> Medicine Group. 2021 November 30;2(11):1163-1167. doi: 10.37871/jbres1365.
Microbial Biofilm Infections in Tissue Implant: A Review
Sajjad Haider1*, Adnan Haider2, Bushra Bano3, Rawaiz Khan4, Nausheen Bukhari5 and Ali Alrahlah6
2Department of Biological Sciences, National University of Medical Sciences, Punjab, Rawalpindi, Pakistan
3Department of Biochemistry, Institute of Basic Medical Sciences, Khyber Medical University, Peshawar, Pakistan
4Engineer Abdullah Bugshan Research Chair for Dental and Oral Rehabilitation, College of Dentistry, King Saud University, Riyadh, Saudi Arabia
5Mohammad College of Medicine, Budni Road, Yaseen Abad, Peshawar, Pakistan
6Restorative Dental Sciences Department, College of Dentistry, King Saud University, Riyadh, , Saudi Arabia
- Biofilms
- Bacteria
- Biofilm infections
- Wound
- Implants
Abstract
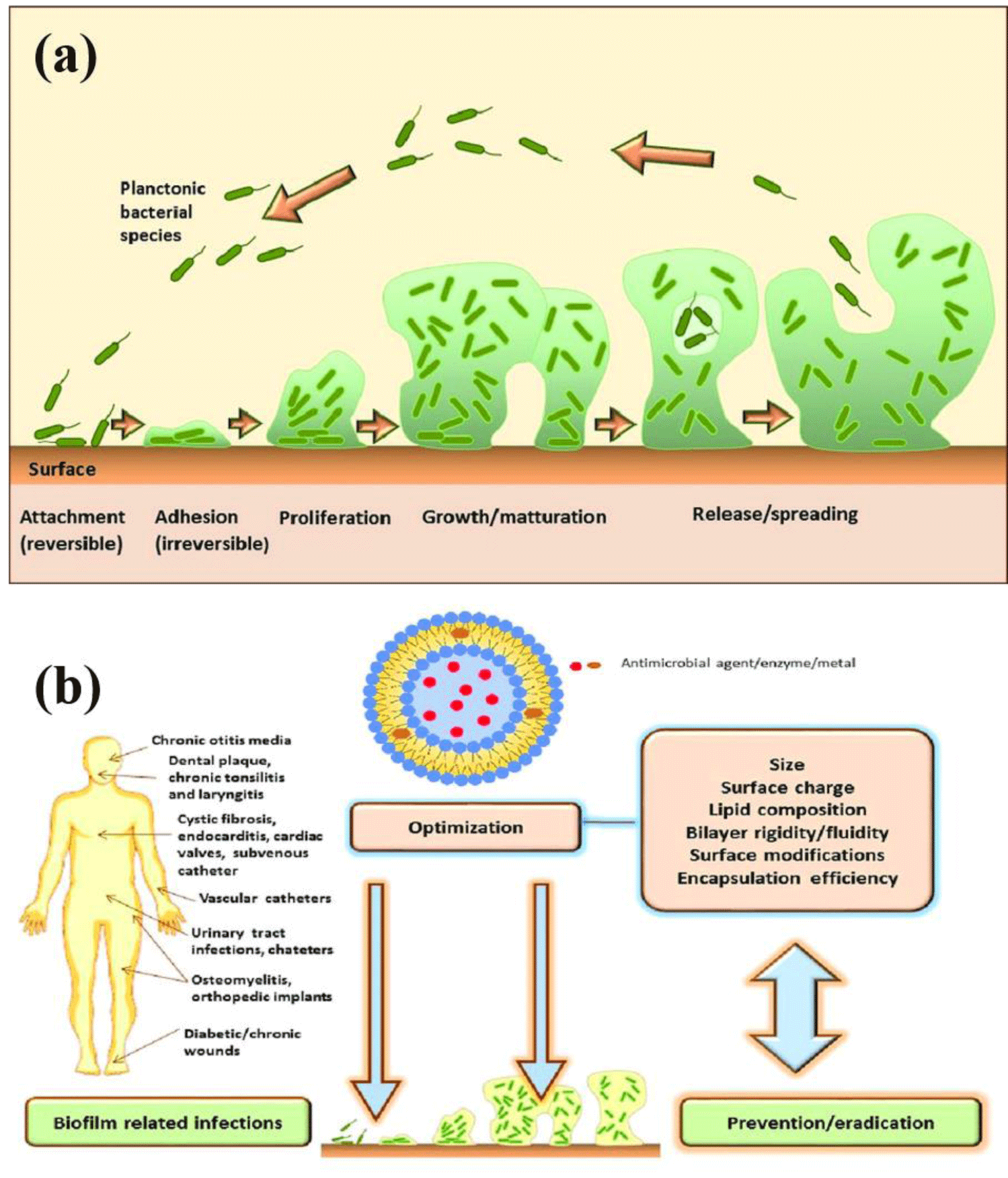
In implant and trauma surgery, implant-related infections are a significant problem. Implant-related infections are becoming more common with the increase in implant procedures. Implantation of implants has become a common and life-saving surgery. The number of hip surgeries performed worldwide is one million per year, and the number of knee surgeries exceeds 250000. More than 30% of hospital patients have one or more vascular catheters that need repair. More than 10% of hospital patients have a fixed urinary catheter. Approximately 2 million nosocomial infections cost over $11 billion each year in the United States. One of the most important risk factors is exposure to intrusive medical devices. Current treatment approaches have serious consequences for individuals and often fail to eradicate the disease. The increased likelihood of infections becoming chronic is due to effective bacterial evasion tactics, with biofilm formation being an important factor in bacterial persistence. The presence of foreign material promotes biofilm formation, contributing to the persistence of infection. Therefore, there is great interest in eradicating the disease in the planktonic phase (free-swimming bacteria) before biofilm transformation occurs and avoiding reinfection after antibiotic or surgical therapy. This mini-review reviews the literature on the implant, associated infections, their mechanism, and strategies used to prevent these infections.
Introduction
Many surgical procedures require the implantation of a tissue-derived or inert implant deep within the body. Although a large proportion of these implants are not colonized by bacteria, a tiny minority develop a biofilm that harbours aggressive germs. Unresolved periprosthetic infections in orthopaedic surgery can result in implant loosening, arthrodesis, amputations, and even death The competition between host macromolecules, bacteria, and tissue cells for surface leads to either successful tissue integration or implant infections. Bacteria can invade the body regardless of the method or sterility of the environment. At the time of closure, pathogenic microorganisms such as S. aureus were found in approximately 90% of clean wounds. Even under laminar airflow conditions, it is difficult to produce a predictably sterile wound. Despite following sanitary rules, a considerable proportion of implant infections emerge biologically [1-3]. Infections are an issue, in the medical field, that relies on foreign body implantation. While a wide range of organisms can be detected in deep orthopedic infections, Gram-positive pathogens such as Staphylococcus aureus (S. aureus) and coagulase-negative staphylococci are the most prevalent [4-8]. Foreign materials reduce the onset of microbial infection and cause local immunosuppression. Improved biomimetic properties may minimize infection caused by implants. Exciting studies are currently underway to reduce implant-related infections [2,3]. The precise release of antibiotics [9-12] or silver ions from the surface of the implant [12,13] are the recently established therapies. While both therapies have a significant benefit, issues such as tissue damage have fueled the long-term hunt for antimicrobial alternatives. This mini-review reviews the literature on the implant, associated infections, their mechanism, and strategies used to prevent these infections.
Biofilm formation
It is a common misconception that bacteria exist as separate individuals in a planktonic phase. However, this misconception has been disproved by recent findings that microorganisms accumulate impetuously on divers of surfaces and attach directly at the base. The surfaces include domestic and industrial pipes, biomaterials, and medical devices such as implants and urinary catheters, as well as tissues (plant and animal) The aggregation of the microbes onto a substrate is known as a biofilm. The aggregation microorganisms may compose of mono- or poly-microbial aggregates that may comprise various bacterial ecosystems. The proximity of microbes enables the interchange of substrates, the dispersion of metabolites, and the removal of hazardous end products, allowing various species to sustain one another. Moreover, the structure of the biofilm community may guard bacteria against antimicrobial agents, shear pressure, and the immune system. There are five phases in the formation of a biofilm: first reversible adhesion (1), irreversible adhesion (2-3), maturation (4), and dispersion (5), as shown in figure 1. The first contact between the migrating planktonic bacteria and the surface is the initial point, and at this phase, it is still reversible. To defend themselves, bacteria form a monolayer and generate an extracellular matrix known as slime. Extracellular polymeric substances include polysaccharides, structural proteins, cellular wastes, and nucleic acids (EPS). The early phases of matrix synthesis are subjugated by extracellular DNA (eDNA), while polysaccharides and structural proteins take over later. Microcolonies form during these phases and proliferate rapidly, exhibiting cell-cell interactions such as quorum sensing. The biofilm now grows in three dimensions and the association is irreversible. In the final stage, some mature biofilm cells detach and disperse as planktonic cells in the environment, presumably to initiate a new cycle of biofilm production [14-16].
Strategies used to reduce infections
All infections are triggered by the adherence of bacteria to the implant surface, which means that the surface properties of the implant and the composition of the microenvironment at the implant-host tissue interface are responsible for triggering the infection. Consequently, implant surfaces with anti-adhesive and antibacterial properties are ideal to prevent implant-associated infections, either by surface coatings or immobilizations or by integrating sustained-release antibacterial agents. Several functionalizing biomaterial surfaces have been developed based on design principles, and their utility in reducing implant-associated infections has been carefully studied.
Surface coatings or immobilizations
Inhibiting the attachment of proteins, biomolecules, and bacteria to implant surfaces is a critical step in infection prevention. PEO, PEG, and their derivatives are hydrophilic polymers that can be used to prepare antiadhesive surfaces. The hydrophilic polymers have vibrant chains that provide a large exclusion volume on surfaces in an aqueous atmosphere (such as the bloodstream) by holding a surrounding aqueous sheet, hence repelling the adhesion of molecular species [17-20], cells [21,22], and bacteria Surfactants are wetting agents that are used to reduce interfacial tension. They have an amphiphilic structure, meaning they have both hydrophobic and hydrophilic segments, so they can be effortlessly adsorbed onto the surface by merely dipping the device into the surfactant solution. Synthetic and bio-surfactants are among the surfactants used in coating to reduce the adhesion of bacteria. Biosurfactants are, however, preferred over synthetic ones. Biosurfactants are microbial substances that have strong surface and emulsifying properties. They have a diverse chemical structure, including glycolipids, lipopeptides, polysaccharide-protein complexes, phospholipids, fatty acids, and neutral lipids [23-25]. Biosurfactants also offer numerous benefits over synthetic surfactants. These include better biodegradability, low toxicity, and efficiency at high temperatures or pH. Biosurfactants are antibacterial and can be utilized to create antibacterial surfaces. Biosurfactants enable the absorption of water-impermeable substrates by reducing the surface tension at the phase boundary, therefore averting the adhesion of bacteria. Albumin is a renowned protein that inhibits microbial adherence. Albumin is a simple protein that is found in both plants and mammals. Surface modifications of albumin include physical and chemical coatings. It has been observed that the adherence of bacteria is reduced in the presence of a physically adsorbed albumin coating [25]. This is distinct from PEG and may be related to albumin's molecular structure.
Controlled release of antibacterial agents
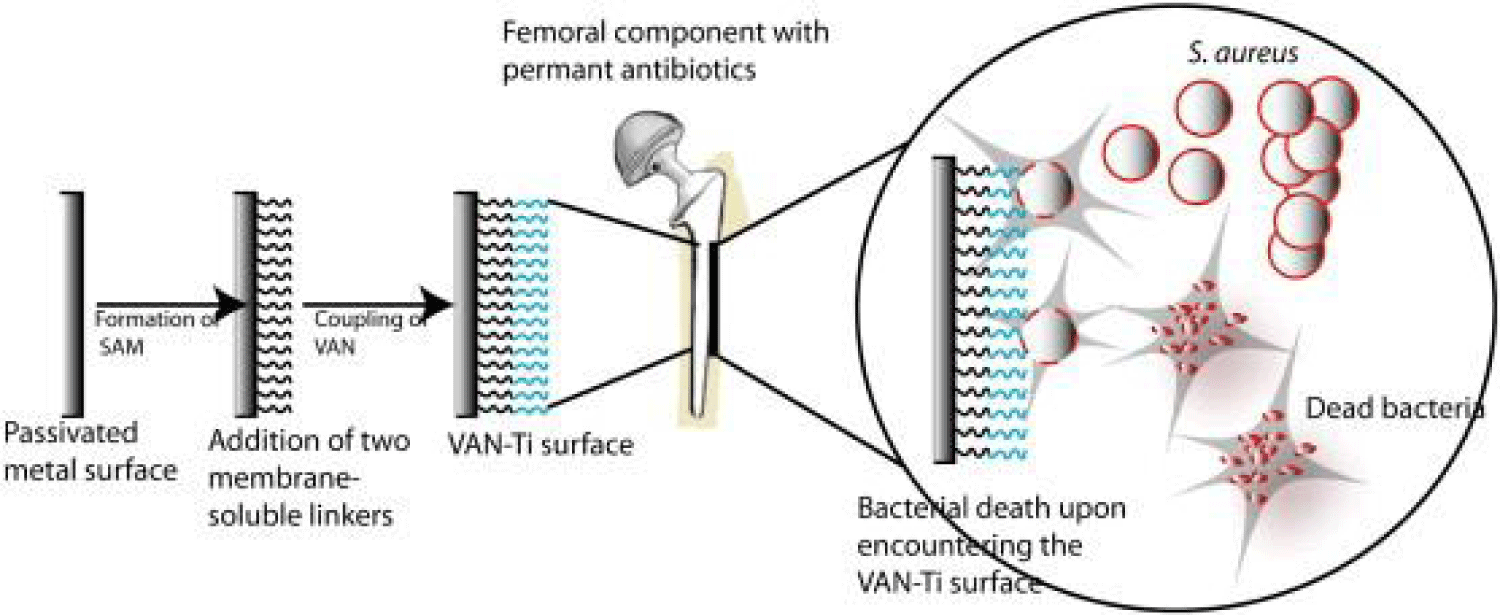
These chemicals, which include silver, titanium, copper, and their derivatives, are used to retard the spread of infection (Figure 2). Silver has long been used as an antibacterial agent. It has long been used in the treatment of burn wound infection. The dynamic form of silver, such as Ag+ or Ag0 nanocrystalline, must be soluble [26]. n solution, Ag0 occurs in a subcrystalline state with fewer than eight atoms, while Ag+ may be present due to dissociation of ionic silver compounds such as silver nitrate and silver sulfadiazine [27]. Because silver ions can easily attach to negatively charged proteins, RNA, and DNA, it poisons respiratory enzymes and components of the microbial electron transport system and impairs some DNA functions. Antibiotics are chemical substances that suppress or eliminate the development of microorganisms including bacteria, fungi, and protozoa. They are either bactericidal or bacteriostatic. Bactericidal directly kills bacteria, but bacteriostats prevent them from multiplying [28]. Systemic antibiotics are the most commonly used therapy to treat infections. They are costly and often fail, leading to complications. An antibody, as we all know, is a "Y-shaped" protein that the immune system uses to recognize and kill foreign entities such as bacteria [29]. The Fab (fragment, antigen-binding) region is located at the tip of the forked section of the "Y." The Fc (Fragment, crystallizable) region has two variable domains that may recognize and bind to epitopes on certain antigens. Opsonization is another term for this process. The Fc region is the stem of the "Y," and it is made up of two hefty chains. Immune system cells such as neutrophils, monocytes, and macrophages may identify it. As a result, the antibody works as a bridge to assist immune system cells in phagocytizing foreign substances. Antibodies have been employed in the treatment of a wide range of disorders, including tumours, infections, asthma, inflammation, arthritis, and osteoporosis [29].
Self-sterilizing surfaces and material
Effective biocides have been described for cationic compounds containing quaternary ammonium or biguanide groups. The cationic groups can engage electrostatically with the bacterial cell surface, rupturing the cell membrane and causing the escape of K+ ions and other cytoplasmic contents [30,31]. The method of forming quaternary ammonium or biguanide groups on the surface of a material can therefore lead to "self-sterilizing" materials for biomedical applications. Quaternary ammonium or phosphonium structures can be generated in two ways: in polymer structures or on the surface of substrates. The first phase consists of adding an alkyl chloride into the polymer backbone, followed by tertiary amines quaternization. The second phase reverses the procedure by incorporating tertiary amine structures into the polymer as pendants, followed by alkyl chloride quaternization. Both options are viable. The antibacterial activity is mainly determined by the size of the alkyl chain of the quaternary ammonium. Long-chain cans lead to an improved antibacterial effect [23].
Bioactive materials
Chitosan is a naturally occurring polymer having amino and hydroxyl as pendant groups. Chitosan is highly biocompatible and biodegradable. These properties make chitosan ideal for a variety of applications, including food preservation, dressings, and scaffolds for tissue engineering [32,33]. Furthermore, chitosan has antimicrobial properties [33]. Chitosan also has antibacterial activity [33]. Although the antibacterial activity mechanism of chitosan and its derivatives is not known, the most logical theory is that positively charged chitosan molecules interact with negatively charged cell membranes, causing proteinaceous and other intracellular components to leak out. In addition, chitosan can bind metal ions, preventing the production of toxins and the growth of microorganisms. In addition, several studies have shown that chitosan can penetrate the membrane, bind to DNA, and interfere with the formation of mRNA and proteins [33].
Conclusion
Implantation of implants has become a common and life-saving surgery. The number of implant-related surgeries performed worldwide is increasing, for an instant, the number of hips implant is one million per year, knee surgeries exceeds 250 000. More than 30% of hospital patients have one or more vascular catheters that need repair. More than 10% of hospital patients have a fixed urinary catheter. Hence, it has become imperative to find a way to reduce implant-related infection. The rising antimicrobial resistance has changed the current research by applying new techniques and therapies to reduce bacterial growth or division that leads to cell death or dormancy, trigger biofilm breakdown or investigate methods to avoid biofilm formation in the first place.
References
- Hickok NJ, Shapiro IM. Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev. 2012 Sep;64(12):1165-1176. doi: 10.1016/j.addr.2012.03.015. Epub 2012 Apr 4. PMID: 22512927; PMCID: PMC3413739.
- Jarvis WR. Selected aspects of the socioeconomic impact of nosocomial infections: morbidity, mortality, cost, and prevention. Infect Control Hosp Epidemiol. 1996 Aug;17(8):552-557. doi: 10.1086/647371. PMID: 8875302.
- Stamm WE. Infections related to medical devices. Ann Intern Med. 1978 Nov;89(5 Pt 2 Suppl):764-769. doi: 10.7326/0003-4819-89-5-764. PMID: 717950.
- Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996 Apr;78(4):512-523. doi: 10.2106/00004623-199604000-00005. PMID: 8609130.
- Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: A retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001 Nov;(392):15-23. PMID: 11716377.
- Sanderson PJ. Infection in orthopaedic implants. J Hosp Infect. 1991 Jun;18 Suppl A:367-375. doi: 10.1016/0195-6701(91)90043-8. PMID: 1679802.
- Darouiche RO. Antimicrobial approaches for preventing infections associated with surgical implants. Clin Infect Dis. 2003 May 15;36(10):1284-1289. doi: 10.1086/374842. Epub 2003 May 9. PMID: 12746774.
- Harris LG, Richards RG. Staphylococci and implant surfaces: a review. Injury. 2006 May;37 Suppl 2:S3-14. doi: 10.1016/j.injury.2006.04.003. PMID: 16651069.
- Anwar H, Dasgupta MK, Costerton JW. Testing the susceptibility of bacteria in biofilms to antibacterial agents. Antimicrob Agents Chemother. 1990 Nov;34(11):2043-2046. doi: 10.1128/AAC.34.11.2043. PMID: 2073094; PMCID: PMC171995.
- Duncan CP, Masri BA. The role of antibiotic-loaded cement in the treatment of an infection after a hip replacement. Instr Course Lect. 1995;44:305-313. PMID: 7797868.
- Zhao L, Wang H, Huo K, Cui L, Zhang W, Ni H, Zhang Y, Wu Z, Chu PK. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials. 2011 Aug;32(24):5706-5716. doi: 10.1016/j.biomaterials.2011.04.040. Epub 2011 May 12. PMID: 21565401.
- Furkert FH, Sörensen JH, Arnoldi J, Robioneck B, Steckel H. Antimicrobial efficacy of surface-coated external fixation pins. Curr Microbiol. 2011 Jun;62(6):1743-1751. doi: 10.1007/s00284-011-9923-3. Epub 2011 Mar 27. PMID: 21442392.
- Juan L, Zhimin Z, Anchun M, Lei L, Jingchao Z. Deposition of silver nanoparticles on titanium surface for antibacterial effect. Int J Nanomedicine. 2010 Apr 15;5:261-7. doi: 10.2147/ijn.s8810. PMID: 20463942; PMCID: PMC2865021.
- d'Enfert C. Biofilms and their role in the resistance of pathogenic Candida to antifungal agents. Curr Drug Targets. 2006 Apr;7(4):465-470. doi: 10.2174/138945006776359458. PMID: 16611034.
- Macassey E, Dawes P. Biofilms and their role in otorhinolaryngological disease. J Laryngol Otol. 2008 Dec;122(12):1273-1278. doi: 10.1017/S0022215108002193. Epub 2008 Apr 11. PMID: 18405407.
- Bai X, Nakatsu CH, Bhunia AK. Bacterial biofilms and their implications in pathogenesis and food safety. Foods. 2021 Sep 8;10(9):2117. doi: 10.3390/foods10092117. PMID: 34574227; PMCID: PMC8472614.
- Rukavina Z, Vanić Ž. Current trends in development of liposomes for targeting bacterial biofilms. Pharmaceutics. 2016 May 24;8(2):18. doi: 10.3390/pharmaceutics8020018. PMID: 27231933; PMCID: PMC4932481.
- Andersson J, Bexborn F, Klinth J, Nilsson B, Ekdahl KN. Surface-attached PEO in the form of activated Pluronic with immobilized factor H reduces both coagulation and complement activation in a whole-blood model. J Biomed Mater Res A. 2006 Jan;76(1):25-34. doi: 10.1002/jbm.a.30377. PMID: 16250010.
- Chen H, Brook MA, Chen Y, Sheardown H. Surface properties of PEO-silicone composites: Reducing protein adsorption. J Biomater Sci Polym Ed. 2005;16(4):531-548. doi: 10.1163/1568562053700183. PMID: 15887658.
- Kim JH, Kim SC. Effect of synthesis temperature of PEO-grafted PU/PS IPNs on surface morphology and in vitro blood compatibility. J Biomater Sci Polym Ed. 2003;14(6):601-614. doi: 10.1163/15685620360674281. PMID: 12901441.
- Groll J, Fiedler J, Engelhard E, Ameringer T, Tugulu S, Klok HA, Brenner RE, Moeller M. A novel star PEG-derived surface coating for specific cell adhesion. J Biomed Mater Res A. 2005 Sep 15;74(4):607-617. doi: 10.1002/jbm.a.30335. PMID: 16035061.
- Nagelschmidt M, Saad S. Influence of polyethylene glycol 4000 and dextran 70 on adhesion formation in rats. J Surg Res. 1997;67(2):113-118. https://tinyurl.com/jku6kjyf
- Bertrand J, Bonin P, Goutx M, Mille G, Gauthier M. The potential application of biosurfactants in combatting hydrocarbon pollution in marine environments. Research in Microbiology; (France). 1994;145(1). https://tinyurl.com/5n9ak2ax
- Kitamoto D, Isoda H, Nakahara T. Functions and potential applications of glycolipid biosurfactants--from energy-saving materials to gene delivery carriers. J Biosci Bioeng. 2002;94(3):187-201. doi: 10.1263/jbb.94.187. PMID: 16233292.
- Rodrigues L, Banat IM, Teixeira J, Oliveira R. Biosurfactants: potential applications in medicine. J Antimicrob Chemother. 2006 Apr;57(4):609-18. doi: 10.1093/jac/dkl024. Epub 2006 Feb 9. PMID: 16469849.
- Atiyeh BS, Costagliola M, Hayek SN, Dibo SA. Effect of silver on burn wound infection control and healing: review of the literature. Burns. 2007 Mar;33(2):139-148. doi: 10.1016/j.burns.2006.06.010. Epub 2006 Nov 29. PMID: 17137719.
- Klasen HJ. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns. 2000 Mar;26(2):131-138. doi: 10.1016/s0305-4179(99)00116-3. PMID: 10716355.
- Walker CB, Karpinia K, Baehni P. Chemotherapeutics: antibiotics and other antimicrobials. Periodontol 2000. 2004;36:146-165. doi: 10.1111/j.1600-0757.2004.03677.x. PMID: 15330947.
- Grainger DW. Controlled-release and local delivery of therapeutic antibodies. Expert Opin Biol Ther. 2004 Jul;4(7):1029-44. doi: 10.1517/14712598.4.7.1029. PMID: 15268671.
- Tashiro T. Antibacterial and bacterium adsorbing macromolecules. Macromolecular Materials and Engineering. 2001;286(2):63-87. https://tinyurl.com/yc4w6h3c
- Sawada H, Tanba K, Tomita T, Kawase T, Baba M, Ide T. Antibacterial activity of fluoroalkylated allyl-and diallyl-ammonium chloride oligomers. J Fluorine Chem. 1997;84(2):141-144. https://tinyurl.com/569td488
- Di Martino A, Sittinger M, Risbud MV. Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005 Oct;26(30):5983-5990. doi: 10.1016/j.biomaterials.2005.03.016. PMID: 15894370.
- Kurita K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar Biotechnol (NY). 2006 May-Jun;8(3):203-226. doi: 10.1007/s10126-005-0097-5. Epub 2006 Mar 17. PMID: 16532368.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.