> Medicine Group. 2021 October 29;2(10):985-998. doi: 10.37871/jbres1341.
The State of Cervical Cancer Screening and HPV Vaccination in Africa: In the Advent of Advanced Health Care
Emmanuel Kwateng Drokow1, Clement Yaw Effah2, Clement Agboyibor3, Gloria Selorm Akpabla4 and Kai Sun5*
2College of Public Health, Zhengzhou University, Zhengzhou, China
3School of Pharmaceutical Sciences, Zhengzhou University, Zhengzhou, China
4Department of Internal Medicine, Tianjin Medical University, Tianjin, China
5Department of Haematology, Zhengzhou University People's Hospital & Henan Provincial People's Hospital Henan, PR China
- Cervical cancer
- HPV
- Screening
- Vaccination
- Africa
Abstract
Cervical cancer if not detected and treated promptly can be lethal to females. In several advanced nations, the relevance of coordinated screening services has been implemented. Nevertheless, most developing nations have not implemented a nationwide screening and vaccination programme accessible to all women owing to inadequate screening and vaccination services coupled with vaccine scepticism, misconception concerning vaccination, and lack of awareness are causing an upsurge in Africa's cervical cancer cases. Cervical cancer could be greatly reduced if comprehensive screening services and HPV vaccination are implemented. In this review, we discussed the cervical cancer incidence in Africa, factors influencing the high rate of cervical cancer in Africa, screening and HPV vaccination programs and the potential intervention and recommendations to reduce the incident and mortality rates of cervical cancer in Africa. Also, we highlighted the disadvantages and advantages of widely accessible screening tests in Africa.
Introduction
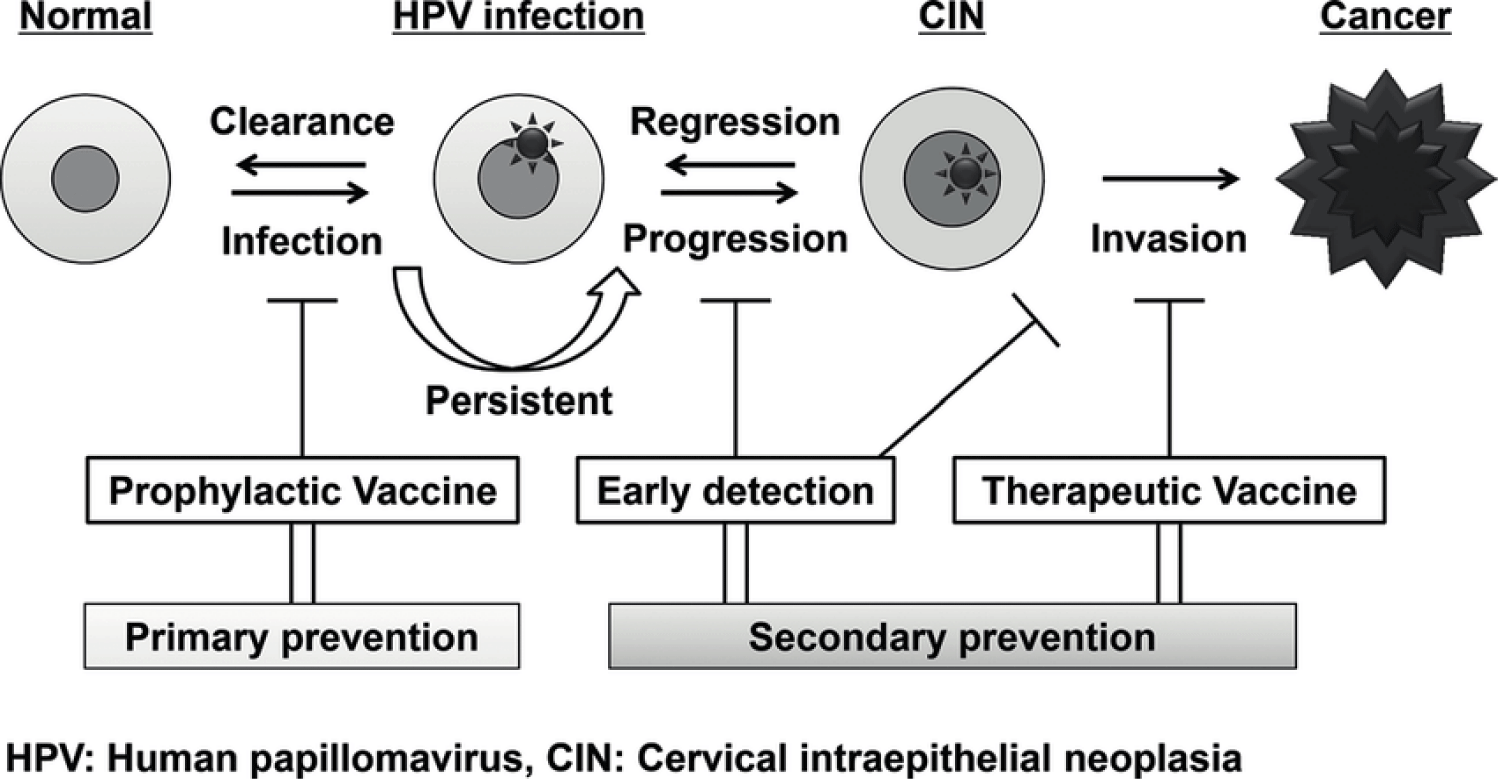
Cervical cancer is most commonly found in the cervix's "transformation zone," a region where columnar cells continually evolve into squamous cells. Human Papilloma Virus can infect any part of the anogenital epithelial tissues. However, the epithelial tissue in the transformation zone is particularly vulnerable to oncogenesis. The transformation zone is the most common location on the cervix for abnormal cells to grow [1]. Adenocarcinoma and squamous cell carcinoma are the two most common histological forms of invasive cervical carcinoma [2], accounting for around 20% and 75% of all histological forms of cervical cancers respectively. Unspecified or other histological types account for the other 5% [2]. Because of their poorer prognosis and aggressive progression compared to adenocarcinoma and squamous cell carcinoma, the 5% of rare histological forms of cervical carcinoma are generally challenging to distinguish histologically, hence, are acknowledged as a unique clinicopathological entity [3,4]. HPV infections can generally be resolved in some months with no medication [5] (Figure 1), and around 90% of infections are resolved within two years [6]. Approximately 10% of chronic infections with oncogenic HPVs grow into precancerous lesions [7], which can develop to invasive cervical carcinoma if not treated. Some non-viral causes [7] for cervical cancer comprises of long-term usage of oral contraceptives, immunodeficiency, multiparity, multiple sex partners and poor nutritional status as well as HPV infections which is the key viral factor [8-10]. Efficient preventive methods are based on a well-understood natural history of cervical cancer growth. Cervical cancer prevention can be achieved via secondary prevention and primary prevention [11].
Both preventive methods seek to break a link in the network of events that leads from HPV infections to the development of cervical cancer.
Secondary prevention centres on screening and this includes HPV testing for high risk HPV types, VIA and Pap smear test. The secondary prevention is defined as an individual or population-based intervention in detecting and treating the disease early in other to achieve a positive health outcome [12]. Secondary prevention of cervical cancer involves early detection of precancerous lesions with techniques such as cytology, Visual Inspection with Acetic Acid (VIA) or Visual Inspection with Lugol’s Iodine (VILI), HPV testing and biomarkers [13]. Incidence and mortality of cervical cancer have decrease to about 80% in advanced countries however, the situation in the developing countries is different [12]. Pap smear cytology is a screening test which has been used widely.
The cervix is accessible anatomically and easily visualized, making sample collection for screening reasonably painless and simple [12]. The efficacy of the Pap smear test in preventing cervical cancer has been proven. VIA and VILI are inexpensive, quick, low-cost point-of-care tests that physicians and qualified non-physicians can utilize in detecting changes with their human eyes [12]. Many cervical cancer-causing HPV forms are detected utilizing HPV detection techniques which detect Messenger Ribonucleic Acid (mRNA) or viral Deoxyribonucleic Acid (DNA). Cervical cancer mortality among females who had a single cycle of HPV testing reduced by 50% in a clinical investigation performed in rural India [14]. There is proof that females in most nations embrace secondary preventive strategies without cultural barriers [12]. Secondary prevention aims to diagnose and treat disease, such that loop electrosurgical excision procedure or cryotherapy are used in treating precancerous lesions [15].
Vaccination is the most highly desired method of primary cervical cancer prevention [16]. On the premise of universal HPV genotype distribution, the current HPV vaccines will decrease oncogenic HPV infections by 90% [16]. Adjuvanted non-infectious recombinant vaccines centered on Virus-Like Particles (VLPs) formed by recombinant expression of major capsid antigen L1 are currently used as prophylactic HPV vaccines. So far, the European Medicines Agency (EMA) and FDA have approved three HPV vaccines against the invasive types of HPV and to prevent HPV-associated diseases. These are the Gardasil and the Cervarix. Merck & Co. manufactured the quadrivalent Human Papilloma Virus (HPV) vaccine, which has been on the market since 2006. The vaccine works against HPV strains responsible for nearly 70% of cervical cancer infections and 90% of anogenital wart infections globally [17].
The HPV vaccine was highlighted as an effective preventive approach by several latest developments. The World Health Organization (WHO), in 2009, proposed that HPV vaccines be integrated into regular national immunization campaigns as a public health requirement [18]. Moreover, according to the 2006 Global Immunization Vision and Strategy (GIVS), one of the objectives is to present new vaccines to all qualified groups within five years of their implementation in nationwide initiatives [19]. Furthermore, significant milestones achieved between 2007 and 2011 made HPV vaccines available to many teenagers in developing nations. The cost involve in the completion of the entire HPV vaccination cycle (roughly $400 US for a three-series dose) [20,21] has been a major obstacle to its acceptability in nations with low resources until recent times when a negotiable price of $4.50 per dose was reached by GAVI, (the Global Alliance for Vaccine Initiatives), for its assisted nations [22]. To accompany this, GAVI in 2013 supported eight campaigns in African nations and it continued for two years (2014-2015) [23]. This is promising since decades have passed between the development of new vaccines and their introduction in developed nations [24].
To the best of our knowledge, the first nation to achieve over 90% coverage of HPV vaccination among teenage females (as in ages 14-25) with a collaboration of Merck pharmaceutical company is Rwanda, and this is believed to be a record in the region [25-27]. In Uganda, 88.9% of females were completely vaccinated via school-based projects assisted by Program for Appropriate Technology in Health (PATH) International and via other means of HPV subsidies [28]. In South Africa, Lesotho, Tanzania and Cameroon [29-32], a similar HPV vaccination pilot program was subsequently launched. African nations hold the greatest burden of cervical cancer worldwide. The global incidence of HPV infections is about 12%; nevertheless, a higher rate of 24% is estimated in Africa [33]. In 2018, the world's newly diagnosed cervical cancer cases were estimated to be 570,000, with 112,000 of these coming from African nations, accounting for nearly 19.6% of the global burden [34,35]. “Over one-third of all cervical cancer deaths globally occur in sub-Saharan Africa, though the region represents only 14% of the world female population [36]”.
The absence of well-structured, comprehensively coordinated preventative programs, such as the Pap smears test, and the incidence of risk factors are major contributors to this burden [37]. HIV co-infection tends to raise the likelihood of HPV infection and development by dramatically decreasing immunity [38]. Pervasive vaccination with the HPV vaccine, particularly if coupled with some preventative initiatives, is anticipated to significantly decrease the burden of cervical cancer [39]. However, the implementation of the HPV vaccination in Africa poses some challenges, attributable to lack of knowledge concerning HPV vaccine and its association to cervical cancer, questions surrounding future fertility and safeness of the vaccine, as well as external factors [40], as demonstrated in recent Cameroun [41] and Rwandan cases [26,27]. These false rumours concerning the adverse or harmful effects which may not be necessarily linked to the vaccine may influence its acceptability among the general public and significantly influence the HPV immunization campaigns, resulting in the termination of the entire initiatives, as currently witnessed in some countries [42,43].
In this review, we discussed the cervical cancer incidence in Africa, factors influencing the high rate of cervical cancer in Africa, screening and HPV vaccination programs and the potential intervention and recommendations to reduce the incident and mortality rates of cervical cancer in Africa. Also, we highlighted the disadvantages and advantages of widely accessible screening tests in Africa.
General Concept of HPV and it’s Vaccine
HPV in the pathogenesis of cervical cancer
The Human Papillomavirus (HPV) is a highly infectious and transmissible virus linked to other diseases and cancers worldwide [44-47]. The HPV viruses are non-enveloped with circular DNA that is double-stranded. The papillomavirus genome is divided into three parts; (1) genomic, (2) late and (3) early regions [48,49]. About 50% of the HPV genome comprises the early region, which includes E7, E6, E5, E4, E2 and E1 [50]. Both E7, E6 and E5 are responsible for cell transformation, E2 is for transcription, while both E2 and E1 are involved in regulating the DNA replication mechanism [50]. Tumour suppressor genes pRB and p53, which are reported to regulate a broad variety of cellular mechanisms, can be turned off by the E7 and E6 viral proteins. E7 and E6 are two genes that play a key role in oncogenesis. A current whole-genome sequencing analysis that evaluated the likelihood of viral genetic variations found that the 98 amino acids of E7, which disrupt the role of the Retinoblastoma Protein (pRB), were essential for HPV 16 oncogenesis and the development of cervical cancer and Cervical Intraepithelial Neoplasia (CIN) [51].
The three phases of persistent high risk HPV infection of the cervix are dormant, permissive, and transforming [52-54]. HPV first foray the basal cells of the epithelial through the minor epithelial tissue breaches [55] and becomes dormant as a nuclear episome. The invaded cell generally dies after virus proliferation. The E7 and E6 genes induce the growth of HPV in cells although they are rarely incorporated into DNA cellularization. This mechanism often allows for high levels of E7 and E6 oncogenes expression. The cells infected by the HPV can escape the immune defence of the hosts because the E7 and E6 proteins expressed in the basal cells are highly regulated. Undoubtedly, T cells and HPV-specific antibodies are found at low rates in a small proportion of HPV-infected females [56,57].
The Late region (L), which comprises L2 and L1, makes up 40% of the genome, whereas the “genomic regulatory region” makes up the rest [50]. The virion's structural proteins are found in the genome's late region [58]. Based on the potential of the virus to contribute to malignant transformations and facilitate infected cells proliferation, HPVs can be subcategorized into high, intermediate and low-risk oncogenic potency [59]. HPV 44, 43, 42, 11 and 6 are low-risk HPVs which causes benign cervical lesions and condylomas, but do not progress to malignant cancers [60]. HPV52, 51, 35, 33 and 31 are among the intermediate oncogenic potential HPVs and the issue of whether they induce cancerous transformations, as well as the high-risk HPV forms, is still being debated. HPV56, 45, 18 and 16 are high oncogenic risk HPVs that often induce neoplastic transformations [61].
HPV transmission
HPV infection is known to be one of the most common infection transmitted sexually. Nonsexual and sexual contact are means through which HPV infections can spread. HPV penetrates the body via skin abrasions, epidermis injuries, mucous membranes and skin-to-skin [62]. HPVs that affect the genital area are often sexually transmitted. In general, epidemiological findings have reported an association between the number of sexual partners and HPV infections, the age at first sexual encounter and the probability of one of the sexual partners having an HPV infection [63,64] As a result, the number of the sexual partners is just as important as the person's sexual conduct in HPV-related cancers such as urethra, penile or cervical cancers [63,65]. HPVs can be transmitted from mother to child via perinatal transmission during birth, which is also seen in transmitting other viral and microbial infections [48].
Horizontal HPV transmission has been observed, with the first case being a 5-year-old boy who contracted HPV2 and developed warts on his anus and hands as a result of genital-finger transmission. Cervical cancer is caused by more than just HPV [66]. HPV is responsible for 90% of cervical cancer, with two-thirds of cases occurring in developing nations [67]. Other risk factors include immune suppression, high parity, tobacco smoking, hormonal oral contraceptives usage, sexually transmitted infections (HIV, herpes simplex virus, Chlamydia trachomatis) [68,69]. Hence, counselling teenagers at a younger age concerning reducing the number of sexual partners, initiation of sexual activities at early age and avoiding the usage of tobacco may aid in reducing cervical cancer. Oxidative Stress (OS) and chronic inflammation have recently been shown to play a role between carcinogenic processes and the pathogenesis of HPV infections [70].
HPV vaccination
Gardasil-9, Gardasil (Merck & Co, PA, USA) and Cervarix (GSK, UK) are the three commercially available vaccines used in preventing HPV-18 and -16-associated cancers and other HPV related infections. Cervarix offers protection against the most prevalent HPV-18 and HPV-16 oncogenic genotypes, which are known to be responsible for causing about 70% of all cases related to cervical cancers [71]. Gardasil targets HPV-11 and HPV-6, which causes about 90% of all cases related to genital warts [72], in addition to targeting HPV-18 and HPV-16 oncogenic genotypes. Gardasil-9 (Merck & Co., PA, USA), a nine-valent vaccine that protects against HPV-58, HPV-53, HPV-45, HPV-33, HPV-31, HPV-18, HPV-16, HPV-11 and HPV-6, was approved by the Food and Drugs Authority in 2014. The five-other new HPV oncogenic genotypes that are targeted by Gardasil-9 can cover HPV strains associated with a further 20% of cervix carcinoma cases. Therefore, Gardasil-9 has the ability to offer protection against about 90% of cervical cancer cases [73]. They are multivalent subunit, recombinant vaccines containing Virus-Like Particles (VLPs) obtained from HPV types 18 and 16 L1 proteins. According to a comparative modelling analysis, if the worldwide policy of combining twice screening in a lifetime and a comprehensive mapped HPV vaccination is implemented, the prevalence of cervical cancer will be decreased by 97% by 2100 [74,75]. The appropriate period for getting the HPV vaccination is before teenage boys and girls become sexually engaging. This is because the vaccine is most effective when there is no evidence of the risk of HPV infections. The global HPV vaccination initiatives averagely, constitute around 30% of the world targeted population, with several countries having limited full-dose coverage [76]. In advanced and developed countries, the HPV vaccination coverage is substantially higher, with approximately 32% of young women between ages 10-20 years completing the entire vaccination cycle by 2014 [76,77]. In countries like Sweden, Denmark and Australia, the vaccination coverage is over 60% [76,78,79]. Since the vaccination campaign was initiated about ten decades ago, findings have shown that the HPV vaccines have significantly decreased the occurrences of cervical pre-cancer in teenage girls [80].
Furthermore, women who were not vaccinated also exhibited a decreased risk of the disease, implying that these women's "herd immunity" resulted in significant protection. Interestingly, most developing nations are still vulnerable, with just around 1% of female adolescents receiving a complete cycle of the HPV vaccines [76]. Nevertheless, HPV vaccination was effectively implemented into national regular immunization programs in some developing nations, such as Rwanda [27], with higher coverage been achieved. South Africa, Lesotho, Tanzania and Cameroun [29-32], have all recently launched HPV vaccination pilot campaigns. Kenya recently became the first African nation to obtain support from the Global Alliance for Vaccine Initiatives (GAVI) in rolling out its HPV vaccination pilot initiatives [23]. GAVI subsequently declared in 2014 that it would support the first state wide implementation of HPV vaccination in Rwanda for females of all qualified ages, in addition to other HPV project implementation in Tanzania, Sierra Leone, Niger, Malawi, Madagascar, Ghana, Zimbabwe, and Mozambique [23]. Unfortunately, HPV vaccination disparity seems to exist between rural and urban residents living in developing nations.
In 2014, the World Health Organizations (WHO) Strategic Advisory Group of Experts (SAGE) advocated a two-dose HPV vaccines strategy for females under the age of 15. The Immunogenicity achieved among girls between the ages of 15-18-years-old who received a two-full dose of quadrivalent HPV vaccines were not inferior to girls who received a full dose of three in the same age group, per an ongoing study reports [81]. A recent study revealed that one dose of HPV vaccine is equally effective as three or two doses of the vaccines [82]. If the effectiveness of a one-dose HPV vaccine can be demonstrated in well-controlled trials, it will lower the cost, which is the main barrier to vaccination campaigns being implemented in developing countries. The literature suggests that the vaccine's effectiveness lasts between six to ten years [83], even though long-term effects are still unknown.
Nevertheless, HPV vaccines can develop serum-neutralizing antibodies of high titers in humans and animals [84]. This vaccination might not be capable of producing substantial therapeutic benefits for established HPV infections that are resistant to antibody-mediated neutralization. Hence, therapeutic vaccines with the potential of eliminating pre-existing lesions or malignant tumour cells and preventing the growth of lesions should be investigated further.
Screening test for cervical cancer
Cancer can be detected at the early stages through screenings. Cervical cancer screening test which includes cervicography, HPV-DNA testing, Pap smear cytology, visual inspection with magnification devices-magnavisualizers, Visual Inspection with Lugol’s Iodine (VILI), and Visual Inspection with Acetic Acid (VIA), which are accessible globally and have demonstrated good efficiency and effectiveness [85]. In the 1930s, Lugol's iodine, which was used to the visually inspect the cervix without magnification, was the first procedure for screening the cervix. VILI was replaced by VIA because in the “single-visit approach”, the moderate specificity of VILI can lead to over- treatment and over-referral. Furthermore, it utilization in postmenopausal women was less accurate [86]. Due to the low specificity and sensitivity of 85.0% and 79.0% respectively of VIA, cervical cytology became the substitute [87]. As a result, detecting abnormal cellular changes at the transformation zone of the cervix have primarily relied on Papanicolaou (Pap) smear tests.
Since the 1950s, Pap smear tests have been the gold standard in detecting early cervical cancer lesions. It has a specificity and sensitivity of 95% and 80%, respectively, for Cervical Intraepithelial Neoplasia (CIN) [88]. The extensive use of Pap smear tests in developing nations poses a number of problems. Due to these problems, VIA, which uses the naked eyes as a screening tool in low-resource environments, was reintroduced. Notwithstanding its low specificity, it is cost-effective, yields results quickly and requires little equipment. Both the VIA and Pap smear test can effectively detect grade 2/3 CIN tumours, which are actual precancerous tumours.
In low-resource environments, the HPV test, alone or when combined with VIA, has the ability to enhance the screening of cervical cancer. However, it is costly, and demands a lengthy time for the results to be ready. It has a lower specificity (84.2 % vs. 94.5%) and higher sensitivity (90.2% vs. 41.4%) than VIA [87]. HPV testing, on the other hand, is superior to cervical cytology or VIA in that it is capable of detecting a significant number of high-risk HPV oncogenic genotypes. Cervical cytology tests, on the other hand, are superb pre-invasive cancer screening techniques. This suggests that rapid HPV testing results may be appropriate for screening and treatment in low-resource settings.
Additionally, the authors assert that, in order to prevent missed diagnoses, it is better and safer to combine two screening tests instead of using a single test alone. Studies are currently ongoing to develop a blood- and urine-based screening tests that are probably less costly and non-invasive [89].
Cervical Cancer Incidence, Screening and HPV Vaccination in Africa
Cervical cancer in Africa
In many advanced nations, cervical cancer is progressively becoming a rare condition. However, this is not the same in most African nations. Cervical carcinoma is the most prevalent cancer among females in Africa, behind breast cancer. In 2018, cervical cancer accounted for 24% of all female cancers, and it is the most common cause of cancer mortality among females in the region [90]. Cervical cancer affects approximately 75% of women residing in rural areas in Africa. Most women with cervical cancer go untreated, owing to a lack of accessibility to healthcare (both geographically and financially). Cervical cancer claims the lives of several women in Africa than any other cancer. Sadly, it strikes them at a period in their lives when they are of economic and social importance to their families.
Cervical cancer incidence in Africa
Cervical cancer cases are increasing progressively in Africa, with over 119,000 new cases and 81,000 mortality in 2018 (Table 1) [34]. This resulted in the region recording the highest mortality and incidence rate. Southern Africa recorded the highest incidence rate (“e.g., Swaziland, with the highest incidence rate), followed by Eastern Africa (Malawi, with the highest mortality rate; and Zimbabwe), and Western Africa (Guinea, Burkina Faso, and Mali”) [34]. In 2020, the region continued to record the highest incidence and mortality rates of cervical cancer, with the rate particularly high in Eastern Africa (“Malawi has the highest mortality and incidence rate worldwide”), followed by Southern and Middle Africa [91].
| Table 1: The mortality and incident rates of cervical cancer in Africa (adapted from sources) [34,91]. | ||||||||
| Northern Africa | Middle Africa | Western Africa | Southern Africa | Eastern Africa | Northern America | Southern Europe | Australia/ New Zealand |
|
| 2018 | ||||||||
| Cases | 7652 | 12635 | 31955 | 14409 | 52633 | 15502 | 9155 | 1114 |
| Mortality | 5243 | 9418 | 23529 | 6480 | 37017 | 5852 | 3512 | 403 |
| Age-standardized incidence/100,000 | 7.2 | 26.8 | 29.6 | 43.1 | 40.1 | 6.4 | 7.8 | 6.0 |
| Age-standardized mortality/100,000 | 5.1 | 21.1 | 23.0 | 20..0 | 30.0 | 1.9 | 2.2 | 1.7 |
| 2020 | ||||||||
| Age-standardized incidence/100,000 | 6.2 | 31.6 | 23.0 | 36.4 | 40.1 | 6.2 | 7.7 | 5.6 |
| Age-standardized mortality/100,000 | 3.7 | 22.7 | 16.6 | 20.6 | 28.6 | 2.1 | 2.3 | 1.6 |
The prevalence rate in African countries is 7-10 times higher than in advanced countries such as New Zealand, Austria and North America [91]. It will interest us in knowing that, due to under-reporting prevalence, the real prevalence of cervical cancer in several countries in Africa is mostly not known. Only a few regions have cancer registries that are effective in operation, but with non-existent or minimal record-keeping. The majority of the figures mentioned in literature are hospital-based, representing a small proportion of women who died from cervical cancer since most women die in the house due to the treatment cost. The low survival rates and high mortality observed in most African countries can be attributable to incomplete treatment related to poverty since most women are not able to afford the medical expenses. Also, low-quality healthcare, loss to follow up, advanced stage of tumours, late clinical presentation, comorbidities such as HIV infection, malaria, anaemia, poor nutrition and poor accessibility to health centres (worst in remote regions, where 75% of women are diagnosed with cervical cancer) are all factors attributable to low survival rates and high mortality of cervical cancer patients in Africa [92-96]. Again, treatment facilities are inadequate, and in circumstances where they are accessible, most women cannot afford these services.
Factors that influence the high incidence rate of cervical cancer in Africa
Socioeconomic factors: Cervical cancer is a more common cancer in women with low socioeconomic backgrounds around the world. Cervical cancer is mainly associated with poor women and poverty [97]. Poverty is widespread in Africa. Bayo, et al. [98] reported that, bad personal hygiene, poor social conditions and high parity were the primary factors associated with cervical cancers among HPV-infected population in Mali. Poverty, in all of its manifestations, is a major obstacle to the treatment and prevention of this cancer.
Socio-cultural factors: HPV one of the fundamental causative agent of cervical cancers, is rife in several African countries. Risk factors such as early sexual activities, early pregnancies, high parity, polygamous marriages and early marriages increase an individual's risk of acquiring HPV infections leading to carcinogenesis of the cervix and these factors are very rampant in most African countries [66,99]. Many ethnic groups in Africa accept and agree to polygamous marriages [100-101].
This act tends to increase the risk of contracting HPV. Polygamy has been found to double the risk of cervical cancer, with the risk increasing as the number of spouses increases. Early sexual activities and high parity are all common practices that have been associated with an increased risk of cervical cancer in African countries. The rise in cases have been attributed to the misconception that the vaccines render women barren, decrease the accessibility of the HPV vaccine, and lack of adherence to gynaecology consultations.
Biological factors: Many people in some African countries are immunosuppressed because of ravage infections (Tuberculosis, HIV, malaria etc.) and malnutrition. Reproductive tract infections are also common in the region. Aside from HPV infections, other Sexually Transmitted Infections (STIs) have been associated with cervical cancer. Neisseria gonorrhoea, Chlamydia trachomatis and Herpes simplex type 2 have all been linked to the development of invasive cervical cancer and Cervical Intraepithelial Neoplasia (CIN) following high-risk HPV infections [102]. The risk of cancer is increased by infections when chronic inflammatory responses are induced. This results in the generation of free radicals, which promote carcinogenesis or tumourigenesis. Cervical cancer is a commonly diagnosed cancer in HIV-positive women and is categorized as an “AIDS-defining disease” [103,104]. AIDS-associated mortality has decreased significantly since the introduction of “Antiretroviral Therapy” (ART), and HIV-positive women's life expectancy has almost equalled that of HIV-negative women [105]. Hence, the population of HIV- infected women has upsurge from 3.3 million to 18.8 million between 1990 to 2018, with 60% of these women living in Southern and Eastern Africa [106]. Persistent HPV infections are most likely to occur in women living with HIV than those without HIV. Kelly, et al. [107] carried out a study among 1395 women living with HIV in South Africa and Burkina Faso. They reported that the percentage of HIV-infected women in Burkina Faso diagnosed with CIN1, CIN2+ and CIN3+ were 94.2%, 5.8% and 2.3%, respectively, while that of South African women were 98.7%, 1.3% and 0.2%.
Stelzle, et al. [108] reported that cervical cancer risk was higher in HIV-positive women (RR 6·07, 95% CI 4·40-8·37). In 2018, 5·8% (95% CI 4·6-7·3) of newly diagnosed cases of cervical cancer were reported in HIV-positive women (33,000 new cases, 95% CI 26000-42,000), with HIV infections (28,000 new cases, 20,000-36,000) contributing to 4·9% (95% CI 3·6-6·4). Eastern Africa and southern Africa were the most affected areas. In Eastern Africa, 27·4% (23·7-31·7) of cervical cancer women (14,000 new cases, 12,000-17,000) were HIV-positive whereas, in Southern Africa, 63·8% (95% CI 58·9-68·1) of cervical cancer women (9200 new cases, 95% CI 8500-9800) were HIV-positive. The age-standardized incidence rates of HIV-related cervical cancer were higher than 20 per 100,000 in ten countries, all in Eastern Africa, Southern Africa and Western Africa.
A study in South Africa by Taku, et al. [109] reported that HIV-positive women with CIN3+ and CIN2+ had an HPV prevalence of 96.6% (142/147) and 93.5%(43/46), respectively. Furthermore, HPV prevalence was substantially higher in women living with HIV than those without HIV, and lastly, multiple types were found to be more common in women living with HIV than those without HIV (p = 0.034). According to other studies from the continent, HIV-positive women are diagnosed with cervical cancer at a younger age than HIV-negative women [110-113]. In a study conducted in Kenya, Gichangi, et al. [114] discovered that women who developed invasive cervical cancer and were less than 35 years old were 2.6 times more susceptible to be HIV-positive than women of equal age without invasive cervical cancer (35% vs. 17%, Odds Ratio: 2.6, p = 0.043).
Human resource factors: The substantial lack of trained medical professionals influences healthcare accessibility in Africa. The world health organization, in 2006, stated that 818,000 medical personnel (midwives, nurses, and doctors) are needed in Africa based on the assumption that 2.28 healthcare personnel are needed per every population of 1000 in each African county [115]. The higher cervical cancer rates in Africa, which is highly preventive, is contributing to the complexity of the problems they are facing.
Awareness and knowledge of cervical cancer in Africa
Although it has not yet been recognized as a major public health issue in Africa, cervical cancer is becoming an increasingly significant issue. A number of studies have indicated that there is a low awareness of the disease in African countries [116-118], which spans across individuals with different levels of literacy. A study by Drokow et al. in Ghana showed that 75.5% of 844 women from four selected cities in Ghana knew about cervical cancer. Out of this percentage, only 55.6%, 14.3%, 21.4% and 35.2% respectively knew that HPV infection, multiparity, early marriage and Immunocompromised/HIV-AIDS were risk factors of cervical cancer [119]. Olubodun, et al. [120] conducted a cross-sectional study among 305 reproductive age women and found that only 12.8% (39) had heard of cervical cancer. The authors concluded that the knowledge of HPV immunization, cervical cancer screening and risk factors was poor among the participants. The study revealed that none of the women had taken the HPV vaccine or had their daughters immunized as well.
Kasa, et al. [100] reported that out of 735 women recruited in their study to assess attitude and knowledge of cervical cancer in North West Ethiopia, only 7.3% of respondents had ever been screened for cervical cancer, while 172 (23.1%) were knowledgeable of the disease and 63% of the respondents had a negative attitude toward the disease. Similar studies in Tanzania, Kenya, Cameroun, Sudan and Ethiopia have reported a low level of awareness and knowledge of cervical cancer in women residing in these countries [121-126]. The time required by primary healthcare professionals in Lagos to detect and refer cervical cancer patients to a tertiary health facility for treatment and management was an essential factor in patients presenting with an advanced stage of the disease. It took an average of 9.35 to 12.9 months for primary healthcare professionals in Lagos to diagnose and refer cervical cancer patients [127]. However, this has improved with time, as recent studies by Dulla, et al. [128] and Ifemelumma, et al. [129] showed that a significant number of healthcare providers are knowledgeable of cervical cancer even though the rate of screening is very low among them.
Cervical cancer screening in Africa
Cervical cancer in its advanced stages is deadly and has serious consequences, including sexual, psychological and physical problems. Interestingly it is rarely screened in African women. None of some 500 women who attended a child and maternal health facility in a rural part of Nigeria had ever been screened [130]. Only 9% of healthcare professionals in two Nigerian healthcare facilities had ever been screened [131,132]. Many women with accessible screening services do not seek screening due to misguided perceptions regarding the risks of getting cervical cancer. Only a small percentage of women in Africa have ever had Pap smear test due to a lack of awareness and understanding of the disease, as well as the lack of accessibility and availability of screening services. This situation is no different among female healthcare workers.
Dulla, et al. [128] reported low screening practice among healthcare professionals in Southern Ethiopia even though they had good knowledge concerning the disease. A study by Adanu, et al. [133] showed that only 2.1% have had a screening test. Awodele, et al. [134] indicated that 12.5% of nurses utilized VIA while 20.5% utilized Pap smear test. Gebreegziabher, et al. [135] showed that Pap smear test among nurses in public health centres in Mekelle, Ethiopia was 10.7%. Advanced countries have over 80% of women accessing screening services, while in developing nations, there are 0.4% to 14% accessing screening services in rural areas and 2% to 20% among urban dwellers [136].
Furthermore, the majority of the screening facilities are situated in tertiary and secondary healthcare centres in urban regions. For instance, Mali lacks healthcare facilities and financial incentives needed for the implementation of regular cervical cancer screenings; as a result, these screening services are presently only available at a small number of district medical facilities, where nursing staff (midwives) or doctors could perform VIA screenings upon demand [137]. Regular Pap smear test is extremely low in Senegal, particularly among those who live in rural locations and older women [138]. Only about three of Senegal's 13 rural areas have begun screening initiatives, implying that only 325,000 of the country's 1,000,000 women living in rural areas have access to this life-saving intervention [139].
HPV prevalence in Africa
A number of studies on HPV prevalence have been carried in some African countries. The majority of reports available in literature concentrated on a particular population in a particular geographic region of a country. Nevertheless, a pooled estimate by the International Agency for Research on Cancer (IARC) found that the age-standardized HPV incidence in females with normal cytology in Europe is about five times lower than in Africa [140,141]. The high HPV incidence in Africa can be attributable to cellular immune impairment caused by HIV, malnutrition, parasitic infections and chronic cervical inflammation, which are mostly endemic on the continent [141]. HPV 18 and 16 are detected in approximately 70% of all cervical cancer cases globally [142]. Mbulawa et al. indicated that HPV-18(9%) and 16(20%) were more prevalent in women living with HIV in South Africa [143].
Tounkara, et al. [144] reported that overall HPV prevalence rates in Mali and Benin were 81.4% and 95.5%, respectively. Again, the top three high risk HPV genotypes in Mali were HPV-52 (12.9%), HPV-51 (14.3%) and HPV-16 (15.7%) whereas that of Benin was HPV-52 (28.8%), HPV-16 (36.6%) and HPV-58 (37.5%). In Mali, the main factors associated with high-risk HPV were HIV infection and duration of sex work, while in Benin, they were gonococcal infection and vaginal douching. Mbulawa, et al. [145] concluded that the prevalence of HPV in young and adolescent South African women was 66.7%. Furthermore, the HPV type frequently detected in South African women were HPV-81 (7.6%), HPV-18 (7.6%), HPV-66(8.6%), HPV-51(8.9%), HPV-58(10.3%) and HPV-16(11.7%). Bacterial vaginosis was the main factor associated with HPV infections.
Ogembo, et al. [146] reported that HPV-18 and 16 accounted for 67.7% of invasive cervical cancer among African women based on their analysis from 23 different African countries with a sample size of 17273 women. Eastern Africa had the highest number of women with HPV infections (n = 6,640), followed by Western Africa (n = 5,600), Southern Africa (n = 3.030) and Northern Africa (n = 1.865). The top ten HPV genotypes prevalent in women with normal cytology were HPV56, 53, 31, 45, 51, 58, 18, 35, 52 and 16 in ascending order. Other studies from the continent have detected some of these HPV genotypes [146]. These epidemiological findings may influence vaccination campaigns. Sadly, the expensive nature of the vaccine is making it inaccessible and unaffordable in several African countries [119,147,148]. It is anticipated that, with the cooperation of countries, pharmaceutical companies and international agencies, the HPV vaccine may be affordable and easily assessed in the various African countries, especially rural communities.
Potential Interventions and Recommendations to Reduce the Incidence and Mortality Rate of Cervical Cancer in Africa
Enhancing and improving awareness of the risk factors, symptoms and possible treatments for cervical cancer ought to be the key aspect of every cervical cancer prevention campaign. First of all, Heads of State, government officials and various health institutions must show a great desire in eradicating this disease. This can be achieved when resources are channelled towards prevention and research and by subsidizing cervical cancer treatment, especially for people living in rural areas. Community leaders and civil societies must be at the forefront of humanitarian interventions and investment projects. Prior to cervical cancer screening and HPV vaccination, town hall forums could be beneficial, as this initiative will show community leaders approval, interest, involvement and cooperation. This provides a welcoming platform for members of the community to seek answers concerning any wrong perceptions and myths surrounding the screening and the vaccination process and further obtain a better understanding of these preventive interventions [149]. The effectiveness of these humanitarian interventions and investment projects depends on the establishment of trust between vulnerable communities and key stakeholders, such as healthcare professionals. Discrimination and stigmatization faced by cancer patients could be eliminated if civil societies and community leaders worked together.
Given that most teenage girls are sexually active, educational initiatives aimed at educating teenage girls about cervical cancer risk factors must begin in elementary school because schools can adequately and efficiently serve as an emplacement to conduct the vaccination campaigns. Also, reaching out to the right targeted age groups (teenagers) in schools would be easier, since most African countries have implemented free basic education policy. Integrating HPV vaccination and cervical cancer screenings into HIV and reproductive healthcare services, which are already in existence, would be a successful interventional approach [150]. This strategy will use previous resources such as transport and personnel, because when integrated with an effective, efficient and well-funded programme, the existing programme may cover the transportation cost and personnel costs (such as allowances and salaries). Nevertheless, if integrated with less efficient and under-funded programme, then the budget for nationwide immunization will require to cover the transportation costs and personnel allowances in order to reach large coverage levels in delivering the vaccines. Community outreach and public health education programs must be created; for example, professionals' midwives and community healthcare professionals must first be educated to help conduct mass educational campaigns and awareness on the objective, procedure, and timing for the Pap smear test.
The "single-visit approach" for cervical cancer prevention employing low technology and low cost must be suggested for some countries on the continent. This approach is effective and cost-effective. Free cervical cancer screenings have been implemented using this method in the Niger Republic. African governments must endorse and participate in ongoing HPV vaccine trials and research as the key preventive measure against this cancer. HPV vaccine as a preventive measure could, in the longer term, help decrease the prevalence of cervical cancer, especially in Africa. The World Bank, PATH, and Global Alliance for Vaccines and Immunization (GAVI) must collaborate with the pharmaceutical companies to reduce the vaccine's cost to render it affordable and accessible in Africa. An efficient approach may be to concentrate vaccination campaigns on HIV-positive women and female sex workers to avoid the possible transfer of the virus to other females by male partners. The majority of teenage girls residing in rural areas of most African countries are not vaccinated. Therefore, a major preventive intervention may also be to target these teenage girls for vaccination. Again, the treatment, vaccination, and screenings for cervical cancer should be significantly subsidized by the government or be free. This is possible if there is financial and political support.
Owing to a lack of regular screening, many females in these resource-strapped nations have advanced cancer, with palliative care being the only viable therapeutic choice. Palliative care is limited in several countries in Africa. It is projected that 20 million individuals in low-and middle income countries suffer from extreme pain which morphine could have been used in alleviating the pain. Morphine to treat extreme pain was unavailable to another 28 million people who did not die [151]. Furthermore, only 0.03% of the licensed opioid analgesics are accessible in low income nations while that of low-and middle income countries is 3.6% [152]. Radiotherapy is commonly used in treating women who are privileged enough to seek treatment. Only private and tertiary health facilities have radiotherapy equipment, with poor maintenance and mostly non-operational [153]. Current studies into “screen and treat” projects in India and South Africa found that HPV-based screening combined with cryotherapy care would significantly decrease the risk of cervical cancers precursor and the disease itself in developing nations [154,155]. The cost of screening and the HPV vaccine is significantly high in several African countries, putting them out of the general public's reach. Cervarix and Gardasil cost between $150 to $190 per dose at retail prices [156]. For instance, an average Ghanaian woman needs about $200 to complete the entire HPV vaccination cycle, which is highly expensive, especially for women in rural communities. Evidently, the major challenge to implementing widely accessible and acceptable cancer screening programs and HPV vaccination in developing nations is cost. Thus, bargaining on the price of the vaccine with drug companies may be one approach to overcome this challenge.
The abilities of vaccine producers and suppliers in developing nations have grown significantly over the past decades for a variety of vaccines; nevertheless, this pattern does not involve HPV vaccines [157]. Manufacturers should invest in the development of HPV vaccines for developing nations, which are rapidly growing and developing economies. Establishing vaccine manufacturing facilities in collaboration with producers and state authorities in countries with proper management should be considered. The foundation for implementing worldwide vaccination campaigns is to develop effective vaccine formulations that take into account the type of HPV genotypes and produce them in sufficient amounts and at reasonable costs. That being said, achieving optimum accessibility and adoption necessitates strong collaborations among international and domestic public health organizations, regulatory agencies and private manufacturers.
Conclusion
Cervical cancer is primarily prevalent in Africa. Cervical cancer could be greatly reduced if comprehensive screening services and HPV vaccination are implemented. Until these initiatives are fully implemented, it is critical to increase awareness regarding the risk factors and preventive measures. Pap smear tests and HPV vaccines are currently out of reach for most Africans due to cost. Millions of women might be saved from a potentially eradicable disease if states, societies, pharmaceutical companies, researchers, private investors, and sponsors work together to build productive synergies. Ultimately, poverty in Africa must be urgently tackled as poverty plays an essential role in treating and preventing this disease.
Author Contributions
EK Drokow wrote and presented the original draft. CY Effah, C Agboyibor, GS Akpabla, were involved, review and editing. K Sun was involved in supervision.
References
- Schiffman M, Wentzensen N. Human papillomavirus infection and the multistage carcinogenesis of cervical cancer. Cancer Epidemiol Biomarkers Prev. 2013 Apr;22(4):553-60. doi: 10.1158/1055-9965.EPI-12-1406. PMID: 23549399; PMCID: PMC3711590.
- Waggoner SE. Cervical cancer. Lancet. 2003 Jun 28;361(9376):2217-25. doi: 10.1016/S0140-6736(03)13778-6. PMID: 12842378.
- Andrae B, Kemetli L, Sparén P, Silfverdal L, Strander B, Ryd W, Dillner J, Törnberg S. Screening-preventable cervical cancer risks: evidence from a nationwide audit in Sweden. J Natl Cancer Inst. 2008 May 7;100(9):622-9. doi: 10.1093/jnci/djn099. Epub 2008 Apr 29. PMID: 18445828.
- Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO Classification of Tumours of Female Reproductive Organs. 6. 4th ed. Lyon: International Agency for Research on Cancer (IARC); 2014.
- Wright TC Jr, Schiffman M. Adding a test for human papillomavirus DNA to cervical-cancer screening. N Engl J Med. 2003 Feb 6;348(6):489-90. doi: 10.1056/NEJMp020178. PMID: 12571255.
- Plummer M, Schiffman M, Castle PE, Maucort-Boulch D, Wheeler CM; ALTS Group. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis. 2007 Jun 1;195(11):1582-9. doi: 10.1086/516784. Epub 2007 Apr 16. PMID: 17471427.
- Schiffman M, Herrero R, Desalle R, Hildesheim A, Wacholder S, Rodriguez AC, Bratti MC, Sherman ME, Morales J, Guillen D, Alfaro M, Hutchinson M, Wright TC, Solomon D, Chen Z, Schussler J, Castle PE, Burk RD. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology. 2005 Jun 20;337(1):76-84. doi: 10.1016/j.virol.2005.04.002. PMID: 15914222.
- Castellsagué X, Muñoz N. Chapter 3: Cofactors in human papillomavirus carcinogenesis--role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr. 2003;(31):20-8. PMID: 12807941.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical carcinoma and reproductive factors: collaborative reanalysis of individual data on 16,563 women with cervical carcinoma and 33,542 women without cervical carcinoma from 25 epidemiological studies. Int J Cancer. 2006 Sep 1;119(5):1108-24. doi: 10.1002/ijc.21953. PMID: 16570271.
- Plummer M, Peto J, Franceschi S; International Collaboration of Epidemiological Studies of Cervical Cancer. Time since first sexual intercourse and the risk of cervical cancer. Int J Cancer. 2012 Jun 1;130(11):2638-44. doi: 10.1002/ijc.26250. Epub 2011 Aug 12. PMID: 21702036; PMCID: PMC3982220.
- Sato S, Itamochi H. Secondary Prevention of Uterine Cervical Cancer. Cervical Cancer: Screening, Treatment and Prevention-Universal Protocols for Ultimate Control. 2018 May;16:59.
- Basu P, Mittal S, Bhadra Vale D, Chami Kharaji Y. Secondary Prevention of Cervical Cancer. International Agency for Research on Cancer. 2017:1-13.
- Aggarwal P. Cervical cancer: Can it be prevented? World J Clin Oncol. 2014 Oct 10;5(4):775-80. doi: 10.5306/wjco.v5.i4.775. PMID: 25302177; PMCID: PMC4129540.
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015 Mar;65(2):87-108. doi: 10.3322/caac.21262. Epub 2015 Feb 4. PMID: 25651787.
- Jeronimo J, Castle PE, Temin S, Denny L, Gupta V, Kim JJ, Luciani S, Murokora D, Ngoma T, Qiao Y, Quinn M, Sankaranarayanan R, Sasieni P, Schmeler KM, Shastri SS. Secondary Prevention of Cervical Cancer: ASCO Resource-Stratified Clinical Practice Guideline. J Glob Oncol. 2016 Oct 12;3(5):635-657. doi: 10.1200/JGO.2016.006577. PMID: 29094101; PMCID: PMC5646891.
- Arrossi S, Temin S, Garland S, Eckert LO, Bhatla N, Castellsagué X, Alkaff SE, Felder T, Hammouda D, Konno R, Lopes G, Mugisha E, Murillo R, Scarinci IC, Stanley M, Tsu V, Wheeler CM, Adewole IF, de Sanjosé S. Primary Prevention of Cervical Cancer: American Society of Clinical Oncology Resource-Stratified Guideline. J Glob Oncol. 2017 Mar 17;3(5):611-634. doi: 10.1200/JGO.2016.008151. PMID: 29094100; PMCID: PMC5646902.
- de Villiers EM. Heterogeneity of the human papillomavirus group. J Virol. 1989 Nov;63(11):4898-903. doi: 10.1128/JVI.63.11.4898-4903.1989. PMID: 2552162; PMCID: PMC251129.
- Human papillomavirus vaccines. WHO position paper. Wkly Epidemiol Rec. 2009 Apr 10;84(15):118-31. English, French. PMID: 19360985.
- WHO and UNICEF. Progress towards global immunization goals - 2011. Geneva: World Health Organization. 2012;35.
- Agosti JM, Goldie SJ. Introducing HPV vaccine in developing countries--key challenges and issues. N Engl J Med. 2007 May 10;356(19):1908-10. doi: 10.1056/NEJMp078053. PMID: 17494923.
- GAVI welcomes lower prices for life-saving vaccines. June 6, 2011.
- GAVI injects new life into HPV vaccine rollout. Lancet. 2013 May 18;381(9879):1688. doi: 10.1016/S0140-6736(13)61058-2. PMID: 23683613.
- GAVI, Millions of girls in developing countries to be protected against cervical cancer thanks to new HPV vaccine deals-2013- Press releases-News-Library-GAVI Alliance. 2013.
- Kane MA, Sherris J, Coursaget P, Aguado T, Cutts F. Chapter 15: HPV vaccine use in the developing world. Vaccine. 2006 Aug 31;24 Suppl 3:S3/132-9. doi: 10.1016/j.vaccine.2006.05.128. PMID: 16950000.
- Binagwaho A, Wagner CM, Gatera M, Karema C, Nutt CT, Ngabo F. Achieving high coverage in Rwanda's national human papillomavirus vaccination programme. Bull World Health Organ. 2012 Aug 1;90(8):623-8. doi: 10.2471/BLT.11.097253. Epub 2012 May 23. PMID: 22893746; PMCID: PMC3417784.
- Binagwaho A, Wagner CM, Nutt CT. HPV vaccine in Rwanda: different disease, same double standard. Lancet. 2011 Dec 3;378(9807):1916. doi: 10.1016/S0140-6736(11)61837-0. PMID: 22137840.
- Ouedraogo N, Müller O, Jahn A, Gerhardus A. Human papillomavirus vaccination in Africa. Lancet. 2011 Jul 23;378(9788):315-6. doi: 10.1016/S0140-6736(11)61164-1. PMID: 21784261.
- LaMontagne DS, Barge S, Le NT, Mugisha E, Penny ME, Gandhi S, Janmohamed A, Kumakech E, Mosqueira NR, Nguyen NQ, Paul P, Tang Y, Minh TH, Uttekar BP, Jumaan AO. Human papillomavirus vaccine delivery strategies that achieved high coverage in low- and middle-income countries. Bull World Health Organ. 2011 Nov 1;89(11):821-830B. doi: 10.2471/BLT.11.089862. Epub 2011 Sep 1. PMID: 22084528; PMCID: PMC3209730.
- Moodley I, Tathiah N, Mubaiwa V, Denny L. High uptake of Gardasil vaccine among 9 - 12-year-old schoolgirls participating in an HPV vaccination demonstration project in KwaZulu-Natal, South Africa. S Afr Med J. 2013 May;103(5):318-21. doi: 10.7196/samj.6414. PMID: 23971122.
- Ladner J, Besson MH, Hampshire R, Tapert L, Chirenje M, Saba J. Assessment of eight HPV vaccination programs implemented in lowest income countries. BMC Public Health. 2012 May 23;12:370. doi: 10.1186/1471-2458-12-370. PMID: 22621342; PMCID: PMC3419135.
- Watson-Jones D, Baisley K, Ponsiano R, Lemme F, Remes P, Ross D, Kapiga S, Mayaud P, de Sanjosé S, Wight D, Changalucha J, Hayes R. Human papillomavirus vaccination in Tanzanian schoolgirls: cluster-randomized trial comparing 2 vaccine-delivery strategies. J Infect Dis. 2012 Sep 1;206(5):678-86. doi: 10.1093/infdis/jis407. Epub 2012 Jun 18. PMID: 22711908; PMCID: PMC3414230.
- Ayissi CA, Wamai RG, Oduwo GO, Perlman S, Welty E, Welty T, Manga S, Ogembo JG. Awareness, acceptability and uptake of human papilloma virus vaccine among Cameroonian school-attending female adolescents. J Community Health. 2012 Dec;37(6):1127-35. doi: 10.1007/s10900-012-9554-z. PMID: 22426995.
- De Vuyst H, Alemany L, Lacey C, Chibwesha CJ, Sahasrabuddhe V, Banura C, Denny L, Parham GP. The burden of human papillomavirus infections and related diseases in sub-saharan Africa. Vaccine. 2013 Dec 29;31 Suppl 5(0 5):F32-46. doi: 10.1016/j.vaccine.2012.07.092. PMID: 24331746; PMCID: PMC4144870.
- Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020 Jul;70(4):313. doi: 10.3322/caac.21609. Epub 2020 Apr 6. Erratum for: CA Cancer J Clin. 2018 Nov;68(6):394-424. PMID: 32767693.
- Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019 Apr 15;144(8):1941-1953. doi: 10.1002/ijc.31937. Epub 2018 Dec 6. PMID: 30350310.
- The Cancer Atlas. 2021. https://bit.ly/3nbttYB
- Cunningham MS, Davison C, Aronson KJ. HPV vaccine acceptability in Africa: a systematic review. Prev Med. 2014 Dec;69:274-9. doi: 10.1016/j.ypmed.2014.08.035. Epub 2014 Oct 24. PMID: 25451327.
- Palefsky JM, Gillison ML, Strickler HD. Chapter 16: HPV vaccines in immunocompromised women and men. Vaccine. 2006 Aug 31;24 Suppl 3:S3/140-6. doi: 10.1016/j.vaccine.2006.05.120. Epub 2006 Jun 23. PMID: 16950001.
- Garnett GP, Kim JJ, French K, Goldie SJ. Chapter 21: Modelling the impact of HPV vaccines on cervical cancer and screening programmes. Vaccine. 2006 Aug 31;24 Suppl 3:S3/178-86. doi: 10.1016/j.vaccine.2006.05.116. PMID: 16950005.
- Wigle J, Coast E, Watson-Jones D. Human papillomavirus (HPV) vaccine implementation in low and middle-income countries (LMICs): health system experiences and prospects. Vaccine. 2013 Aug 20;31(37):3811-7. doi: 10.1016/j.vaccine.2013.06.016. Epub 2013 Jun 15. PMID: 23777956; PMCID: PMC3763375.
- Ebosse Y. Mama Fouda authorizes a vaccine that kills. Le Soir Hebd. Yaounde, Cameroon: The Evening Weekly; 2010.
- Larson HJ, Brocard P, Garnett G. The India HPV-vaccine suspension. Lancet. 2010 Aug 21;376(9741):572-3. doi: 10.1016/S0140-6736(10)60881-1. PMID: 20728739.
- Khan KS, Ter Riet G, Glanville J, Sowden AJ, Kleijnen J. Undertaking systematic reviews of research on effectiveness: CRD's guidance for carrying out or commissioning reviews. NHS Centre for Reviews and Dissemination. 2001.
- Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019 Jan 12;393(10167):169-182. doi: 10.1016/S0140-6736(18)32470-X. PMID: 30638582.
- Leeds IL, Fang SH. Anal cancer and intraepithelial neoplasia screening: A review. World J Gastrointest Surg. 2016 Jan 27;8(1):41-51. doi: 10.4240/wjgs.v8.i1.41. PMID: 26843912; PMCID: PMC4724586.
- Näsman A, Du J, Dalianis T. A global epidemic increase of an HPV-induced tonsil and tongue base cancer - potential benefit from a pan-gender use of HPV vaccine. J Intern Med. 2020 Feb;287(2):134-152. doi: 10.1111/joim.13010. Epub 2019 Dec 9. PMID: 31733108.
- Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017 Apr;40(2):80-85. Epub 2017 Apr 3. PMID: 28368072.
- Tulay P, Serakinci N. The role of human papillomaviruses in cancer progression. J Cancer Metastasis Treat. 2016;2:201-213.
- Shah A, Malik A, Garg A, Mair M, Nair S, Chaturvedi P. Oral sex and human papilloma virus-related head and neck squamous cell cancer: a review of the literature. Postgrad Med J. 2017 Nov;93(1105):704-709. doi: 10.1136/postgradmedj-2016-134603. Epub 2017 Aug 4. PMID: 28778951.
- Yeo-Teh NSL, Ito Y, Jha S. High-Risk Human Papillomaviral Oncogenes E6 and E7 Target Key Cellular Pathways to Achieve Oncogenesis. Int J Mol Sci. 2018 Jun 8;19(6):1706. doi: 10.3390/ijms19061706. PMID: 29890655; PMCID: PMC6032416.
- Mirabello L, Yeager M, Yu K, Clifford GM, Xiao Y, Zhu B, Cullen M, Boland JF, Wentzensen N, Nelson CW, Raine-Bennett T, Chen Z, Bass S, Song L, Yang Q, Steinberg M, Burdett L, Dean M, Roberson D, Mitchell J, Lorey T, Franceschi S, Castle PE, Walker J, Zuna R, Kreimer AR, Beachler DC, Hildesheim A, Gonzalez P, Porras C, Burk RD, Schiffman M. HPV16 E7 Genetic Conservation Is Critical to Carcinogenesis. Cell. 2017 Sep 7;170(6):1164-1174.e6. doi: 10.1016/j.cell.2017.08.001. PMID: 28886384; PMCID: PMC5674785.
- Doeberitz Mv, Vinokurova S. Host factors in HPV-related carcinogenesis: cellular mechanisms controlling HPV infections. Arch Med Res. 2009 Aug;40(6):435-42. doi: 10.1016/j.arcmed.2009.06.002. Epub 2009 Sep 5. PMID: 19853183.
- Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond). 2006 May;110(5):525-41. doi: 10.1042/CS20050369. PMID: 16597322.
- Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, Stanley MA. The biology and life-cycle of human papillomaviruses. Vaccine. 2012 Nov 20;30 Suppl 5:F55-70. doi: 10.1016/j.vaccine.2012.06.083. PMID: 23199966.
- Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc Natl Acad Sci U S A. 2009 Dec 1;106(48):20458-63. doi: 10.1073/pnas.0908502106. Epub 2009 Nov 17. PMID: 19920181; PMCID: PMC2787115.
- Reuschenbach M, Waterboer T, Wallin KL, Einenkel J, Dillner J, Hamsikova E, Eschenbach D, Zimmer H, Heilig B, Kopitz J, Pawlita M, Doeberitz Mv, Wentzensen N. Characterization of humoral immune responses against p16, p53, HPV16 E6 and HPV16 E7 in patients with HPV-associated cancers. Int J Cancer. 2008 Dec 1;123(11):2626-31. doi: 10.1002/ijc.23837. PMID: 18785210.
- de Jong A, van Poelgeest MI, van der Hulst JM, Drijfhout JW, Fleuren GJ, Melief CJ, Kenter G, Offringa R, van der Burg SH. Human papillomavirus type 16-positive cervical cancer is associated with impaired CD4+ T-cell immunity against early antigens E2 and E6. Cancer Res. 2004 Aug 1;64(15):5449-55. doi: 10.1158/0008-5472.CAN-04-0831. PMID: 15289354.
- Fischer M, Uxa S, Stanko C, Magin TM, Engeland K. Human papilloma virus E7 oncoprotein abrogates the p53-p21-DREAM pathway. Sci Rep. 2017 Jun 1;7(1):2603. doi: 10.1038/s41598-017-02831-9. PMID: 28572607; PMCID: PMC5453983.
- Khan A, Junaid M, Kaushik AC, Ali A, Ali SS, Mehmood A, Wei DQ. Computational identification, characterization and validation of potential antigenic peptide vaccines from hrHPVs E6 proteins using immunoinformatics and computational systems biology approaches. PLoS One. 2018 May 1;13(5):e0196484. doi: 10.1371/journal.pone.0196484. PMID: 29715318; PMCID: PMC5929558.
- Biedermann K, Dandachi N, Trattner M, Vogl G, Doppelmayr H, Moré E, Staudach A, Dietze O, Hauser-Kronberger C. Comparison of real-time PCR signal-amplified in situ hybridization and conventional PCR for detection and quantification of human papillomavirus in archival cervical cancer tissue. J Clin Microbiol. 2004 Aug;42(8):3758-65. doi: 10.1128/JCM.42.8.3758-3765.2004. PMID: 15297527; PMCID: PMC497646.
- Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F; WHO International Agency for Research on Cancer. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005 Apr;6(4):204. doi: 10.1016/s1470-2045(05)70086-3. PMID: 15830458.
- Yuan J, Ni G, Wang T, Mounsey K, Cavezza S, Pan X, Liu X. Genital warts treatment: Beyond imiquimod. Hum Vaccin Immunother. 2018 Jul 3;14(7):1815-1819. doi: 10.1080/21645515.2018.1445947. Epub 2018 Apr 9. PMID: 29505317; PMCID: PMC6067868.
- Kjaer SK, Chackerian B, van den Brule AJ, Svare EI, Paull G, Walbomers JM, Schiller JT, Bock JE, Sherman ME, Lowy DR, Meijer CL. High-risk human papillomavirus is sexually transmitted: evidence from a follow-up study of virgins starting sexual activity (intercourse). Cancer Epidemiol Biomarkers Prev. 2001 Feb;10(2):101-6. PMID: 11219765.
- Castellsagué X, Ghaffari A, Daniel RW, Bosch FX, Muñoz N, Shah KV. Prevalence of penile human papillomavirus DNA in husbands of women with and without cervical neoplasia: a study in Spain and Colombia. J Infect Dis. 1997 Aug;176(2):353-61. doi: 10.1086/514052. PMID: 9237700.
- Omone OM, Kozlovszky M. HPV and Cervical Cancer Screening Awareness: A Case-control Study in Nigeria. In 2020 IEEE 24th International Conference on Intelligent Engineering Systems (INES). IEEE. 2020;145-152.
- Banister CE, Liu C, Pirisi L, Creek KE, Buckhaults PJ. Identification and characterization of HPV-independent cervical cancers. Oncotarget. 2017 Feb 21;8(8):13375-13386. doi: 10.18632/oncotarget.14533. PMID: 28077784; PMCID: PMC5355105.
- de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017 Aug 15;141(4):664-670. doi: 10.1002/ijc.30716. Epub 2017 Jun 8. PMID: 28369882; PMCID: PMC5520228.
- Deivendran S, Marzook KH, Radhakrishna Pillai M. The role of inflammation in cervical cancer. Adv Exp Med Biol. 2014;816:377-99. doi: 10.1007/978-3-0348-0837-8_15. PMID: 24818731.
- Dugué PA, Rebolj M, Garred P, Lynge E. Immunosuppression and risk of cervical cancer. Expert Rev Anticancer Ther. 2013 Jan;13(1):29-42. doi: 10.1586/era.12.159. PMID: 23259425.
- Ebrahimi S, Soltani A, Hashemy SI. Oxidative stress in cervical cancer pathogenesis and resistance to therapy. J Cell Biochem. 2018 Nov 13. doi: 10.1002/jcb.28007. Epub ahead of print. PMID: 30426530.
- de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, Vallejos CS, de Ruiz PA, Lima MA, Guimera N, Clavero O, Alejo M, Llombart-Bosch A, Cheng-Yang C, Tatti SA, Kasamatsu E, Iljazovic E, Odida M, Prado R, Seoud M, Grce M, Usubutun A, Jain A, Suarez GA, Lombardi LE, Banjo A, Menéndez C, Domingo EJ, Velasco J, Nessa A, Chichareon SC, Qiao YL, Lerma E, Garland SM, Sasagawa T, Ferrera A, Hammouda D, Mariani L, Pelayo A, Steiner I, Oliva E, Meijer CJ, Al-Jassar WF, Cruz E, Wright TC, Puras A, Llave CL, Tzardi M, Agorastos T, Garcia-Barriola V, Clavel C, Ordi J, Andújar M, Castellsagué X, Sánchez GI, Nowakowski AM, Bornstein J, Muñoz N, Bosch FX; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010 Nov;11(11):1048-56. doi: 10.1016/S1470-2045(10)70230-8. Epub 2010 Oct 15. PMID: 20952254.
- Garland SM, Steben M, Sings HL, James M, Lu S, Railkar R, Barr E, Haupt RM, Joura EA. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009 Mar 15;199(6):805-14. doi: 10.1086/597071. PMID: 19199546.
- Yang DY, Bracken K. Update on the new 9-valent vaccine for human papillomavirus prevention. Can Fam Physician. 2016 May;62(5):399-402. Epub 2016 May 12. PMID: 27255620; PMCID: PMC4865336.
- Canfell K, Kim JJ, Brisson M, Keane A, Simms KT, Caruana M, Burger EA, Martin D, Nguyen DTN, Bénard É, Sy S, Regan C, Drolet M, Gingras G, Laprise JF, Torode J, Smith MA, Fidarova E, Trapani D, Bray F, Ilbawi A, Broutet N, Hutubessy R. Mortality impact of achieving WHO cervical cancer elimination targets: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020 Feb 22;395(10224):591-603. doi: 10.1016/S0140-6736(20)30157-4. Epub 2020 Jan 30. PMID: 32007142; PMCID: PMC7043006.
- Cheng L, Wang Y, Du J. Human Papillomavirus Vaccines: An Updated Review. Vaccines (Basel). 2020 Jul 16;8(3):391. doi: 10.3390/vaccines8030391. PMID: 32708759; PMCID: PMC7565290.
- Bruni L, Diaz M, Barrionuevo-Rosas L, Herrero R, Bray F, Bosch FX, de Sanjosé S, Castellsagué X. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. 2016 Jul;4(7):e453-63. doi: 10.1016/S2214-109X(16)30099-7. Erratum in: Lancet Glob Health. 2017 Jul;5(7):e662. PMID: 27340003.
- de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017 Aug 15;141(4):664-670. doi: 10.1002/ijc.30716. Epub 2017 Jun 8. PMID: 28369882; PMCID: PMC5520228.
- Sabeena S, Bhat PV, Kamath V, Arunkumar G. Global human papilloma virus vaccine implementation: An update. J Obstet Gynaecol Res. 2018 Jun;44(6):989-997. doi: 10.1111/jog.13634. Epub 2018 Mar 8. PMID: 29517117.
- Ährlund-Richter A, Cheng L, Hu YOO, Svensson M, Pennhag AAL, Ursu RG, Haeggblom L, Grün N, Ramqvist T, Engstrand L, Dalianis T, Du J. Changes in Cervical Human Papillomavirus (HPV) Prevalence at a Youth Clinic in Stockholm, Sweden, a Decade After the Introduction of the HPV Vaccine. Front Cell Infect Microbiol. 2019 Mar 20;9:59. doi: 10.3389/fcimb.2019.00059. PMID: 30949454; PMCID: PMC6435486.
- Giuliano AR, Joura EA, Garland SM, Huh WK, Iversen OE, Kjaer SK, Ferenczy A, Kurman RJ, Ronnett BM, Stoler MH, Bautista OM, Moeller E, Ritter M, Shields C, Luxembourg A. Nine-valent HPV vaccine efficacy against related diseases and definitive therapy: comparison with historic placebo population. Gynecol Oncol. 2019 Jul;154(1):110-117. doi: 10.1016/j.ygyno.2019.03.253. Epub 2019 Apr 11. PMID: 30982556.
- Bhatla N, Nene BM, Joshi S, Esmy PO, Poli URR, Joshi G, Verma Y, Zomawia E, Pimple S, Prabhu PR, Basu P, Muwonge R, Hingmire S, Sauvaget C, Lucas E, Pawlita M, Gheit T, Jayant K, Malvi SG, Siddiqi M, Michel A, Butt J, Sankaran S, Kannan TPRA, Varghese R, Divate U, Willhauck-Fleckenstein M, Waterboer T, Müller M, Sehr P, Kriplani A, Mishra G, Jadhav R, Thorat R, Tommasino M, Pillai MR, Sankaranarayanan R; Indian HPV vaccine study group. Are two doses of human papillomavirus vaccine sufficient for girls aged 15-18 years? Results from a cohort study in India. Papillomavirus Res. 2018 Jun;5:163-171. doi: 10.1016/j.pvr.2018.03.008. Epub 2018 Mar 22. PMID: 29578097; PMCID: PMC6047463.
- Sonawane K, Nyitray AG, Nemutlu GS, Swartz MD, Chhatwal J, Deshmukh AA. Prevalence of Human Papillomavirus Infection by Number of Vaccine Doses Among US Women. JAMA Netw Open. 2019 Dec 2;2(12):e1918571. doi: 10.1001/jamanetworkopen.2019.18571. PMID: 31880792; PMCID: PMC6986697.
- Kjaer SK, Nygård M, Dillner J, Brooke Marshall J, Radley D, Li M, Munk C, Hansen BT, Sigurdardottir LG, Hortlund M, Tryggvadottir L, Joshi A, Das R, Saah AJ. A 12-Year Follow-up on the Long-Term Effectiveness of the Quadrivalent Human Papillomavirus Vaccine in 4 Nordic Countries. Clin Infect Dis. 2018 Jan 18;66(3):339-345. doi: 10.1093/cid/cix797. PMID: 29029053.
- Haque A, Kouriba B, Aïssatou N, Pant A. Eliminating Cervical Cancer in Mali and Senegal, Two Sub-Saharan Countries: Insights and Optimizing Solutions. Vaccines (Basel). 2020 Apr 14;8(2):181. doi: 10.3390/vaccines8020181. PMID: 32295116; PMCID: PMC7349839.
- Sankaranarayanan R, Anorlu R, Sangwa-Lugoma G, Denny LA. Infrastructure requirements for human papillomavirus vaccination and cervical cancer screening in sub-Saharan Africa. Vaccine. 2013 Dec 29;31 Suppl 5:F47-52. doi: 10.1016/j.vaccine.2012.06.066. PMID: 24331747.
- ACCP. Visual screening approaches: Promising alterative screening strategies. Cervical Cancer Prevention Fact Sheet. October 2002.
- Arbyn M, Sankaranarayanan R, Muwonge R, Keita N, Dolo A, Mbalawa CG, Nouhou H, Sakande B, Wesley R, Somanathan T, Sharma A, Shastri S, Basu P. Pooled analysis of the accuracy of five cervical cancer screening tests assessed in eleven studies in Africa and India. Int J Cancer. 2008 Jul 1;123(1):153-60. doi: 10.1002/ijc.23489. PMID: 18404671.
- Ghosh P, Gandhi G, Kochhar PK, Zutshi V, Batra S. Visual inspection of cervix with Lugol's iodine for early detection of premalignant & malignant lesions of cervix. Indian J Med Res. 2012 Aug;136(2):265-71. PMID: 22960894; PMCID: PMC3461739.
- Sahasrabuddhe VV, Gravitt PE, Dunn ST, Brown D, Allen RA, Eby YJ, Smith K, Zuna RE, Zhang RR, Gold MA, Schiffman M, Walker JL, Castle PE, Wentzensen N. Comparison of human papillomavirus detections in urine, vulvar, and cervical samples from women attending a colposcopy clinic. J Clin Microbiol. 2014 Jan;52(1):187-92. doi: 10.1128/JCM.01623-13. Epub 2013 Nov 6. PMID: 24197879; PMCID: PMC3911475.
- Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, Bray F. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020 Feb;8(2):e191-e203. doi: 10.1016/S2214-109X(19)30482-6. Epub 2019 Dec 4. PMID: 31812369; PMCID: PMC7025157.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021 May;71(3):209-249. doi: 10.3322/caac.21660. Epub 2021 Feb 4. PMID: 33538338.
- Odida M, Schmauz R, Lwanga SK. Grade of malignancy of cervical cancer in regions of Uganda with varying malarial endemicity. Int J Cancer. 2002 Jun 10;99(5):737-41. doi: 10.1002/ijc.10384. PMID: 12115509.
- Adoch W, Garimoi CO, Scott SE, Okeny GG, Moodley J, Komakech H, Walter FM, Mwaka AD. Knowledge of cervical cancer risk factors and symptoms among women in a refugee settlement: a cross-sectional study in northern Uganda. Confl Health. 2020 Dec 3;14(1):85. doi: 10.1186/s13031-020-00328-3. PMID: 33292345; PMCID: PMC7713037.
- Mboumba Bouassa RS, Prazuck T, Lethu T, Jenabian MA, Meye JF, Bélec L. Cervical cancer in sub-Saharan Africa: a preventable noncommunicable disease. Expert Rev Anti Infect Ther. 2017 Jun;15(6):613-627. doi: 10.1080/14787210.2017.1322902. Epub 2017 May 5. PMID: 28440679.
- Moelle U, Mathewos A, Aynalem A, Wondemagegnehu T, Yonas B, Begoihn M, Addissie A, Unverzagt S, Jemal A, Thomssen C, Vordermark D, Kantelhardt EJ. Cervical Cancer in Ethiopia: The Effect of Adherence to Radiotherapy on Survival. Oncologist. 2018 Sep;23(9):1024-1032. doi: 10.1634/theoncologist.2017-0271. Epub 2018 Mar 22. PMID: 29567823; PMCID: PMC6192604.
- Ononogbu U, Almujtaba M, Modibbo F, Lawal I, Offiong R, Olaniyan O, Dakum P, Spiegelman D, Blattner W, Adebamowo C. Cervical cancer risk factors among HIV-infected Nigerian women. BMC Public Health. 2013 Jun 14;13:582. doi: 10.1186/1471-2458-13-582. PMID: 23767681; PMCID: PMC3728111.
- Anorlu RI. Cervical cancer: the sub-Saharan African perspective. Reprod Health Matters. 2008 Nov;16(32):41-9. doi: 10.1016/S0968-8080(08)32415-X. PMID: 19027621.
- Bayo S, Bosch FX, de Sanjosé S, Muñoz N, Combita AL, Coursaget P, Diaz M, Dolo A, van den Brule AJ, Meijer CJ. Risk factors of invasive cervical cancer in Mali. Int J Epidemiol. 2002 Feb;31(1):202-9. doi: 10.1093/ije/31.1.202. PMID: 11914322.
- Muwonge R, Ngo Mbus L, Ngoma T, Gombe Mbalawa C, Dolo A, da Ganda Manuel M, Nouhou H, Nacoulma M, Mwaiselage J, Koulibaly M, Bayo S, Nsonde Malanda J, De Vuyst H, Herrero R, Sankaranarayanan R, Keita N; IARC Multicentre Study Group on Cervical Cancer Early Detection. Socio-demographic and reproductive determinants of cervical neoplasia in seven sub-Sahara African countries. Cancer Causes Control. 2016 Dec;27(12):1437-1446. doi: 10.1007/s10552-016-0823-5. Epub 2016 Nov 7. PMID: 27822586.
- Kasa AS, Tesfaye TD, Temesgen WA. Knowledge, attitude and practice towards cervical cancer among women in Finote Selam city administration, West Gojjam Zone, Amhara Region, North West Ethiopia, 2017. Afr Health Sci. 2018 Sep;18(3):623-636. doi: 10.4314/ahs.v18i3.20. PMID: 30602995; PMCID: PMC6307012.
- Rocha JW. Culture and its influence on an increase of cervical cancer cases in Angola. Brazilian Journal of Oncology. 2020;16:1-5.
- de Abreu AL, Malaguti N, Souza RP, Uchimura NS, Ferreira ÉC, Pereira MW, Carvalho MD, Pelloso SM, Bonini MG, Gimenes F, Consolaro ME. Association of human papillomavirus, Neisseria gonorrhoeae and Chlamydia trachomatis co-infections on the risk of high-grade squamous intraepithelial cervical lesion. Am J Cancer Res. 2016 Jun 1;6(6):1371-83. PMID: 27429850; PMCID: PMC4937739.
- Kharsany AB, Karim QA. HIV Infection and AIDS in Sub-Saharan Africa: Current Status, Challenges and Opportunities. Open AIDS J. 2016 Apr 8;10:34-48. doi: 10.2174/1874613601610010034. PMID: 27347270; PMCID: PMC4893541.
- WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach (second edition)-annex 10, WHO clinical staging of HIV disease in adults, adolescents and children. June 2016.
- May MT, Gompels M, Delpech V, Porter K, Orkin C, Kegg S, Hay P, Johnson M, Palfreeman A, Gilson R, Chadwick D, Martin F, Hill T, Walsh J, Post F, Fisher M, Ainsworth J, Jose S, Leen C, Nelson M, Anderson J, Sabin C; UK Collaborative HIV Cohort (UK CHIC) Study. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS. 2014 May 15;28(8):1193-202. doi: 10.1097/QAD.0000000000000243. PMID: 24556869; PMCID: PMC4004637.
- Joint United Nations Programme on HIV/AIDS. HIV estimates with uncertainty bounds. July 2020;1990-2019.
- Kelly HA, Chikandiwa A, Sawadogo B, Gilham C, Michelow P, Lompo OG, Omar T, Zan S, Magooa P, Segondy M, Nagot N, Meda N, Delany-Moretlwe S, Mayaud P; HARP Study Group. Diagnostic accuracy of cervical cancer screening and screening-triage strategies among women living with HIV-1 in Burkina Faso and South Africa: A cohort study. PLoS Med. 2021 Mar 4;18(3):e1003528. doi: 10.1371/journal.pmed.1003528. PMID: 33661957; PMCID: PMC7971880.
- Stelzle D, Tanaka LF, Lee KK, Ibrahim Khalil A, Baussano I, Shah ASV, McAllister DA, Gottlieb SL, Klug SJ, Winkler AS, Bray F, Baggaley R, Clifford GM, Broutet N, Dalal S. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob Health. 2021 Feb;9(2):e161-e169. doi: 10.1016/S2214-109X(20)30459-9. Epub 2020 Nov 16. Erratum in: Lancet Glob Health. 2021 Feb;9(2):e119. PMID: 33212031; PMCID: PMC7815633.
- Taku O, Mbulawa ZZA, Phohlo K, Garcia-Jardon M, Businge CB, Williamson AL. Distribution of Human Papillomavirus (HPV) Genotypes in HIV-Negative and HIV-Positive Women with Cervical Intraepithelial Lesions in the Eastern Cape Province, South Africa. Viruses. 2021 Feb 11;13(2):280. doi: 10.3390/v13020280. PMID: 33670231; PMCID: PMC7916956.
- Lekoane KMB, Kuupiel D, Mashamba-Thompson TP, Ginindza TG. The interplay of HIV and human papillomavirus-related cancers in sub-Saharan Africa: scoping review. Syst Rev. 2020 Apr 22;9(1):88. doi: 10.1186/s13643-020-01354-1. PMID: 32321580; PMCID: PMC7178989.
- Chambuso RS, Shadrack S, Lidenge SJ, Mwakibete N, Medeiros RM. Influence of HIV/AIDS on Cervical Cancer: A Retrospective Study From Tanzania. J Glob Oncol. 2016 Jun 1;3(1):72-78. doi: 10.1200/JGO.2015.002964. PMID: 28717744; PMCID: PMC5493231.
- Reddy D, Njala J, Stocker P, Schooley A, Flores M, Tseng CH, Pfaff C, Jansen P, Mitsuyasu RT, Hoffman RM. High-risk human papillomavirus in HIV-infected women undergoing cervical cancer screening in Lilongwe, Malawi: a pilot study. Int J STD AIDS. 2015 May;26(6):379-87. doi: 10.1177/0956462414539149. Epub 2014 Jun 13. PMID: 24928579; PMCID: PMC4363075.
- Wu ES, Urban RR, Krantz EM, Mugisha NM, Nakisige C, Schwartz SM, Gray HJ, Casper C. The association between HIV infection and cervical cancer presentation and survival in Uganda. Gynecol Oncol Rep. 2019 Nov 19;31:100516. doi: 10.1016/j.gore.2019.100516. PMID: 31886403; PMCID: PMC6921151.
- Gichangi PB, Bwayo J, Estambale B, De Vuyst H, Ojwang S, Rogo K, Abwao H, Temmerman M. Impact of HIV infection on invasive cervical cancer in Kenyan women. AIDS. 2003 Sep 5;17(13):1963-8. doi: 10.1097/00002030-200309050-00015. PMID: 12960829.
- World Health Organization. Working Together for Health: The World Health Report 2006; World Health Organization: Geneva, Switzerland, 2018.
- Ruddies F, Gizaw M, Teka B, Thies S, Wienke A, Kaufmann AM, Abebe T, Addissie A, Kantelhardt EJ. Cervical cancer screening in rural Ethiopia: a cross- sectional knowledge, attitude and practice study. BMC Cancer. 2020 Jun 17;20(1):563. doi: 10.1186/s12885-020-07060-4. PMID: 32552740; PMCID: PMC7298871.
- Moucheraud C, Kawale P, Kafwafwa S, Bastani R, Hoffman RM. "It is big because it's ruining the lives of many people in Malawi": Women's attitudes and beliefs about cervical cancer. Prev Med Rep. 2020 Apr 8;18:101093. doi: 10.1016/j.pmedr.2020.101093. PMID: 32322461; PMCID: PMC7168763.
- Nyamambi E, Murendo C, Sibanda N, Mazinyane S. Knowledge, attitudes and barriers of cervical cancer screening among women in Chegutu rural district of Zimbabwe. Cogent Social Sciences. 2020;6(1):1766784.
- Drokow EK, Zi L, Han Q, Effah CY, Agboyibor C, Sasu E, Akpabla GS, Foli F, Sun K. Awareness of Cervical Cancer and Attitude Toward Human Papillomavirus and Its Vaccine Among Ghanaians. Front Oncol. 2020 Sep 8;10:1651. doi: 10.3389/fonc.2020.01651. PMID: 33014828; PMCID: PMC7506130.
- Olubodun T, Odukoya OO, Balogun MR. Knowledge, attitude and practice of cervical cancer prevention, among women residing in an urban slum in Lagos, South West, Nigeria. Pan Afr Med J. 2019 Mar 18;32:130. doi: 10.11604/pamj.2019.32.130.14432. PMID: 31223418; PMCID: PMC6561126.
- Kimondo FC, Kajoka HD, Mwantake MR, Amour C, Mboya IB. Knowledge, attitude, and practice of cervical cancer screening among women living with HIV in the Kilimanjaro region, northern Tanzania. Cancer Rep (Hoboken). 2021 Mar 19:e1374. doi: 10.1002/cnr2.1374. Epub ahead of print. PMID: 33739611.
- Adewumi K, Nishimura H, Oketch SY, Adsul P, Huchko M. Barriers and Facilitators to Cervical Cancer Screening in Western Kenya: a Qualitative Study. J Cancer Educ. 2021 Jan 7. doi: 10.1007/s13187-020-01928-6. Epub ahead of print. PMID: 33411253.
- Georges K, Armel Herve NK, Simo Richard T, Jeremie MA, Charlette N. Knowledge and behavior of women on cervical cancer in the northern region of Cameroon 2. 2017.
- Almobarak AO, Elbadawi AA, Elmadhoun WM, Elhoweris MH, Ahmed MH. Knowledge, Attitudes and Practices of Sudanese Women Regarding the Pap Smear Test and Cervical Cancer. Asian Pac J Cancer Prev. 2016;17(2):625-30. doi: 10.7314/apjcp.2016.17.2.625. PMID: 26925654.
- Eshete M, Abdulwuhab Atta M, Yeshita HY. Cervical Cancer Screening Acceptance among Women in Dabat District, Northwest Ethiopia, 2017: An Institution-Based Cross-Sectional Study. Obstet Gynecol Int. 2020 Feb 7;2020:2805936. doi: 10.1155/2020/2805936. PMID: 32089698; PMCID: PMC7029298.
- Anya SE, Oshi DC, Nwosu SO, Anya AE. Knowledge, attitude, and practice of female health professionals regarding cervical cancer and Pap smear. Niger J Med. 2005 Jul-Sep;14(3):283-6. PMID: 16350698.
- Anorlu RI, Orakwue CO, Oyeneyin L, Abudu OO. Late presentation of patients with cervical cancer to a tertiary hospital in Lagos: what is responsible? Eur J Gynaecol Oncol. 2004;25(6):729-32. PMID: 15597852.
- Dulla D, Daka D, Wakgari N. Knowledge about cervical cancer screening and its practice among female health care workers in southern Ethiopia: a cross-sectional study. Int J Womens Health. 2017 May 22;9:365-372. doi: 10.2147/IJWH.S132202. PMID: 28579837; PMCID: PMC5446960.
- Ifemelumma CC, Anikwe CC, Okorochukwu BC, Onu FA, Obuna JA, Ejikeme BN, Ezeonu OP. Cervical Cancer Screening: Assessment of Perception and Utilization of Services among Health Workers in Low Resource Setting. Int J Reprod Med. 2019 Feb 3;2019:6505482. doi: 10.1155/2019/6505482. PMID: 30854395; PMCID: PMC6377970.
- Anorlu RI, Banjo AA, Odoemhum C. Cervical cancer and cervical cancer screening: level of awareness in women attending a primary health care facility in Lagos. Nigeria Postgraduate Medical Journal. 2000;70(1):25-28.
- Ayinde OA, Omigbodun AO. Knowledge, attitude and practices related to prevention of cancer of the cervix among female health workers in Ibadan. J Obstet Gynaecol. 2003 Jan;23(1):59-62. doi: 10.1080/0144361021000043272. PMID: 12623487.
- Anya SE, Oshi DC, Nwosu SO, Anya AE. Knowledge, attitude, and practice of female health professionals regarding cervical cancer and Pap smear. Niger J Med. 2005 Jul-Sep;14(3):283-6. PMID: 16350698.
- Adanu RM, Seffah JD, Duda R, Darko R, Hill A, Anarfi J. Clinic visits and cervical cancer screening in accra. Ghana Med J. 2010 Jun;44(2):59-63. doi: 10.4314/gmj.v44i2.68885. PMID: 21327005; PMCID: PMC2994147.
- Awodele O, Adeyomoye AA, Awodele DF, Kwashi V, Awodele IO, Dolapo DC. A study on cervical cancer screening amongst nurses in Lagos University Teaching Hospital, Lagos, Nigeria. J Cancer Educ. 2011 Sep;26(3):497-504. doi: 10.1007/s13187-010-0187-6. PMID: 21222192; PMCID: PMC3161190.
- Gebreegziabher M, Asefa NG, Berhe S. Factors affecting the practices of cervical cancer screening among female nurses at public health institutions in Mekelle town, Northern Ethiopia, 2014: a cross-sectional study. Journal of Cancer Research. 2016.
- Aswathy S, Quereshi MA, Kurian B, Leelamoni K. Cervical cancer screening: Current knowledge & practice among women in a rural population of Kerala, India. Indian J Med Res. 2012 Aug;136(2):205-10. PMID: 22960886; PMCID: PMC3461731.
- Teguete I, Muwonge R, Traore CB, Dolo A, Bayo S, Sankaranarayanan R. Can visual cervical screening be sustained in routine health services? Experience from Mali, Africa. BJOG. 2012 Jan;119(2):220-6. doi: 10.1111/j.1471-0528.2011.03122.x. Epub 2011 Sep 6. PMID: 21895956.
- Fall NS, Tamalet C, Diagne N, Fenollar F, Raoult D, Sokhna C, Lagier JC. Feasibility, Acceptability, and Accuracy of Vaginal Self-Sampling for Screening Human Papillomavirus Types in Women from Rural Areas in Senegal. Am J Trop Med Hyg. 2019 Jun;100(6):1552-1555. doi: 10.4269/ajtmh.19-0045. PMID: 30994102; PMCID: PMC6553900.
- de la Statistique AN. de la Démographie (ANSD) Sénégal. Sénégal, Calverton, Maryland: ANSD and Macro International, Inc. 2010.
- Mboumba Bouassa RS, Gubavu C, Veyer D, Robin L, Gravier A, Hocqueloux L, Prazuck T, Péré H, Bélec L; ANRS ImmiPap Study Group. High Prevalence of Cervical High-Risk Human Papillomavirus Harboring Atypical Genotypes in Human Immunodeficiency Virus -Infected and -Uninfected First-Generation Adult Immigrant Women Originating from Sub-Saharan Africa and Living in France. J Immigr Minor Health. 2021 Apr;23(2):308-319. doi: 10.1007/s10903-020-01074-7. PMID: 32816173; PMCID: PMC7914190.
- Clifford GM, Gallus S, Herrero R, Muñoz N, Snijders PJ, Vaccarella S, Anh PT, Ferreccio C, Hieu NT, Matos E, Molano M, Rajkumar R, Ronco G, de Sanjosé S, Shin HR, Sukvirach S, Thomas JO, Tunsakul S, Meijer CJ, Franceschi S; IARC HPV Prevalence Surveys Study Group. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet. 2005 Sep 17-23;366(9490):991-8. doi: 10.1016/S0140-6736(05)67069-9. PMID: 16168781.
- Piroozmand A, Mostafavi Zadeh SM, Madani A, Soleimani R, Nedaeinia R, Niakan M, Avan A, Manian M, Moradi M, Eftekhar Z. The Association of High Risk Human Papillomaviruses in Patients With Cervical Cancer: An Evidence Based Study on Patients With Squamous Cell Dysplasia or Carcinoma for Evaluation of 23 Human Papilloma Virus Genotypes. Jundishapur J Microbiol. 2016 Feb 17;9(4):e32728. doi: 10.5812/jjm.32728. PMID: 27279992; PMCID: PMC4895315.
- Mbulawa ZZ, Coetzee D, Williamson AL. Human papillomavirus prevalence in South African women and men according to age and human immunodeficiency virus status. BMC Infect Dis. 2015 Oct 26;15:459. doi: 10.1186/s12879-015-1181-8. PMID: 26502723; PMCID: PMC4624185.
- Tounkara FK, Téguété I, Guédou FA, Keita B, Alary M. Prevalence and Factors Associated With HIV and Sexually Transmitted Infections Among Female Sex Workers in Bamako, Mali. Sex Transm Dis. 2020 Oct;47(10):679-685. doi: 10.1097/OLQ.0000000000001231. PMID: 32932403.
- Mbulawa ZZA, van Schalkwyk C, Hu NC, Meiring TL, Barnabas S, Dabee S, Jaspan H, Kriek JM, Jaumdally SZ, Muller E, Bekker LG, Lewis DA, Dietrich J, Gray G, Passmore JS, Williamson AL. High human papillomavirus (HPV) prevalence in South African adolescents and young women encourages expanded HPV vaccination campaigns. PLoS One. 2018 Jan 2;13(1):e0190166. doi: 10.1371/journal.pone.0190166. PMID: 29293566; PMCID: PMC5749739.
- Ogembo RK, Gona PN, Seymour AJ, Park HS, Bain PA, Maranda L, Ogembo JG. Prevalence of human papillomavirus genotypes among African women with normal cervical cytology and neoplasia: a systematic review and meta-analysis. PLoS One. 2015 Apr 14;10(4):e0122488. doi: 10.1371/journal.pone.0122488. PMID: 25875167; PMCID: PMC4396854.
- Keehn DC, Chamberlain RM, Tibbits M, Kahesa C, Msami K, Soliman AS. Using Key Informants to Evaluate Barriers to Education and Acceptability of the HPV Vaccine in Tanzania: Implications for Cancer Education. J Cancer Educ. 2020 May 26:10.1007/s13187-020-01773-7. doi: 10.1007/s13187-020-01773-7. Epub ahead of print. PMID: 32451878; PMCID: PMC7688505.
- Mabeya H, Odunga J, Broeck DV. Mothers of adolescent girls and Human Papilloma Virus (HPV) vaccination in Western Kenya. Pan Afr Med J. 2021 Feb 4;38:126. doi: 10.11604/pamj.2021.38.126.21359. PMID: 33912296; PMCID: PMC8051220.
- O'Donovan J, O'Donovan C, Nagraj S. The role of community health workers in cervical cancer screening in low-income and middle-income countries: a systematic scoping review of the literature. BMJ Glob Health. 2019 May 13;4(3):e001452. doi: 10.1136/bmjgh-2019-001452. PMID: 31179040; PMCID: PMC6528769.
- Ayoub N, Sunwoo JB, Starmer HM. Implementation of a targeted HPV educational program in a population with HIV. World J Otorhinolaryngol Head Neck Surg. 2019 Feb 23;5(2):105-111. doi: 10.1016/j.wjorl.2018.09.006. PMID: 31334489; PMCID: PMC6617159.
- Knaul FM, Bhadelia A, Rodriguez NM, Arreola-Ornelas H, Zimmermann C. The lancet commission on palliative care and pain reliefdfindings, recommendations, and future directions. Lancet Glob Heal. 2018;6.
- van der Plas WY, Benjamens S, Kruijff S. The increased need for palliative cancer care in Sub-Saharan Africa. Eur J Surg Oncol. 2020 Jul;46(7):1373-1376. doi: 10.1016/j.ejso.2020.03.212. Epub 2020 Mar 27. PMID: 32265092.
- Denny L, Anorlu R. Cervical cancer in Africa. Cancer Epidemiol Biomarkers Prev. 2012 Sep;21(9):1434-8. doi: 10.1158/1055-9965.EPI-12-0334. Epub 2012 Jul 17. PMID: 22806169.
- Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, Hingmire S, Malvi SG, Thorat R, Kothari A, Chinoy R, Kelkar R, Kane S, Desai S, Keskar VR, Rajeshwarkar R, Panse N, Dinshaw KA. HPV screening for cervical cancer in rural India. N Engl J Med. 2009 Apr 2;360(14):1385-94. doi: 10.1056/NEJMoa0808516. PMID: 19339719.
- Denny L, Kuhn L, Hu CC, Tsai WY, Wright TC Jr. Human papillomavirus-based cervical cancer prevention: long-term results of a randomized screening trial. J Natl Cancer Inst. 2010 Oct 20;102(20):1557-67. doi: 10.1093/jnci/djq342. Epub 2010 Sep 30. PMID: 20884893.
- Clendinen C, Zhang Y, Warburton RN, Light DW. Manufacturing costs of HPV vaccines for developing countries. Vaccine. 2016 Nov 21;34(48):5984-5989. doi: 10.1016/j.vaccine.2016.09.042. Epub 2016 Oct 19. PMID: 27771183.
- Munira SL, Hendriks JT, Atmosukarto II, Friede MH, Carter LM, Butler JRG, Clements ACA. A cost analysis of producing vaccines in developing countries. Vaccine. 2019 Feb 21;37(9):1245-1251. doi: 10.1016/j.vaccine.2018.11.050. Epub 2019 Jan 14. PMID: 30651198.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.