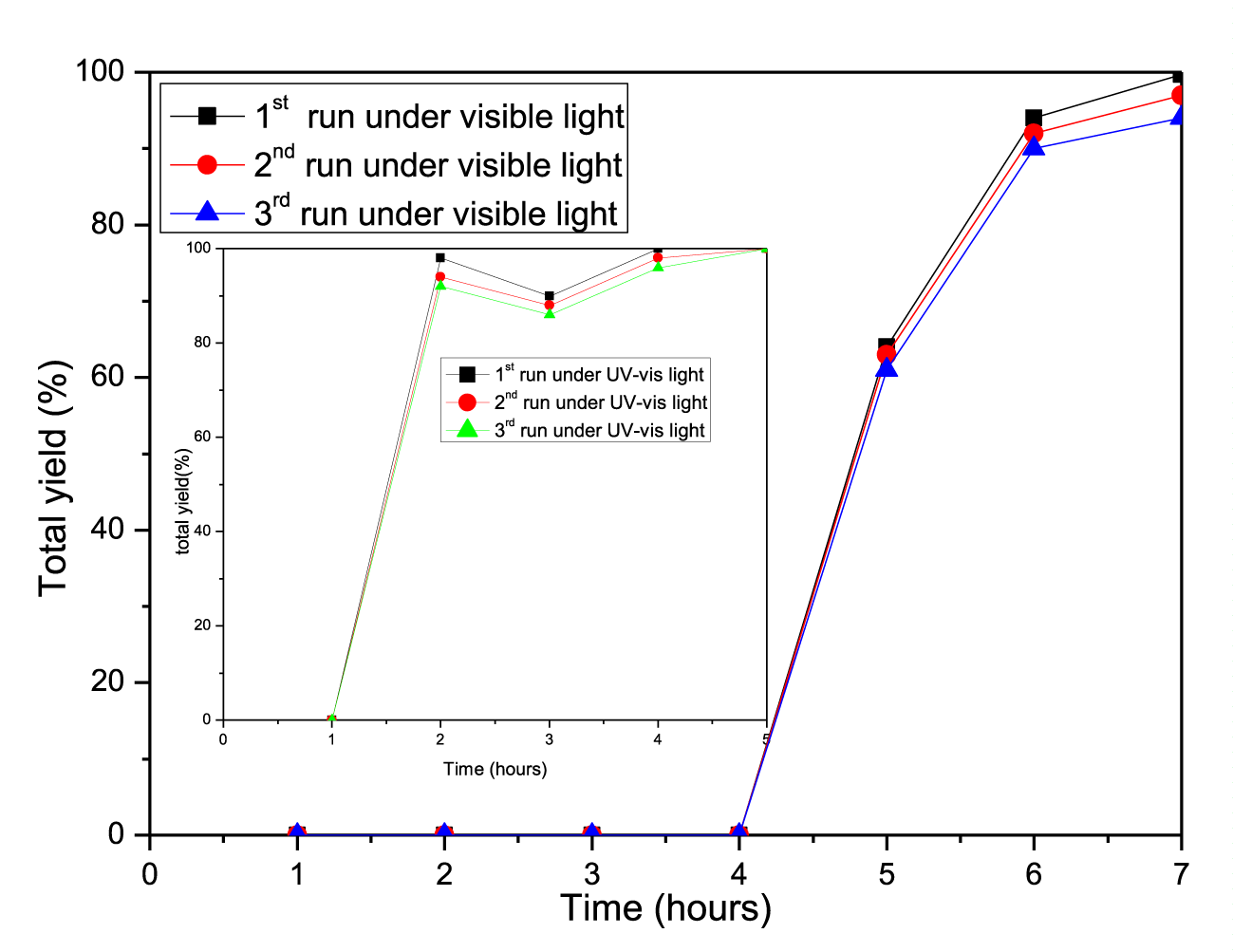
To be economical, the catalyst needs to be turn over several times, even hundreds of equivalence of internal alkenes. In order to achieve these turnover numbers, one efficient catalyst recycling loop was needed. In the photocatalytic DBI of terminal alkene to internal alkene, 12-TPA as catalyst was filtered and separated from the alkenes mixture, then dissolved in deionized water. 12-Tungstophosphoric Acid (12-TPA) containing 6 crystal water generated after the solution was evaporated at 100°C, and regenerated to be used in another double bond isomerization of terminal alkene to internal alkene. After 10 turnovers of 12-TPA in DBI of 1-hexene, 1-hexene’s conversion rate was decreased by 10% (from 100% to 90%), as shown in figure 8.
In addition to 1-hexene (1-C6H12), olefin isomerization has been carried out from α-alkenes such as1-heptene (1-C7H14), 1-octene (1-C8H16), 1-Decene (1-C10H20), 1-Dodecene (1-C12H24), 1-Tetradecene (1-C14H28) and 1-Octadecene (1-C18H36) to internal alkenes. As shown in table 1 12-TPA was used as an efficient catalyst in double bond isomerization of such terminal alkenes, and with the increase of carbon chain number in α-alkene, temperature and time required for olefin DBI reaction increase gradually, which resulted from that the longer the c=c double bond moves from terminal position to the interior one, the longer time required for DBI and the higher the activation energy required for DBI reaction of α-alkene.
| Table 1: C=C isomerization of Linear Alpha Olefins (LAO) catalyzed by 12-TPAs as photocatalyst. | |||||||
| No. | α-Alkenes | Temperature/°C | Time/h | Conversion/% | Internal Alkenes | Yield /% | Other isomers/% |
| 1 | 1-Hexene | 30 | 4 | 100 | 2-Hexene | 70 | 0 |
| 3-Hexene | 30 | ||||||
| 2 | 1-Heptene | 80 | 10 | 65 | 2-Heptene* | 57 | 0 |
| 3-Heptene | 8 | ||||||
| 3 | 1-Octene | 30 | 9 | 50 | 2-Octene | 50 | 0 |
| 4 | 1-Decene | 110 | 14 | 100 | 2-Decene | 31 | 0 |
| 3-Decene | 28 | ||||||
| 5-Decene | 41 | ||||||
| 5 | 1-Dodecene | 110 | 12 | 100 | 2-Dodecene | 20 | 9 |
| 3-Dodecene | 24 | ||||||
| 5-Dodecene | 47 | ||||||
| 6 | 1-Tetradecene | 110 | 14 | 45 | 7-Tetradecene | 25 | 20 |
| 7 | 1-Octadecene | 90 | 12 | 48 | 5-Octadecene | 36 | 12 |
| Reaction Condition: α-alkenes as reactant, 12-Tungstophosphate acid as photocatalyst, Xenon lamp as radiation source. Yield, Conversion, E/Z ratio, reaction temperature/T and reaction time/t were listed in the table. The reaction temperature was kept by a magnetic stirring heater. Conversion and E/Z ratio were determined by GC-MS analysis. * E/Z ratio of 2-Heptene of 1:1. |
|||||||
Additionally, for geometry isomerization from E-2-heptene to Z-2-heptene, the radical Intermediate (I) which is similar to the above mentioned reaction mechanism resulted from the charge-transfer tungstyl’s interaction with 1-heptene, participates in formation of Z-2-heptene and form the same quantity of Z-2-heptene, shown in the table 1.
Experimental Results
Preparation of photo catalyst
50 g sodium tungstate (Na2WO4·2H2O, Sigma-Aldrich), 5.6g concentrated phosphoric acid (H3PO4, 85%wt) and 46mL concentrated Hydrochloric Acid (HCl, 32%wt) were mixed under moderate magnetic stirring at 60°C till it became shallow yellow, then colorless after naturally cooled to room temperature. Both of 40mL ethyl ether and the solutions as above were added into a separating funnel, and then the funnel was then inverted repeatedly. The separating funnel is set aside to allow for the complete separation of the phases to form three layers. The bottom layer (heteropoly acid and ether compounds) has been removed firstly for further extraction, the middle layer (sodium chloride and hydrochloride aqueous solution) has been removed secondly, and finally the upper layer (ethyl ether) is poured out through the top into another container. After the solution of heteropoly acid and ether compounds were extracted for 5 times, and then dried in the air to form crystals and calcites at 120°C to become solid catalyst.
Characterization technique of photo catalyst
Sample was heated at 100°C for 24 h in a half-closed vessel before thermal analysis. Thermal patterns of the sample were recorded in a STA 6000 apparatus by ramping heating (20 K•min−1) the sample in a static air atmosphere or nitrogen one.
XRD analysis was carried out at room temperature in a Panalytical X’Pert Pro Multipurpose Diffractometer using monochromatic Cu Kα X-ray radiation at 40 kV and 40mA. The samples were all calcined at 100°C in a furnace for 12 h before exposed to X-ray radiation. The samples’ diffraction patterns were compared with those included in the JCPDS data base. Specific surface areas using the BET method with N2 adsorption at 77 K and pore size distribution by means of N2 adsorption–desorption isotherms were measured in an ASAP-2000 apparatus from Micromeritics.
Infrared spectra of the samples in KBr pellets were performed by a FTIR-8400 Shimadzu spectrometer and recorded with 2 cm-1 resolution and 200 scans. Raman spectra were recorded at room temperature on the samples in a nearly backscattering geometry using an ISA Labram micro-Raman apparatus. The excitation line was the 632.8 nm of a He–Ne laser. The laser power on the sample was kept low (about 1mW) to avoid thermal effects.
Double bond isomerization on linear alpha olefins by photo catalyst
A cylindrical quartz flask was used as a batch reactor, which was completely sealed and had a magnetic stirring to mix up reactant and dry nitrogen line to flush up it. Certain amount of catalyst was weighed and imported into the flask, which always remained at the bottom of the flask in the process of experiments and never suffered from effective loss due to agitation. In these experiments, we used 2 g of catalyst and 20 mL of 1-hexene (or other olefins). An electromagnetic mixer was used to heat and control temperature of reactant in the flask. And a universal arc lamp housings 500 Watt family was used to beam the reactant in the flask. In each experiment, we added 1-Decene and catalyst into the flask, and then flushed the flask with nitrogen at room temperature to fill with nitrogen gas in the space above the oleic acid in the flask throughout whole the experiment. We draw out 0.1 ml of samples of 1-hexene by inserting a long syringe needle through the rubber stopper at the opening of the flask once the experiment ran at the targeted time. We diluted each sample with acetone as 10 times, and then injected it into GC-MS and FTIR.
We made each analysis by using a split injection mode on SHIMADZU gas chromatography – mass spectrometer, the column was SHIM-5MScolumn with 30m×0.25mm×0.25μm. Injection Temperature: 200°C; Carrier gas: He.
Mass spectrometer detector was that Ion temperature was 200, Interface Temperature was 250°C, and solvent cut time was 1min.
Conclusion
Photocatalytic C=C isomerisation of terminal linear alkenes with tungstophosphate acids as catalyst was used in production of corresponding internal alkenes in general high selectivity and high yields.
Acknowledgement
The authors would like to thank the Oxford University Innovation in University of Oxford for financial support.
References
- Shuangquan, Dapeng, Lusi L, Zhen G, Yuanting C, Armando B, Yanhui Y. Highly selective 1-heptene isomerization over vanadium grafted mesoporous molecular sieve catalyst. Chemical Engineering Journal. 2010;165:916-923. https://tinyurl.com/2s4havcw
- TJ Mooibroek, EC Wenker, W Smit, I Mutikainen, M Lutz, E Bouwman. Homogeneous hydrogenation and isomerization of 1-octene catalyzed by nickel (ii) complexes with bidentate diarylphosphane ligands. Inorg Chem. 2013;52:8190-8201. https://tinyurl.com/ycys8ukn
- Modhera B, Chakraborty M, Parikh P, Bajaj Hari C. 1-Hexene isomerization over nano-crystalline zeolite beta: effects of metal and carrier gases on catalytic performance. Catal Lett. 2009;132:168-173. https://tinyurl.com/yckwhbfn
- Shi G, Shen J. Skeletal isomerization of 1-Hexene over sulfided Co/Co-MCM-41 catalysts. Energ Fuels. 2008;23:320-326. https://tinyurl.com/ydhxkzsw
- Rao Y, Kang J, Antonelli D. 1-Hexene isomerization over sulfated mesoporous Ta oxide: The effects of active site and confinement. J Am Chem Soc. 2007;130:394-395. https://tinyurl.com/2p94pa4n
- Rao, Kang J, Trudeau M. Investigation of the catalytic activities of sulfated mesoporous Ti, Nb, and Ta oxides in 1-hexene isomerization. J Catal. 2009;266:1-8. https://tinyurl.com/cwttcpvv
- Marci E, Bellardita, Parisi, Colbeau, Sorgues, Liotta, Palmisano. Phys Chem Chem Phys. 2013;15:13329-13342.
- Ruya RO, John F. Photocatalytic oxidation of aqueous 1,2-Dichlorobenzene by polyoxometalates supported on the NaY zeolite. J Phys Chem B. 2002;106:4336-4342. https://tinyurl.com/4pztrvsn
- Wang, Yang. Recent advances in polyoxometalate-catalyzed reactions. 2015;115:4893-4962. Chem Rev. https://tinyurl.com/4ay3mek4
- Anpo M, Yamada, Coluccia, Zecchina, Che B. Photocatalysed isomerization of butenes on MgO powders with coordinatively unsaturated surface ions J Chem Soc. 1989;85(3):609-620. https://tinyurl.com/2p95smzh
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.