> Environmental Sciences. 2021 October 21;2(10):945-953. doi: 10.37871/jbres1336.
Status of Chinese Carp Fisheries in Tunisian Freshwater Reservoirs: Threats and Opportunities
Sami Mili1*, Rim Ennouri1, Manel Fatnassi1, Tahani Chargui1, Hajer Zarrouk1 and Houcine Laouar2
2Technical Center of Aquaculture, 5. Rue du Sahel Montfleury, 1009 Tunis, Tunisia
- Management
- Fisheries
- Chinese carps
- Ctenopharyngodon idella
- Aristichthys nobilis
- Hypophthalmichthys molitrix
- Freshwater reservoirs
- Tunisia
Abstract
The current paper aims to diagnose the state of fisheries of three Chinese carp species whish have been introduced in Tunisian reservoirs since 1981: (silver carp Hypophthalmichthys molitrix, herbivorous carp Ctenopharyngodon idella and bighead carp Aristichthys nobilis) and seeks to study the benefits and risks associated with their introduction.
Chinese carps cannot reproduce naturally in freshwater reservoirs. Eventually, artificial breeding operations and seeding of the dams with farm-produced fry are carried out by the Technical Centre of Aquaculture every year.
Statistical analyses have shown a strong correlation between the landed quantity and the number of fries stocked each year. The impact assessment showed that the risks and benefits associated with the introduction of the three species are variable. Regarding their benefits, it was clear that the herbivorous carp has provided effective and sustainable control of the extensive development of aquatic vegetation in the eutrophic reservoirs and canal systems. The value of the other two species, though, remains less obvious, particularly for the bighead carp. The consequences of their introduction on ecosystems and native species seem to be negligible, especially when the densities are low.
Eventually, it seems judicious to increase the stocking of the herbivorous carp, silver carp and bighead carp in Tunisian reservoirs.
Introduction
The Tunisian experience in the exploitation of reservoirs by fishing activities goes back to the 1960s. Several attempts to introduce various species of Asian, European and African origins have been made since then. These introductions have been conducted either because of these species’ commercial value, such as the pike-perch Sander lucioperca, the catfish Silurus glanis and the black bass Micropterus salmoides, or their use as forage fish, it is the case with the roach Rutilus rubilio and rudd Scardinius erythrophtalmus [1]. Reservoirs are actively managed through the annual stocking of fry (mullet, Chinese carp, zander and black bass) and the transfer of broodstock (roach, rudd, zander and tilapia). Production in these reservoirs has increased over the last twenty years from 840-1350 [2].
In 2019 alone, the number of boats was estimated at 250 which corresponding to the fishing capacity [2], with 500 fishermen (2 fishermen per boat) as stipulated by law. This activity is dependent on obtaining a fishing license from the regional administration (the Regional Commissariats for Agricultural Development). The species commonly caught are the hogfish Lisa ramada, the bighead mullet Mugil cephalus, the common carp and the zander [2].
Species introductions represent one of the major current threats to biodiversity, along with pollution, overexploitation and habitat loss or destruction [3-5]. There are various reasons why fish is being introduced into reservoirs: aquaculture, commercial or recreational fishing, aquaristics and wetland management [5,6]. Indeed, more than 1205 cases of species introduction for aquaculture have been recorded worldwide [7]. However, the vast majority of introduced species fail to become established and those that do generally have little impact on endogenous species or the native environment [8,9]. The main disturbances described on native species and ecosystems are hybridization, predation, competition, extirpation, spread of diseases or parasites, disruption of habitats, biogeochemical cycles and food webs [6,10-12]. The interaction between the introduced species and ecosystems determines the fate of the introduction. This introduction also depends on the intensity of stocking, the number of species introduced and the frequency of their introductions [5,13,14].
The Tunisian freshwater fauna has been studied showing the presence of 21 species with 5 groups introduced and established in Tunisian reservoirs [15-19]. It looks like this is a weak number when compared to the 13000 species of freshwater fish described worldwide [20]. Of these species, 3 were introduced from Asia and 7 from Europe [1,17,19]. The majority of the ichthyological fauna currently living in Tunisian reservoirs was introduced during the last 50 years [1,15,19]. It is important to note that the impact of fish species introductions in Tunisian reservoirs is not documented. This is the case of the three Chinese carp species: the silver carp Hypophthalmichthys molitrix, the herbivorous carp Ctenopharyngodon idella and the bighead carp Aristichthys nobilis, which were introduced to over 70 countries [21,22] for experimental purposes of aquatic plant, phytoplankton control [15,23,24] and biological water quality control [25,26].
These three carp species have similar biological features: they are fast-growing eurioic animals that can reach very large sizes (>100 cm) and weights (> 20 kg) [5]. However, their diet differs to a great extent: the herbivorous carps mainly consume macrophytes and filamentous algae; the silver carps are mainly phytoplanktonophagous and the bighead carps are zooplanktonophagous [27]. These species are of considerable economic importance at the global level as they alone account for more than 10 million tons produced each year [5]. Eventually, they have been introduced into many countries over the past 70 years for biological control and aquaculture [5]. The natural reproduction of these carps was observed in a few countries whose ecosystems are similar to these species’ native environment and it has been demonstrated that they reproduce with difficulty when placed outside their natural habitat. These carps, in fact, require primarily large rivers with strong current (1 m/s), large and rapid water level variations (1-2 m) and optimal temperatures between 20 and 25°C in summer [5]. Since these conditions do not exist in Tunisia, these carps are unlikely to reproduce naturally.
The present paper intends to provide an overview of the current status of the Chinese carp fisheries in the Tunisian reservoirs and to investigate both benefits and threats associated with their introductions. In addition, it represents an analysis of the catches of the Chinese carps introduced in Tunisia: the herbivorous carp C. idella, the silver carp H. molitrix and the bighead carp A. nobilis.
Materials and Methods
Presentation of the Chinese carp species
C. idella, which is native to East Asia [28], was introduced into Tunisia in 1981 from Hungary [29]. According to Teletchea and Le Doré [5], the herbivorous carp tolerates temperatures ranging from 0 to 33°C. This species prefers well-oxygenated waters, but can endure very low oxygen concentrations, up to 0.2 mg/l [28,30]. C. idella larvae feed on zooplankton, and when they reach the size of 20 mm they become exclusively herbivorous [28,31-33]. This species can grow to a maximum size of 120 cm with a weight varying between 5 and 10 kg [32,34]. At the ecological level, C. idella is considered beneficial as it selectively removes aquatic vegetation.
H. molitrix (Valenciennes, 1844) is native of large rivers in southern Asia, eastern China and the far east of Russia [5]. This freshwater species lives in a temperature between 6 and 28°C [5]. It has low dissolved oxygen requirements and can tolerate oxygen levels close to zero for short periods [30]. H. molitrix can live in brackish waters with salinity between 5 and 12‰ according to its developmental stage. This species prefers open and well eutrophic areas and occupies the upper and middle zones in the water column [35]. H. molitrix is a passive predator of zooplankton and a passive grazer of phytoplankton [36]. Silver carp measures on average 40-60 cm [30,32]. It also seems to have a fairly long lifespan [35]. The ecological value of this species resides in the cleaning of reservoirs and water bodies from harmful algae through water filtration [36].
A. nobilis (Richardson, 1845) is native to eastern China, eastern Siberia and extreme North Korea. This species is a eurothermic fish, with ability to tolerate water temperatures ranging from 0.5 to 38°C [35]. A. nobilis is able to withstand salinity up to 15-20‰ [35]. Kottelat and Freyhof [32] indicate that this species feeds mainly on zooplankton and algae. It can also ingest large amounts of detritus, including mineral particles and organic substances [35]. It can grow to nearly 1.5 m in length and 40 kg in weight [32,35,37]. The ecological value of A. nobilis consists mainly in its ability to reduce accelerated eutrophication.
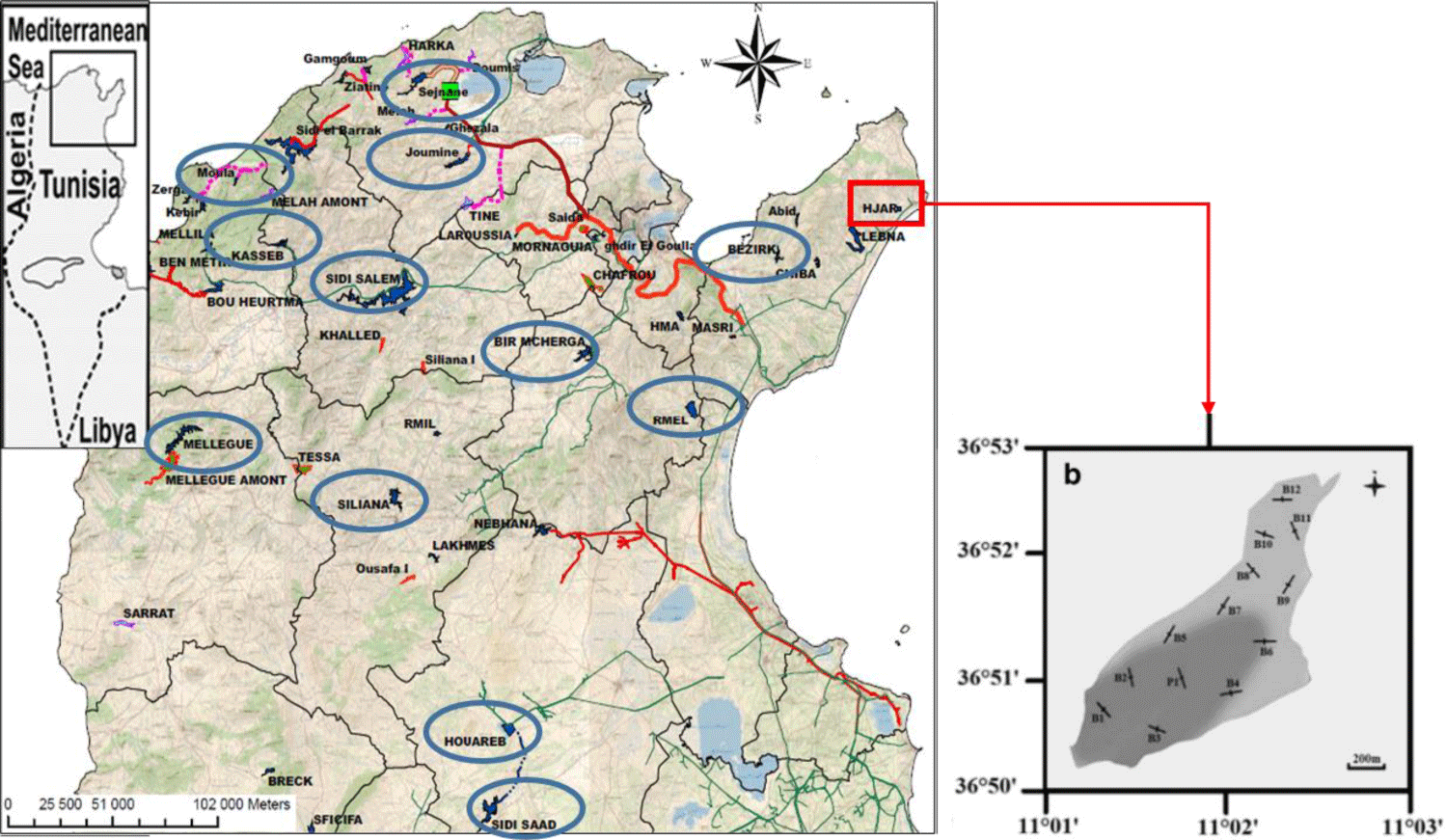
The objective of this paper is to provide an appraisal of the current state of the three Chinese carp fisheries, namely the herbivorous carp C. idella, the bighead carp A. nobilis and the silver carp H. molitrix in the Tunisian reservoirs (Figure 1), considering both benefits and risks associated with their introductions.
To achieve the aforementioned objectives of this article, we analyzed data provided by the Technical Centre of Aquaculture (TCA) and the General Directorate of Fisheries and Aquaculture for the period between 2009 (the date of the center creation and the beginning of the stocking of dams with produced fry) and 2018. Fishing operations were conducted in 2018 in one typical reservoir to estimate the percentage of Chinese carps in the total carp production. Secondly, the quantity of stocked fry was correlated to the production. As a next step, we investigated the effects related to the introductions of these species with reference to previous studies.
The fish were caught in Lahjar reservoir (36° 50′ 18″ N/36° 52′ 51″ N and 11° 01′ 14″ E/11° 02′ 37″ E) prior to the Chinese carps spawning season in May 2018 (Figure 1) [19]. In order to catch carps, 20 specially designed fishing nets were constructed. The monofilament gill nets were of different mesh sizes: 40, 50, 60, and 70 mm from knot to knot. These meshes were chosen according to the studies of Fridman [38] and Smith [39], which relate the fish length to net mesh size. We set the smallest mesh size at 40 mm in order to allow the young specimens to escape. Net dimensions were 4 × 50 m. Thread diameter was 0.23 mm for all nets. The hanging ratio was 0.5 for horizontal mesh sizes and for lateral ropes. A total of 8 kg of lead was fixed in the footrope and 60 floats (Ø = 60 mm) were attached to the headline [40].
History of carp introduction in Tunisia
A careful examination of the INSTOP archives showed that the three species of Chinese carps, being investigated, were introduced in Tunisian reservoirs in 1981 for experimental purposes of aquatic plant, phytoplankton control and biological control of water quality [15]. This introduction also aimed to provide an alternative source of animal protein for the rural population and to increase their income. It should be noted that this introduction was preceded by that of the common carp Cyprinus carpio communis, imported from Germany and France in 1965-1966 [41].
These species have shown great adaptation to Tunisian limnic ecosystems [15]. The results of the successful introduction of the Chinese carps led the fish managers to carry out artificial breeding operations and to set up the pilot continental fish farming station of Boumhel. For this, the Technical Centre of Aquaculture (TCA) ensured the artificial breeding of the Chinese carp species and the stocking of the reservoirs since 2009.
Results
Stocking
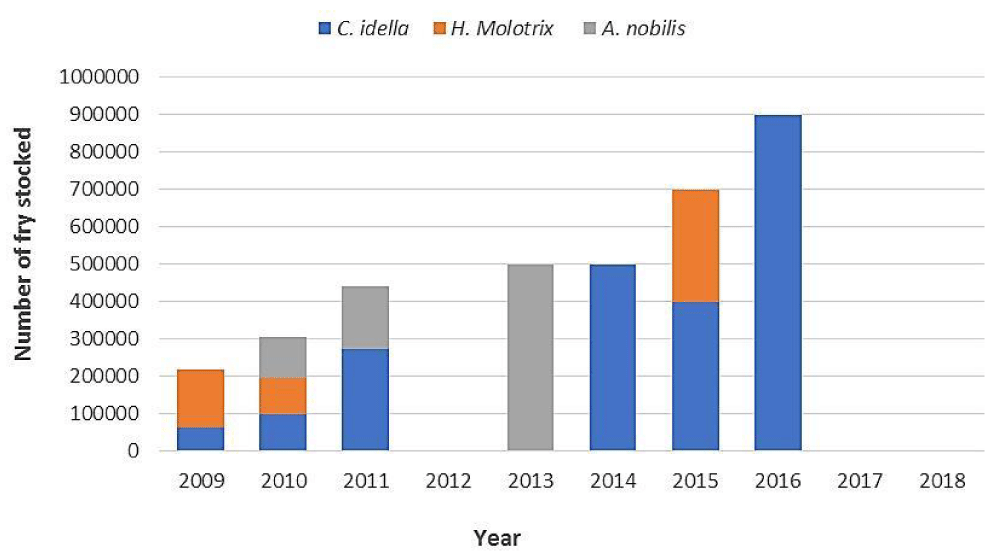
The Technical Centre of Aquaculture conducts annual breeding operations for the Chinese carps by using hormones and stimulants such as Luteinizing Release Hormone (LH), Human Chorionic Gonadotropin (HCG) or pituitary extract from common carps [42]. Chinese carps are stocked at the larval stage (age 5 days; size between 4 and 5 mm and weight 0.1 g) due to the ease of their transport and handling at this phase. However, the average mortality rate at this age is estimated at 50% [42]. Figure 2 summarizes the quantities of Chinese carp stocked in Tunisian reservoirs. The quantities stocked vary annually depending on the demand of the reservoir operators and the availability of brood stock.
In 2012, stocking did not take place due to security concerns in the country after the revolution. In 2017, seeding became fee-based causing resistance from operators to order Chinese carp fry.
Production
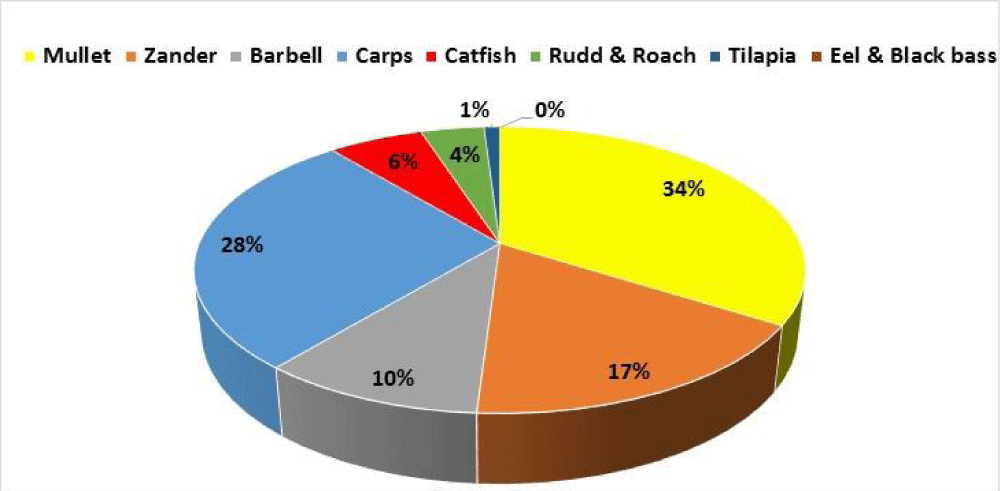
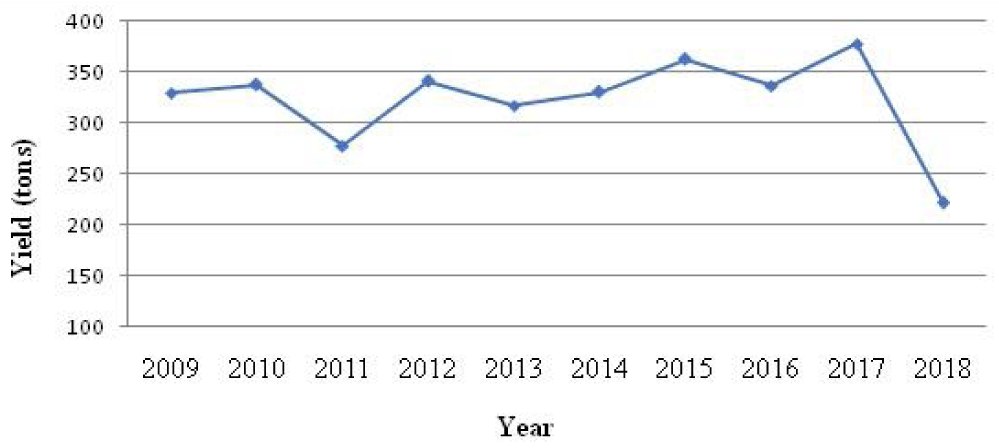
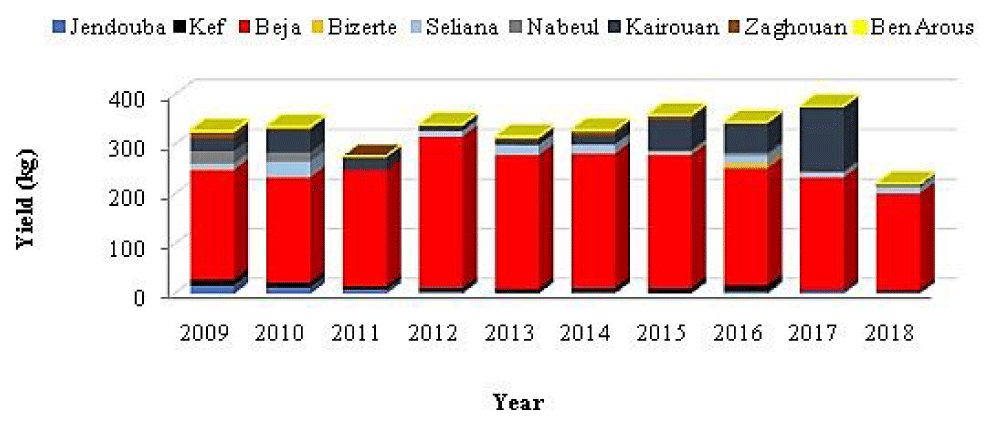
Carp production data were collected from the fishing districts and the General Directorate of Fisheries and Aquaculture. Data related to carp by species are missing; the quantities of Chinese carps and common carps are grouped together in the production statistics. The landed Tunisian carps (all species: common and Chinese) represent 28% of the Tunisian freshwater fish farming production (Figure 3) and they do not exceed 380 tonnes per year (Figure 4).
At the regional level, the governorate of Beja contributes with 60% of the total Tunisian carp production (Figure 5). This production ranged between 196 tons in 2018 and 304.5 tons in 2012 with a significant annual variability (p > 0.05) which can be explained by the fluctuation of the seeding operations as well as the state of the stocks of this resource at the level of the reservoirs in Tunisia.
Carp production in a typical reservoir
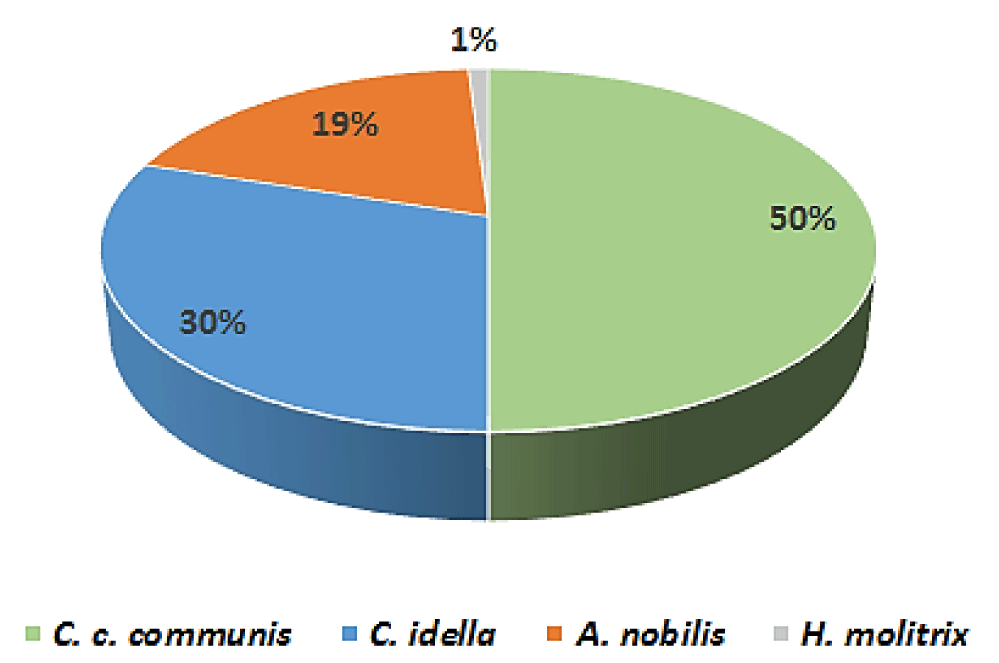
The four experimental fishing operations carried out in a typical reservoir (in the Cap Bon region) with a seasonal frequency in 2018 allowed us to determine the catch rate of the Chinese carps compared to common carps. The analysis of the data collected during one year showed that common carps represent 50% of the total carp production of this reservoir, while the Chinese carps constitute the other 50%: 30% of herbivorous carps, 19% of bighead carps and 1% of silver carps (Figure 6).
The conducted fishing operations allowed us to capture A. nobilis with a weight varying between 8 and 17 kg and herbivorous carps with weight ranging from 3 to 7 kg. The silver carps which were caught range in weight from 1.75 to 3.5kg. The results of fishing operations confirm that the herbivorous carp is more abundant than the other two Chinese carp species.
Stocking-production correlation
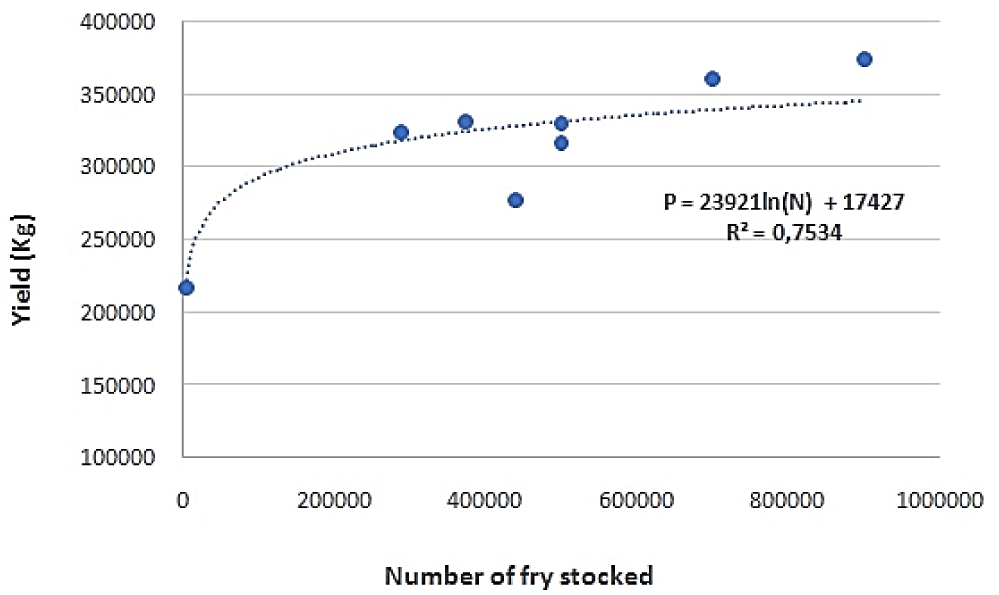
Statistical analyses showed a strong correlation (R² = 0.7534) between the quantities of the landed carps (all species included "P") and the number of stocked fry "N" each year in Tunisian reservoirs. This correlation is governed by the following equation (Figure 7): p = 23921ln (N)+17427.
This result shows that the stocking of Chinese carp fry determines the total carp production.
Discussion
The stocking of Chinese carp fry impacts the total carp production. An increase in this production can be reported in the instance of reservoir’ stocking, while a stability in the production of the settled common carp is noticed when no stoking take place.
It is noted that the absence of the Chinese carps, despite stocking, in some reservoirs results from the death of the stocked fry by lack of food or due to deacclimation. In addition, predatory fish or other animals can cause serious stock losses. The total absence of submerged aquatic macrophytes and the scarce levels of emergent aquatic vegetation means that there is a serious lack of food for these omnivorous and herbivorous species [15].
Benefits of the Chinese carp introduction
The Chinese carps (H. molitrix, C. idella and A. nobilis) have a number of advantages for farming. Firstly, they have a rapid growth rate and can reach a very large size. They can be produced at low cost and may be fed on natural and artificial food [43]. In addition, juveniles can be obtained by large-scale artificial reproduction at low cost. Production management of these species is simple and the rearing period is short [5,44]. It should be noted that in many countries, the Chinese carp species are reared together in polyculture (i.e., 2 or 3 species) in association with other species (integrated aquaculture) such as common carp, tilapia, black bass or catfish [43,45]. Unlike carnivorous species, the production of the Chinese carps does not require the use of oil or animal meal, which is obviously a very important advantage for the sustainable development of aquaculture [5,43,46,47].
In addition, these species are highly valued in many regions and are of considerable importance for human consumption [5,31,35,44]. They have a high protein concentration and a low lipid concentration [5,48,49]. Their flesh is considered tasty and it constitutes an important source of protein for human consumption [5,31,50-52]. However, small bighead carp species do not have very good meat quality [53].
Besides, Chinese carps have a complementary feeding activity: C. idella control macrophytes, H. molitrix and A. nobilis control phytoplankton blooms that may result from macrophyte depletion [5,27,35,54,55]. Ctenopharyngodon idella can control overgrowth of aquatic vegetation and replace the (costly) practice of frequent mowing and the use of chemical treatments [5,30,50,56,57]. The benefits of using C. idella to control the development of aquatic vegetation include longevity of the method, constant consumptive activity against the growth of unwanted weeds and very low costs on the long-term [5,28,31,51,52(A&B),58]. Moreover, this species does not show any aggression towards other fish [50]. H. molitrix are also used for their ability to clean reservoirs and other water bodies from harmful algae [5,44,59]. They can also consume toxic blue-green algae whose toxins have a harmful impact on both animals and humans [35]. Furthermore, it has been reported that silver and bighead carps can limit zooplankton and phytoplankton populations by reducing their concentrations in aquaculture facilities [5,35,37,54,60-63].
About half of the ingested nutrients contained in aquatic plants are used and digested by carps, the other half passes through the digestive tract in part digested and, in another part, fragmented [5,31,49,64]. These nutrient-rich feces can either remain in the water body or accumulate in the sediment [64]. This increase in nutrient abundance can also lead to an increase in phytoplankton and consequently in the whole food chain: zooplankton and zoobenthos, which are favorable to planktivorous species [5,31,64]. The introduction of carp may also indirectly favor the increase in growth and survival of fish species due to the increase in planktivorous or faecal-feeding species [31]. In polyculture, there is an increase in the production of faecal-feeding fish [52]. The presence of H. molitrix, C. idella and A. nobilis can have a beneficial effect in polyculture on the growth of common carp or tilapia as these benthic fish cause the resuspension of organic matter [35]. In conclusion, the rearing of these species is a very efficient and sustainable way to transform aquatic plants into animal protein [5]. In Tunisia, Chinese carps have been introduced to control the proliferation of aquatic vegetation due to eutrophication in various water bodies [15]. In addition, a study in Morocco combining silver and grass carps showed that these species can improve domestic wastewater quality and grow well providing an important meat source [5,65]. Also, polyculture of A. nobilis with other species, especially H. molitrix, can significantly improve the ecological benefit of silver carp culture. This combination improves water quality by continuously removing plankton and decreasing the likelihood of sudden plankton die-off in the fish culture [5,35,53]. In conclusion, the three Chinese carps seem to have a positive effect on the native fish fauna and the ecosystem [5,66].
Risks associated with the Chinese carp introduction
The introduction of C. idella into an aquatic system can affect directly aquatic macrophytes, various living items including plankton, benthic macroinvertebrates, fish, some vertebrates and indirectly water quality [5,28,30,31,52,58,67,68]. Herbivorous carps consume all vegetation, which should provide important food reserves for some mammals, waterbirds, amphibians, reptiles and various invertebrates [68].
Macrophytes serve as refuges and egg-laying sites for many species [5,30,31,68,69]. Thus, it has been shown that a very strong decrease in macrophytes can affect the reproduction of certain species such as pike (Esox lucius) or perch (Perca fluvatilis) [28]. In addition, submerged macrophytes are important for nutrient dynamics, invertebrate-fish interactions and water quality [5,68]. Furthermore, the decrease in macrophytes allows for a significant increase in phytoplankton, as there is less competition for light and nutrients [58]. In addition, it has been shown that H. molitrix and A. nobilis lead to an increase in nanoplankton concentration, a decrease in zooplankton populations and consequently an increase in water turbidity [5,35,70-72]. This significant change in phytoplankton is beneficial to smaller species. There may be direct food competition between these introduced carps and native species that depend on plankton in young or adult stages [5,44,71]. Studies made in US (Missouri and Illinois rivers) have shown a negative impact of the introduction of A. nobilis on plankton-eating species native to both rivers, such as bullfish (Ictiobus cyprinellus), spoonbill (Polyodon spathula) [60,73] or the hickory shad Dorosoma cepedianum [74,75].
In addition, the introduction of C. idella, A. nobilis and H. molitrix can lead to a change (increase or decrease) in nutrient concentration, a decrease in dissolved oxygen and an increase in sediments nitrogen due to a surplus of feces. These effects in turn lead to an increase in water turbidity following an undesired increase in phytoplankton biomass production [5,35,48,70]. The latter can contribute to eutrophication of the water. Changes are most often observed when density is high [5,28]. In fact, Domaizon and Devaux [36] show that water quality improvement is linked to the introduction of moderate biomass.
Overall, it is not clear how to accurately assess the impact of these species’ introduction into reservoirs. Several authors have confirmed that current knowledge does not allow to accurately predict the long-term effects of the Chinese carp introduction. The negative impacts of introduction of C. idella, A. nobilis and H. molitrix are complex to evaluate and depend on many factors, such as the native fish community, the structure and the ecosystem functioning [5,28,31,35,52,58,64,68,71]. However, it is clear that the density of fish introduced into a reservoir strongly controls the impacts.
Interestingly, since these three carps are not known to hybridize with native species, the risk of genetic consequences on the native group remains low [5,35,37].
Another important factor which has been investigated is their vulnerability to disease. It has been reported that these carps are susceptible to many diseases and parasites, including viruses, bacteria, fungi, protozoa, trematodes, nematodes [5,43]. Several studies investigated disease control under growing conditions and the most studied illness is haemorrhagic disease which is caused by a viral agent. These studies helped introduce effective preventive measures, particularly a vaccine, which has been developed and successfully applied [43].
Not to forget that the Chinese carps are rather elusive species, very good jumpers and good fighters. They present a danger to fishermen due to their dynamic behavior when they are caught [5,35,44,48,67].
In relation with the environment, in general, these species would not have major negative impacts [5,53].
This article has shown that the Chinese carp species are exclusively confined to reservoirs in Tunisia. No specimen of carp was caught in running water. Consequently, the impacts of these species are strictly limited to the enclosed waters of the reservoirs. Currently, the reproduction of these carps is impossible in natural environment in Tunisia because they have a very particular reproduction biological requirement. Indeed, the breeding of these species necessitates large rivers with very important variations of water level during spring and summer, a high temperature and a strong flow. Since reproduction is unlikely to occur in Tunisia, their expansion depends exclusively on the stocking of fry and larvae in reservoirs. Furthermore, in case any problem arises it is easy to fix it through capturing these species with gillnets or purse seines given their size. The benefits and risks associated with the introduction of the three Chinese carps are different. C. idella provides effective and sustainable control of overgrowth of aquatic vegetation and thus replaces mowing and the use of chemicals. Its consequences on the ecosystem and on native species are negligible or even insignificant if low densities are used. The benefit of the other two species is less obvious, especially for A. nobilis that can be exploited for its flesh. Moreover, the impacts (or risks) on the ecosystems of H. molitrix and A. nobilis are complex to demonstrate.
In conclusion, the information provided by this paper will allow to:
- Help fisheries managers, environmental administrators and other users to improve the quality of fishing statistics collection in Tunisian reservoirs;
- Encourage the stocking of Tunisian reservoirs with the three species of Chinese carp given their economic and ecological values (fight against aquatic plants, phytoplankton and biological control of water quality); and
- Study the mortality rate of the Chinese carp species stocked in Tunisian dams.
Acknowledgment
This work is part of a collaborative project involving the Technical Centre of Aquaculture, the Higher Institute of Fisheries and Aquaculture of Bizerte and the General Directorate of Fisheries and Aquaculture in Tunisia.
We are most thankful to the Institution of Agricultural Research and Higher Education (IRESA) which provided financial support for the impact research project (AMBISEPT) from which stems this research paper.
References
- Laouar H. Évaluation des ressources halieutiques dans les retenues de barrages de Tunisie: Inter-calibration des échantillonnages acoustiques et aux filets maillants. Thèse de Doctorat en Sciences Agronomiques à l’Institut National Agronomique de Tunisie. 2019;133.
- DGPA. Annuaire des statistiques de pêche de la Direction Générale de la pêche et de l’Aquaculture. Tunis: Direction Générale de la pêche et de l’Aquaculture. 2019;143.
- Hall SR, Mills EL. Exotic species in large lakes of the world. Aquat Ecosyst Health Manag. 2000;3:105-135. doi: 10.1080/14634980008656995
- Olden JD, Hogan ZS, Vander Zanden MJ. Small fish, big fish, red fish, blue fish: Size-biased extinction risk of the world’s freshwater and marine fishes. Glob Ecol Biogeogr. 2007;16:694-701. doi: 10.1111/j.1466-8238.2007.00337. x
- Teletchea F, Le Doré Y. Etude sur l’élevage des carpes dites chinoises en France et évaluation de leur possible reproduction naturelle dans les cours d’eau Français. 2016;92.
- Savini, D, Occhipinti-Ambrogi A, Marchini A, Tricarico E, Gherardi F, Olenin S, Gollasch S. The top 27 animal alien species introduced into Europe for aquaculture and related activities. J Appl Ichthyol. 2010;26:1-7. doi: 10.1111/j.1439-0426.2010. 01503.x
- Casal CM. Global documentation of fish introduction: The growing crisis and recommendations for action. Biol Invasions. 2006;8:3-11. doi: 10.1007/s10530-005-0231-3
- Sax DF, Brown JH. The paradox of invasion. Glob Ecol Biogeogr. 2000; 9:363-371. https://tinyurl.com/4yn5am6d
- Copp GH, Bianco PG, Bogutskaya NG, Eros T, Falka I, Ferreira MT, Fox MG, Freyhof J, Gozlan RE, Grabowska J, Kovac V, Moreno-Amich R, Naseka AM, Penaz M, Povz M, Przybylski M, Robillard M, Russell IC, Stakenas S, Sumer S, Vila-Gispert A, Wiesner C. To be, or not to be, a non-native freshwater fish? J Appl Ichthyol. 2005;21:242-262. doi: 10.1111/j.1439-0426.2005.00690. x
- Crivelli AJ. Are fish introductions a threat to endemic freshwater fishes in the northern Mediterranean region? Biol Conserv. 1995;72:311-319. doi: 10.1016/0006-3207(94)00092-5
- Moyle PB, Light T. Biological invasions of freshwater: Empirical rules and assembly theory. Biol Conserv. 1996;78:149-161. doi: 10.1016/0006-3207(96)00024-9
- Eby LA, Roach WJ, Crowder LB, Stanford JA. Effects of stocking-up freshwater food webs. Trends Ecol Evol. 2006;21:576-584. doi: 10.1016/j.tree.2006.06.016
- Shea K, Chesson P. Community ecology theory as a framework for biological invasions. Trends Ecol Evol. 2002;17:170-176. doi: 10.1016/S0169-5347(02)02495-3
- Facon B, Genton BJ, Shykoff J, Jarne P, Estoup A, David P. A general eco-evolutionary framework for understanding bioinvasions. Trends Ecol Evol. 2006 Mar;21(3):130-5. doi: 10.1016/j.tree.2005.10.012. Epub 2005 Nov 8. PMID: 16701488.
- Losse GF, Nau W, Winter M. Le développement de la pêche en eau douce dans le nord de la Tunisie. Rapport Technique, projet tuniso-allemand de pêche continentale. Deutsche Gesellshaft fur TechnischeZusammenarbeit (GTZ) Eschborn, Allemagne. 1991;403.
- Kraïem MM. Systématique, biogéographie et bioécologie de Barbus callensis, Valencienne, 1842 (Poissons, Cyprinidae) de Tunisie. Thèse de Doctorat d’Etat, Université de Tunis II. 1994;227.
- Djemali I. Evaluation de la biomasse piscicole dans les plans d’eau douce tunisiens : approche analytique et acoustique. Thèse de Doctorat en Sciences Agronomiques à l’Institut National Agronomique de Tunisie. 2005;180. https://tinyurl.com/8xy6seu
- Mili S, Ennouri R, Laouar H, Missaoui H. Fisheries in the Tunisians dams: Diagnosis of the current situation and development opportunities. FAO Fish and Aqua Proceedings. 2015;39:95-106.
- Mili S, Ennouri R, Dhib A, Laouar H, Missaoui H, Aleya L. Characterization of fish assemblages and population structure of freshwater fish in two Tunisian reservoirs: Implications for fishery management. Environ Monit Assess. 2016;188:1-11. doi: 10.1007/s10661-016-5364-6
- Lévêque C, Oberdorff T, Paugy D, Stiassny MLJ, Tedesco PA. Global diversity of fish (Pisces) in freshwater. Hydrobiologia. 2008;595:545-567. doi: 10.1007/s10750-007-9034-0
- Fabrice T. Fish Domestication: An Overview. Animal Domestication. 2019;22. doi: 10.5772/intechopen.79628
- Lu G, Wang C, Zhao J, Liao X, Wang J, Luo M, Zhu L, Bernatzhez L, Li S. Evolution and genetics of bighead and silver carps: Native population conservation versus invasive species control. Evol Appl. 2020;1-20. doi: 10.1111/eva.12982
- Muller HP, Krist H, Rhouma A. Versucheüber die Verwendbarkeit von pflanzenfressenden FischenzurBekämpfung von Wasserpflanzen, Algen und plankton in eutrophierten Talsperren Tunesiens. 1982;1-41.
- Sullivan CJ, Weber MJ, Pierce CL, Camacho CA. A comparison of Grass Carp population characteristics upstream and downstream of Lock and Dam 19 of the Upper Mississippi River. J Fish Wildl Manag. 2020;11(1):99-111; e1944-687X. doi: 10.3996/062019-JFWM-046
- Otte G. Prüfung der Verwendbarkeit von pflanzenfressenden Fischen in eutrophierten Talsperren Tunesiens. GTZ, Eschborn. 1985;1-41.
- Cheng L, Opperman JJ, Tickner D, Speed R, Guo Q, Chen D. Managing the Three Gorges Dam to Implement Environmental Flows in the Yangtze River. Front Environ Sci. 2018;6:64. doi: 10.3389/fenvs.2018.00064
- Islam S, Bhadra A, Rahman A, Khan MM. Pond management and fish polyculture technique in lalmonirhat of Bangladesh. Int j zool stud. 2019 May 27;4(4):52-54.
- Shireman JV, Smith CR. Synopsis of biological data on the grass carp, Ctenopharyngodon idella (Cuvier et Valenciennes, 1844). Food and Aquaculture Organization Sypnosis No. 1983;135:86.
- Muller R. Grass carp and silver carp in Switzerland. I V CEI, Abstracts. 1982;p 207.
- Bruslé J, Quignard JP. Biologie des poisons d’eau douce européens. Editions Tec & Doc, Paris. 2001;p 625.
- Pípalová I. A review of grass carp use for aquatic weed control and its impact on water bodies. J Aquat Plant Manage. 2006;44:1-12.
- Kottelat M, Freyhof J. Handbook of European freshwater fishes. Kottelat, Cornol, Suisse et Freyhof, Berlin, Allemange; 2007; p646.
- Farid S, Ouizgane A, Majdoubi FZ, Hasnaoui M, Droussi M. Food diet of grass carp fries (Ctenopharyngodon idella, Valenciennes 1844) in Deroua fish farming ponds, Morocco. J Wat Env Sci. 2019 Dec;3(1):499-511.
- Teletchea F, Fostier A, Le Bail PY, Jalabert B, Gardeur JN, Fontaine P. STOREFISH: A new database dedicated to the reproduction of temperate freshwater teleost fishes. Cybium. 2007;31:227-235. doi: 10.26028/cybium/2007-312-017
- Kolar CS, Chapman DC, Courtenay WR, Housel CM, Williams JD, Jennings DP. Asian carps of the genus Hypophthalmichthys (Pisces, Cyprinidae) – A biological synopsis and environmental risk assessment. US Geological survey, La Crosse, Wis. Report to US Fish and Wildlife Service per Interagency Agreement. 2005;185.
- Domaizon I, Devaux J. Nouvelle approche des biomanipulations des réseaux trophiques aquatiques. Introduction d’un poisson phytoplanctonophage, la carpe argentée (Hypophthalmichthys molitrix). Année Biologique. 1999;38:91-106. doi: 10.1016/S0003-5017(99)80028-2
- DFO. Carp Status Report. DFO Can Sci Advis Sec Sci Advis Rep. 2005/001; 2005;15.
- Fridman AL. Calculations for fishing gear design. Oxford: Osney Mead. 1986.
- Smith A. Factors to be taken into account in the decision on minimum mesh sizes and the measurement of mesh size. Report of the national workshop on fisheries monitoring control and surveillance in support of fisheries management, Rome, FAO; 2001;19.
- Mili S, Ennouri R, Laouar H, Aleya L. Optimization of fishing for stock enhancement of Rutilis rutilus and Scardinius erythropththalmus in forage fish-deficient Tunisian reservoirs. Environ Monit Assess. 2017;189:610. doi: 10.1007/s10661-017-6326-3
- Rhouma A. Etude biologique et élevage du mulet en Tunisie et comparaison avec une espèce d’eau douce (la carpe). Mémoire de fin d’études du 3ème cycle de l’INAT. Institut National Agronomique de Tunisie. Tunis, Tunisie. 1975;131.
- TCA. Lutte biologique contre la prolifération des plantes aquatiques dans les plans d’eau douce en Tunisie. Echos de l'aquaculture. 2013;2:10-12.
- FAO. Cultured Aquatic Species Information Programme Ctenopharyngodon idellus. Cultured Aquatic Species Fact Sheets. Texte by Weimin, M. In: Département des pêches et de l’aquaculture de la FAO, Rome; c2009-2011a.
- FAO. Cultured Aquatic Species Information Programme Hypophthalmichthy smolitrix. Cultured Aquatic Species FactSheets. Text by Yang N. In: Département des pêches et de l’aquaculture de la FAO, Rome. c2009-2011b.
- Peng L, Xue X, Liao J, Zhao J, Tang Q, Lin Q, Zhang Q, Han BP. Potential impact of population increases of non-native tilapia on fish catch and plankton structure: A case study of Tangxi Reservoir in southern China. Aquatic Invasions. 2021;16:329-348. doi: 10. 3391/ai.2021.16.2.08
- Tacon AGJ, Metian M. Fishing for feed or fishing for food: Increasing global competition for small pelagic forage fish. Ambio. 2009;38:294-302. doi: 10.1579/08-a-574.1
- Tacon AGJ, Metian M, Turchini GM, De Silva SS. Responsible aquaculture and trophic level implications to global fish supply. Reviews in Fisheries Science. 2010;18:94-105. doi: 10.1080/10641260903325680
- Galveston Bay Foundation. Grass carp in Galveston bay. What you need to know about this exotic and invasive species. 2002;4.
- Ashraf M, Zafar A, Rauf A, Mehboob S, Qureshi NA. Nutritional values of wild and cultivated silver carp (Hypophthalmichthys molitrix) and grass carp (Ctenopharyngodon idella). International Int J Agric Biol. 2011;13:210-214.
- Dabbadie L, La carpe Amour, Ctenopharyngodon idella (Valenciennes, 1844). Quelques données bibliographiques sur sa biologie, sa culture et son introduction en dehors de sa zone d’origine. 1994;15.
- Masser MP. Using grass carp in aquaculture and private impoundments. Southern Regional Aquaculture Center Publication No. 3600. 2002. In Mavruk S, Avsar D. Non-native fishes in the Mediterranean from the Red Sea by way of the Suez Canal. Rev Fish Biol Fish. 2008;18:251-262. doi: 10.1007/s11160-007-9073-7
- (52A) Cudmore B, Mandrak NE. Biological synopsis of grass carp (Ctenopharyngodon idella). Can MS Rpt Fish Aquat Sci. 2004;2705:44. (52B) Berday N, El Hamouri B, Zamane Y, Zaoui D. Élevage de la carpe argentée (Hypophthalmichthys molitrix Val.) et de la carpe herbivore (Ctenopharyngodon idella Val.) dans des eaux usées domestiques épurées. Actes De L'IAV Hassan II. 2004;24:99-106.
- FAO. Cultured Aquatic Species Information Programme Hypophthalmichthys nobilis. Cultured Aquatic Species Fact Sheets. Text by Weimin M. In : Département des pêches et de l’aquaculture de la FAO, Rome. c2008-2011.
- Jennings DP. Carpe à grosse tête (Hypophthalmichthys nobilis): un synopsis biologique. US Fish and service wildlife Biol Rep. 1988;88(29).
- Kestemont P. Different systems of carp production and their impacts on their environment. Aquaculture. 1995;129:347-373. doi: 10.1016/0044-8486(94)00292-V
- Stott B, Cross DG. A note on the effect of lowered temperatures on the survival of eggs and fry of the grass carp Ctenopharyngodon idella (Valenciennes, 1844). J Fish Biol. 1973;5:649-658. doi: 10.1111/j.1095-8649.1973.tb04501.x
- Kırkağaç M, Demir N. The effects of grass carp (Ctenopharyngodon idella Val., 1844) on water quality, plankton and benthic macroinvertebrates in a spring pond. Turk. J Fish Aquat Sci. 2006;6:7-15.
- Kırkağaç M, Demir N. The effects of grass carp on aquatic plants, plankton and benthos in ponds. J Aquat Plant Manage. 2004;42:32-39.
- Lin Q, Chen Q, Peng L, Xiao L, Lei L, Jeppesen E. Do bigheaded carp act as a phosphorus source for phytoplankton in (sub) tropical Chinese reservoirs? Water Research. 2020;180:115841. doi: 10.1016/j.watres.2020.115841
- Schrank SJ. Bighead carp threatens Missouri river fisheries. Aquatic Nuisance Digest. 1999;3:26-28.
- Collins SF, Wahl DH. Invasive planktivores as mediators of organic matter exchanges within and across ecosystems. Oecologia. 2017;184:521-530. doi: 10.1007/s00442-017-3872-x
- Tumolo BB, Flinn MB. Top-down effects of an invasive omnivore: detection in long-term monitoring of large-river reservoir chlorophyll-a. Oecologia, 2017;185:293-303. doi: 10.1007/s00442-017-3937-x
- Ochs CA, Pongruktham O, Killgore KJ, Hoover JJ. Phytoplankton Prey Selection by Hypophthalmichthys molitrix Val. (Silver Carp) in a Lower Mississippi River Backwater Lake. Southeastern Naturalist. 2019;18(1),113 129. doi: 10.1656/058.018.0108
- Pípalová I, Květ J, Adámek Z. Limnological changes in a pond ecosystem caused by grass carp (Ctenopharyngodon idella Val.) low stocking density. Czech J Anim Sci. 2009;54:31-45.
- Berday N, El Hamouri B, Zamane Y, Zaoui D. Élevage de la carpe argentée (Hypophthalmichthys molitrix Val.) et de la carpe herbivore (Ctenopharyngodon idella Val.) dans des eaux usées domestiques épurées. Actes Inst Agron Vet (Maroc). 2004;24:99-106.
- Lenhardt M, Markovic G, Hegedis A, Maletin S, Cirkovic M, Markovic Z. Non-native and translocated fish species in Serbia and their impact on the native ichthyofauna. Rev Fish Biol Fish. 2011;21:407-421. doi: 10.1007/s11160-010-9180-8.
- Quesada R. Les dessous noirs de l’Amour blanc. Le Courrier de l’environnement de l’INRA. 2004;51:61-63.
- Dibble ED, Kovalenko K. Ecological impact of grass carp: A review of the available data. J Aquat Plant Manage. 2009;47:1-15.
- Le Louarn H. Amour blanc. L’atlas des poissons d’eau douce de France. P. Keith et J. Allardi (coordinateurs). Patrimoines Naturels, 47, Paris, SPN/IEGB/MNHN; 2001;158-159.
- Bernstein NP, Olson JR. Ecological problems with Iowa’s invasive and introduced fishes. Jour. Iowa Acad. Sci. 2001;4:185-209.
- Radke RJ, Kahl U. Effects of a filter-feeding fish [silver carp, Hypophthalmichthys molitrix (Val.)] on phyto-and zooplankton in a mestrophic reservoir: results from an enclosure experiment. Fresh water Biology. 2002 Dec;47:2337-2344. doi: 10.1046/j.1365-2427.2002.00993.x
- Shen R, Gu X, Chen H, Mao Z, Zeng Q, Jeppesen E. Silver carp (Hypophthalmichthys molitrix) stocking promotes phytoplankton growth by suppression of zooplankton rather than through nutrient recycling: An outdoor mesocosm study. Freshwater Biology. 2021 Feb 08;66(6):1074-1088. doi: 10.1111/fwb.13700
- Schrank SJ, Guy CS. Age, growth, and gonadal characteristics of adult bighead carp, Hypophthalmichthys nobilis, in the lower Missouri river. Environmental Biology of Fishes. 2002;64:443-450. doi: 10.1023/A:1016144529734
- Irons KS, Sass GG, McClelland MA, Stafford JD. Reduced condition factor of two native fish species coincident with invasion of non-native Asian carps in the Illinois River, U.S.A. Is this evidence for competition and reduced fitness? Journal of Fish Biology. 2007;71:258-273. doi: 10.1111/j.1095-8649.2007.01670.x
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.