> Biology Group. 2021 October 20;2(10):929-938. doi: 10.37871/jbres1334.
Accuracy of Roche SARS-CoV-2 Rapid Antigen Test in Nasopharyngeal Swab: Clinical Impression Matters
Khin Phyu Pyar1*, Khine Khine Su2, Kyaw Wunna3, Myo Thant4, Kaung Myat5, Aung Aung5, Zar Ni Htet Aung5, Nyan Lin Maung5, Aung Phyoe Kyaw5, Min Lynn Zaw Oo6, Kyaw Zwa Tun6, Kyaw Ko Ko Aung6, Kyaw Thu6, Thein Soe Tun6, Nyan Ye Oo6, Chan Nyein Latt6, Thi Han Tun7, Si Thu Myint8, Aung Phyo Oo8, Win Ko Ko Min8, Kyaw Khine Win9, Hein Wai Yan9, Thet Mg Oo10 and Win Myint Tin10
2Professor and Head, Department of Microbiology, Defence Services Medical Academy, Myanmar
3Senior Consultant Microbiologist, Department of Microbiology, No. (1) Defence Services General Hospital (1000-Bedded), Myanmar
4Consultant Biochemist, Department of Biochemistry, Defence Services Medical Research Center, Myanmar
5Senior Consultant Physician, No. (1) Defence Services General Hospital (1000-Bedded), Myanmar
6Consultant Physician, No. (1) Defence Services General Hospital (1000-Bedded), Myanmar
7Public Health Specialist, No. (1) Defence Services General Hospital (1000-Bedded), Myanmar
8Consultant Microbiologist, Department of Microbiology, Defence Services Medical Academy, Myanmar
9Microbiologist, Department of Microbiology, Defence Services Medical Academy, Myanmar
10Senior Medical Technologist, Department of Microbiology, No. (1) Defence Services General Hospital (1000-Bedded), Myanmar
Abstract
Background: In COVID-19 pandemic, the diagnosis and treatment must be as early as possible to save the life of each patient. Moreover, screening of asymptomatic carriers, close contacts or healthy subjects must not be delay to prevent transmission to publics. For confirmation of diagnosis of SARS-CoV-2 infection, nasopharyngeal swab must be tested either by real-time Reverse Transcription Polymerase Chain Reaction (RT-PCR) tests or Rapid Antigen Test (RAT). RAT is faster, easier and cheaper; thus, it is suitable for health service in developing country.
Objectives: The aim of this study was to assess the diagnostic accuracy of Roche SARS-CoV-2 Rapid Antigen Test (RAT) in diagnosing SARS-CoV-2 infection.
Methods: Hospital based exploratory study was done in out-patient department and fever clinic, and molecular laboratory of No. (1) Defence Services General Hospital. Nasopharyngeal swabs were taken, and the Roche SARS- CoV-2 RAT was conducted in parallel with RT-PCR test (reference standard).
Results: Among the 932 patients/subjects recruited, RT-PCR was positive in 468 individuals, corresponding to a prevalence of 50.2%. The RAT was positive in 363 patients (60.4%), false positive in 120 patients; it was negative in 569 individuals (39.6%), false negative in 225 patients. The overall sensitivity of the RAT was 51.9% (95% Confidence Interval [CI] 47.29-56.53) and, the specificity was 74.1% (95% CI 69.9-78.07); positive predictive value was 66.9% and negative predictive value was 60.5%. The sensitivity varied with Ct value; 78% in clinical samples with Ct values < 20, 57.5% in those with Ct values between 21 and 25, 41.8% in samples with Ct values between 26 and 30, and, 36.4% in samples with Ct value > 30.
Conclusion: The accuracy of the SARS-CoV-2 Roche RAT in diagnosing SARS-CoV-2 infections was inferior to RT-PCR and manufacturer’s data. The sensitivity was with low Cycle threshold values < 20 which were inversely related to the viral load. RAT test should be used in association with clinical impression of physicians. In hospital setting especially in emergency department, the role of RAT should be reconsidered in those patients presenting with anosmia and some cases of dyspnoea, late symptoms in the course of disease, as the RAT results would be false negative. Other errors may arise if the operator for RAT has to handle more than recommended tests per hour especially in the peak of epidemics.
Keywords: SARS-CoV-2 Rapid Antigen Test (RAT); Nasopharyngeal swab; Reverse Transcription Polymerase Chain Reaction (RT-PCR); Sensitivity; Specificity; Cycle Threshold (CT)
Background
Coronavirus disease 2019 (COVID-19) has been spreading to the whole world since December 2019. As of end of June, 2021, the total number of confirmed cases was 27,000 in Myanmar [1]. Being a developing country, it was a great burden on healthcare system in all aspects: prevention, diagnosis, treatment and rehabilitation. Rapid Antigen Tests (RAT) are very simple; they do not need special technical expertise and molecular laboratory unlike a standard laboratory-based Reverse Transcription-Polymerase Chain Reaction (RT-PCR) test [2]. In fact, they can be done at grass root level even at home as self-testing. Regarding duration of test, the results can be obtained in 30 minutes whereas RT- PCR test takes 6-8 hours [2]. Therefore, rapid tests play an important role for clinicians; early diagnosis of clinically suspicious cases and treatment are critical in saving the lives of patients. Moreover, early diagnosis of contacts of patients and timely isolation can prevent further spread of COVID-19 infection; high impact during epidemics. Furthermore, screening at main entry point to Myanmar like airport, Toll gate and border points with rapid tests can limit cross country/border spread. In addition to time factor, viral transport media is required for RT-PCR test which has added value on cost; total cost per one test for RT- PCR test is six times higher than that of RAT. Rapid antigen tests are better option because SARS-CoV-2 nasopharyngeal swab RT-PCR tests are expensive and not easily available at grass-root level. There is a high demand for SARS-CoV-2 testing by RAT to identify COVID-19 cases. The diagnostic accuracy of Roche SARS-CoV-2 RAT in nasopharyngeal swab should be assessed in Myanmar.
The COVID-19 tests by Reverse Transcription Polymerase Chain Reaction (RT-PCR) technology have been carried out in various molecular laboratory in different States and Division of Myanmar: the National Health Laboratory in Yangon, Department of Medical Research (Lower Myanmar); Yangon Division, Public Health Laboratories (Mandalay; Mandalay Division, Mawlamyaing; Mon State, Taunggyi; Southern Shan State, and Muse; Northern Shan State), No. (1) Defence Services General Hospital (1000-bedded), Yangon, No. (2) Defence Services General Hospital (1000-bedded), Nay Pyi Daw; capital of Myanmar, No. (3) Military Hospital (300-bedded) Kyaing Ton; Eastern Shan State, and No. (17) Military Hospital (100-bedded), Sittwe; Rakhine State. The nasopharyngeal swab from village and small town is sent in viral transport media; thus, more time consuming.
For the molecular diagnosis of COVID-19 infection, the gold standard test, a Reverse Transcription Polymerase Chain Reaction (RT-PCR) has been done in molecular laboratory at No. (1) Defence Services General Hospital (1000-bedded), since February 2020; it is one of the reference laboratories for molecular tests for COVID-19 in Myanmar. It was approved in April 2020 by National Health Laboratory, Union of Myanmar; then, it was accredited by Royal College of Pathology of Australia in 2021. All the nasopharyngeal swabs from clinically suspicious cases, contact of the patients and healthy persons from Yangon region have been sent to it on daily basis for more than one year; and, the average test sample per day is 1,000 to 1,500. RT-PCR tests have several limitations; they require qualified operator; the tests must be done in molecular laboratory with BSL 3; they need special equipment and machine; they are expensive; and, it takes several hours to get the results. Faster, cheaper, and easier to use alternative tools are required in developing countries. Thus, it is necessary to deploy SARS-CoV-2 RAT for nasopharyngeal swab especially in peak of epidemics. Among the various Antigen-Detecting Rapid Diagnostic Tests (Ag-RAT), non-expensive and user friendly one is preferable. On the other hand, it must have good accuracy. Several reports mentioned various accuracy of SARS-CoV-2 RAT for nasopharyngeal swabs [2-4]. Some findings suggested that the accuracy was better if it was used in symptomatic cases especially in early stage of disease where the viral load was high [5-7]. Another report pointed out that the results of RAT should be combine with clinical scenario to get better diagnosis [7]. In addition, the report on specificity of RAT on asymptomatic cases were very limited.
Therefore, the accuracy of RAT was important for early diagnosis in clinical setting as well as decision making in public health aspect. The rapid test should have high specificity; better for exclusion of diagnosis. On the other hand, the sensitivity should not be too low. According to manufacturer’s information on SARS-CoV-2 Roche RAT, the diagnostic sensitivity and specificity in nasopharyngeal swabs collected from both symptomatic and asymptomatic patients were 96.5 and 99.7%, respectively [8].
For better management of this novel threat, exploring detailed knowledge on accuracy of the SARS-CoV-2 Roche RAT in diagnosing SARS- CoV-2 infections in both clinically suspected cases and healthy subjects was still required. In Myanmar, there is no previous study regarding the accuracy of RAT especially in relation to symptoms. It is necessary to investigate them in Myanmar, where the sensitivity and specificity of SARS-CoV-2 Roche RAT in nasopharyngeal swab may differ from the manufacturer’s data; the findings may not be the same as that of other countries. The aim of the study has therefore been to assess the accuracy of SARS-CoV-2 Roche RAT in diagnosing SARS-CoV-2 infections in both clinically suspected cases and healthy subjects in Myanmar.
Methods
Study design, setting, and population
It was a hospital and PCR laboratory based exploratory study in out-patient department and fever clinic, and molecular laboratory of No (1) Defence Services General Hospital (1000-bedded), Yangon, Myanmar. Nasopharyngeal swabs were obtained, and the Roche RAT was conducted in parallel with RT-PCR test (reference standard).
Both clinically suspicious patients and asymptomatic/ healthy subjects were recruited between June and July 2021. Inclusion criteria were: (a) clinically suspicious cases with SARS-CoV-2 infection; (b) age over 18 years; and (c) informed consent. Exclusion criteria were: (1) those cases who did not take nasopharyngeal swab for RT-PCT; and (2) those who did not give informed consent.
Study processes, handling of data, and samples
The subjects were recruited at two sites: emergency department (patients) and out-patient department (healthy subjects) of No. (1) Defence Services General hospital, Yangon. They were explained by physicians on call for the procedure of nasopharyngeal swab- two sets: one for Roche SARS- CoV-2 Rapid Antigen Test (RAT) and the other for RT-PCR. After getting informed consent, nasopharyngeal swab was taken by trained technicians/operator under supervision of physicians. All technicians/operator had completed a training course that was prepared according to established guidelines on swab collection [9]. The procedure was supervised during the first few days of practice. Swabs were collected using nylon flocked swabs, it was placed in a 3 ml Viral Transport Medium (HiMedia, India), and sent to molecular laboratory which was situated 3 minutes walking distance; then, it was stored at 4°C and processed within 6-12 hours (RT-PCR).
The swab for RAT was done immediately after taking the sample by the trained technician/operator; and, the result was recorded in the proforma. The result of RAT was informed to attending physician and not to molecular microbiologist. The symptoms were recorded if the patients were in emergency unit; the reasons for test were noted if the healthy subjects were in out-patient department. The proforma was filled. Coded clinical data and laboratory test results were stored in separate databases and analysis was done.
Determination of the rapid antigen test
Using the same sample material, the Roche SARS- CoV-2 rapid antigen test was conducted by a trained medical laboratory technician who was unaware of the RT-PCR results. Quality control was performed daily, and the manufacturer’s instructions were strictly followed (package leaflet; Roche Diagnostics, Mannheim, Germany). In brief, three drops of the extracted sample were applied to the specimen well of the test device and the test result was completed within 15-30 minutes. The result was only considered valid if the control line was visible. Even faint test lines were considered positive.
Reverse transcription polymerase chain reaction for SARS-CoV-2 detection
The gold standard test was a Reverse Transcription Polymerase Chain Reaction (RT-PCR) laboratory-based assay done in molecular laboratory at No. (1) Defence Services General Hospital (1000-bedded); a reference laboratory for molecular tests for COVID-19 approved/ accredited by National Health Laboratory from Union of Myanmar and Australia in 2020. At the molecular laboratory, viral RNA was extracted within 35 minutes using the MagaBio plus Virus DNA/RNA purification kit (Bioer, China) and Bioer Automatic Nucleic Acid Extraction Machine (Gene Pure Pro, China) according to the manufacturer’s instructions. For SARS-CoV-2 RNA detection, 5 µl of RNA template was tested, using the TaqPath 1-Step SARS-CoV-2 detection kit (Thermofisher Scientific, USA). These detection kit contain primers and probes targeting the ORF1ab gene, Spike gene and N gene. The samples were amplified in Applied Biosystem 7500 Fast Thermocycler instrument (Thermofisher, USA). The PCR program consisted of 25°C for 2 min (UNG incubation), 53°C for 10 min (Reverse transcription), 95°C for 2 min (Polymerase activation) followed by 40 cycles of 95°C for 3 sec and 60°C for 30 sec. Samples were reported as SARS-CoV-2 RNA detected when at least 2 targets were detected with typical exponential growth curves with Cycle Threshold (CT) less than 37.
Statistical analysis
Patient characteristics were presented as numbers (percentages) or mean (standard deviation), as appropriate. Two-by-two tables were created using RT-PCR results as the reference standard test and the Roche SARS- CoV-2 rapid antigen test as the index test. Sensitivities and specificities were calculated accordingly. RT-PCR was considered as a reference standard against the Roche SARS- CoV-2 RAT. Data were presented over- all and in salient subgroups. For sensitivity analysis, diagnostic accuracy measures were calculated for additional Cycling Thresholds (CT) of the RT-PCR. A prevalence of 10% and a power of 0.8 were considered as verifying sensitivity of 90%. Confidence intervals were also calculated [10]. Analyses were performed using SPSS version 23.
Sensitivity: The sensitivity was calculated as the number of specimens identified as positive by the Roche SARS-CoV-2 RAT divided by the number of specimens identified as positive by the RT-PCR reference assay, and expressed as a percentage.
Specificity: The specificity was calculated as the number of specimens identified as negative by the Roche SARS-CoV-2 RAT divided by the number of specimens identified as negative by the RT-PCR reference assay, and expressed as a percentage.
Accuracy: The accuracy was calculated as the proportion of Roche SARS-CoV-2 RAT results that agreed with the RT-PCR results (positive and negative), and expressed as a percentage.
The sensitivity, specificity, and accuracy calculations were performed using the proportion command in STATA® 15, which also generated the 95% Confidence Intervals (CIs).
Comparison of Cycle Threshold (CT) values with antigen assay results
The relationship between the viral load measured as the RT-PCR Ct value and rapid antigen detection was analysed. Ct values were categorized as strongly positive (Ct ≤ 20) indicating abundant target nucleic acid in the sample, moderately positive (Ct = 21-25), weakly positive (Ct = 26-30), and very weakly positive (Ct = > 30); and, they were compared with the Roche SARS- CoV-2 RAT results.
Results
Sensitivity, specificity, positive predictive value and negative predictive value are provided with 95% confidence interval.
Initial recruitment consisted of 18,709 patients/healthy subjects, and 932 individuals having both RAT and RT-PCR were eventually included in this study as shown in figure 1. The majority of patients presented with symptoms consistent with COVID-19 (n = 578; 62.0%); one-third of study population was asymptomatic cases (n = 354; 38%). Three hundred healthy subjects were referred because of exposure to infected individuals; and, 54 healthy subjects were for screening (eg. travel requirements). The mean age was 52.14 years (standard deviation, SD 17.68); 535 individuals (57.4%) were male.
Table 1 reveals clinical performance of RAT. True positive (both RAT and RT-PCR positive) was 26.1% (243/932) and true negative (both RAT and RT-PCR negative) was 36.9% (344/932); false positive (RAT+/PCR-) was 12.9% (120/932) and false negative (RAT-/PCR+) was 24.1% (225/932). The sensitivity of RAT was higher in patients with any symptom (57.07%). The sensitivity was lower in asymptomatic healthy subjects (33%). RT-PCR was positive in 468 cases, corresponding to a prevalence of 50.2%. The RAT was positive in 363 patients (39.6%), and negative in 569 individuals (60.4%). The overall sensitivity of the rapid antigen test was 51.9% (95% Confidence Interval [CI] 47.29-56.53) and, the specificity was 74.1% (95% CI 69.9-78.07); positive predictive value was 66.9% and negative predictive value was 60.5%. The sensitivity increased to 78% in the case of a high viral load. In asymptomatic individuals, the sensitivity was 33.0% and specificity was 86.2%. In symptomatic individuals, the sensitivity was 57.1% and specificity was 59.5%.
| Table 1: Clinical performance of RAT. | |||
| RT-PCR positive |
RT-PCR negative |
||
| RAT | RAT positive | 243 (26.1%) | 120 (12.9%) |
| RAT negative | 225 (24.1%) | 344 (36.9%) | |
| Sensitivity | 51.92 % (47.29-56.53) | ||
| Specificity | 74.14 % (69.9-78.07) | ||
| Positive predictive value | 66.94 % (62.91-70.74) | ||
| Negative predictive value | 60.46 % (57.84-63.02) | ||
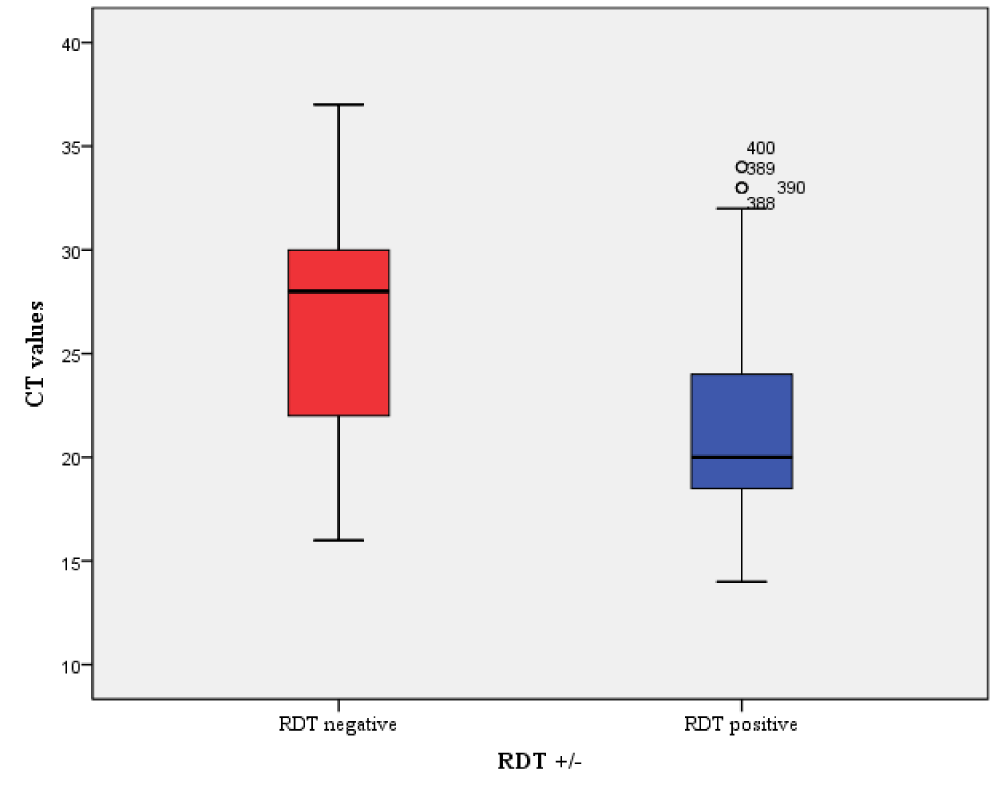
Figure 2 shows variation in sensitivity with Ct value from RT-PCR. Progressive decline in performance was observed as Cycle Threshold (CT) values of different SARS-CoV-2 gene targets increased. The sensitivity varied with Ct value: 78.4 % in clinical samples with Ct values <20; between 58.5% in those with Ct values between 21 and 25; 31.8% in samples with Ct values between 26 and 30; and, 17.9% in samples with Ct value > 31.
In subgroup analysis of false negative cases with Ct value < 20 (n = 33), the likely explanation was noted in 22 cases as demonstrated in tables 2 & 3. Firstly, the nasopharyngeal swab sample seemed to be not properly obtained in 20 cases: 10 consecutives negative RAT cases over 198 tested nasopharyngeal swabs on 18 July and 10 consecutives negative RAT cases over 250 tested nasopharyngeal swabs on 21 July because of staff physical exhaustion and work overload during peak of third wave in our hospital. Highest RAT false positive cases were noted on 18 July (15 cases) and 21 July (14 cases) when there was very high work load. Similar consecutive discrepancy between two results did not occur consecutively in the remaining days in July which were less crowded. The staffs/operators had to take average 300-600 nasopharyngeal swab sample per day at least for RT-PCR in July 2021; moreover, they performed RAT too.
| Table 2: RAT results and RT-PCR Ct value category (n = 425). | ||||
| RT-PCR Ct value category | ||||
| Strongly positive < 20 |
Moderately positive 21-25 |
Weakly positive 26-30 |
Very weakly positive > 30 (31-40) |
|
| RAT Positive | 120 | 62 | 35 | 10 |
| RAT Negative | 33 | 44 | 75 | 46 |
| total | 153 | 106 | 110 | 56 |
| Table 3: Reasons for RAT false negativity in cases with RT-PCR Ct < 20 (n = 33). | |||
| Number of cases | Supporting evidence | Remark | |
| Possible human error, the samples weren’t properly obtained | 5 | Consecutive negative RAT over 33 tests on 18 July | Staff exhaustion/ work overload (False positive cases 15) |
| Anosmia as presentation | 2 | Clinical course may be more than 5 days | |
| Possible human error | 5 | Consecutive negative RAT over 33 tests on 21 July | Staff exhaustion/ work overload (False positive cases 14) |
| Hypoxia as presenting symptom | 10 | Clinical course may be more than 7 days | Common RAT negativity |
| No obvious reason | 11 | ||
| Total | 33 | ||
Secondly, in another10 false negative cases with Ct value less than 20, the clinical presentation was hypoxia, late in the course of disease. Moreover, the patients on oxygen therapy either nasal canular or mask were found to be less co-operative during nasopharyngeal swab procedure. Thirdly, anosmia was the only presenting symptom in two cases in this category and their clinical course may be late. Finally, clear explanation was not found in remaining 11 cases.
Discussion
This study aimed to assess the diagnostic accuracy of Roche RAT in diagnosing SARS-CoV- 2 infection in both fever clinic (emergency department) and out-patient department of No. (1) Defence Services General Hospital (1000-bedded) in third wave in Myanmar. Among the 932 patients/healthy subjects included, 578 had symptoms and 354 did not experience symptoms.
The clinical performance data by manufacturer Roche was 90% clinical sensitivity and 99% clinical specificity [8]. However, in this study the overall sensitivity of the Roche RAT was 51.9%, lower than manufacturer’s data and other studies [2,3]. The same sensitivity results with different brand of RAT were recorded in Pakistan [4] and French [11]; therefore, not all available SARS-CoV-2 RAT had a sensitivity of ≥ 80 - 90%. Therefore, they suggested a confirmatory RT-PCR test in cases with negative RAT. The explanations on variation in sensitivity of RAT were interesting: (1) viral load [7,12-14]; (2) unsufficient amount of viral protein in nasopharyngeal swab [15]; (3) presence or absence of symptom [7,14,16]; (4) symptom onset days (sample collection timing in relation to symptoms) [5,7,14]; (5) skills of the operator in taking nasopharyngeal swab and doing RAT [15,17]; (6) quality of storage of nasopharyngeal swab sample particularly viral transport media; (7) handling of swab [18]; (8) cross contamination; (9) cross reaction with drugs and chemicals; (10) hook effect; (11) viral mutation; (12) reader error [6,19]; and, (13) effect of work overload on physical performance of health care workers during epidemic [20-22].
According to [5], the sensitivity of RAT was better with symptomatic patients who were at the early stages of the disease course. One of the review paper on RAT advised that RAT should be prospective, and the interpretation should include symptoms and timing-symptom onset days [16]. In this study, the sensitivity of the RAT was relatively higher in patients with any symptom (57.07%); it was lower in asymptomatic individuals (33%). It confirmed the fact that the performance of RAT was better with symptomatic patients [5,7]. In view of timing-symptom onset days, the sensitivity of RAT was 58% in early symptom onset days group (less than 7 days) and 55% in late symptom onset days group (more than 7 days); specificity was 53% and 63% respectively in this study. The average incubation period for COVID-19 is 5-6 days although it can be up to 14 days; the patients experienced symptom following incubation period - usually on day 5. Researchers estimated that people became infectious 2-3 days before they developed symptoms. The relationship between SARS-CoV-2 viral load and infectivity remains a matter of open debate; and, patients with low Ct value have high viral load and are very infectious. Though previous report explained that the sensitivity of RAT was high in those with symptoms particularly in early symptom onset group [7,14,16], this study did not show significant difference with timing-symptom onset days.
Concerning the skills of operators and technical factors, proper training of staffs/operator training was done prior to this study both for nasopharyngeal swab taking procedure and RAT tests. As emphasized by Pollock, et al. [17], inter-operator agreement was observed and corrected. During the study period, both nasopharyngeal swabs taking procedure and RAT tests were done under supervision of on-call physicians and the investigators.
Nevertheless, the reports on accuracy of RAT rarely mentioned possible impact of physical exhaustion of health care workers/operators especially during peak days of epidemics; both physical and mental effect [20-22]. It would be more pronounced if there were shortage of health care workers/operators. In this study, 10 consecutives false negative cases over 198 tested nasopharyngeal swabs on 18 July, and the same number of cases over 250 tested nasopharyngeal swabs on 21 July were possibly due to physical exhaustion of staffs/operators and work overload (handling large volume) during peak of third wave in our hospital. There was no such consecutive discrepancy between two results, RAT and RT-PCR, occurring consecutively in the remaining 29 days in July which were less crowded; average RAT tests per day was 150. A skilled laboratorian can perform and read 20 RAT tests per hour [17]; however, in this study, the operator had to take nasopharyngeal swab 300-600/day for both RAT and RT-PCR, and RAT procedure too. Therefore, some form of support should be given to staffs like provision of more man power to reduce overload; thus, false negative cases [23,24].
Variation in nasopharyngeal sampling was an explanation for one of false negative results as greater variation in Ct values in nasopharyngeal swab specimens was noted in two specimens: collected from inpatients by health care workers and their own specimens taken by their selves [15]. In addition, because RAT was qualitative, the followings weakness would change results of RAT: insufficient sample if the contact time to nasopharyngeal wall was very short; and, variation in waiting time to see test line on the kit- less than 10 minutes or more than 30 minutes. Osmanodja, et al. [18] mentioned discrepancy of RAT results owing to variation in skills; difference in self taken swab at home and professional worker taken swab [19]. In addition, accuracy of RAT results also depended on the physical fitness of the operator. It was again supported by two events where the RAT results of first operator in A&E department were negative; and, the same brand of RAT was repeated as clinical impression was too strong for COVID-19 in the COVID-19 quarantine ward, only 30 minutes apart, revealed positive. Their results of RT-PCR were positive and their Ct value was 18. Therefore, a confirmatory RT-PCR test was mandatory if RAT were negative particularly in clinically suspicious cases. Furthermore, Khandker, et al. [25] suggested to repeat RAT to reduce false negative rate. Moreover, researchers from Italy suggested that RAT might be used in association with clinical signs of patients to reduce the number of RT-PCR testing [7]. Thus, it confirmed the Belgium study; it pointed out that RAT could spare RT-PCR which should be reserved molecular resources for more seriously ill patients [26].
The WHO recommended a minimum of 80% sensitivity and 97% specificity for antigen-detecting rapid diagnostic testing- RAT. In reality, especially in peak epidemics, adequate human resources in addition to thorough training could lead to higher accuracy of RAT; avoiding both faulty technique in operating the assay, and collecting the nasopharyngeal swab. Furthermore, clinical skills of the physicians must be sharp; repeating the RAT only 30 minutes apart could confirm the diagnosis and saved the life as explained above in this study. Even in RT-PCR test, we could not rely 100% on its accuracy [27]. Thus, a negative RAT result did not rule out the presence of SARS-CoV-2 infection, and he should be treated with great caution, especially in asymptomatic individuals [28].
Another important cause of false-negative test results was due to viral mutation; we had suspicious cases of delta strain in third wave because the patients presented with possible delta symptoms: running nose, sneezing, headache and sore throat. The analysis of variant strain from false negative sample will be done in the near future. During third wave of epidemic in Myanmar, the people were commonly using over the counter medication: cough syrup, analgesics, antihistamine, antibiotics, vitamins and anti-oxidants which might impair the results of RAT. Apart from possible exogenous substances, endogenous molecules could clog the membrane at the cassettes’ conjugate pad in high concentrations. Certain ‘sandwich’ LFIAs gave rise to false-negative results when samples were saturated with antigen: the so-called Hook effect [29].
The sensitivity of the SARS-CoV-2 Roche RAT in diagnosing SARS-CoV-2 infections in this study was inferior to that of RT-PCR pointing out the need for RT-PCR. It was confirmed by Uganda study where field evaluation of RAT exhibited less than optimal performance; therefore, their opinion was “RAT would be used in places where the access to molecular testing was poor but RT-PCR was still required” [30].
Viral load is inversely related with Ct value. Progressive decline in performance was observed as Cycle Threshold (CT) values of different SARS-CoV-2 gene targets increased. In this study, as predicted, the diagnostic sensitivity was highly dependent on viral load; it ranged 78% in samples with Ct < 20, but then decreased to 17% in those with higher Ct values. Thus, the clinical performance data by manufacturer Roche RAT clinical sensitivity was much higher than this study; 89.6 % at Ct ≤ 30, and 93.1 % at Ct ≤ 27. Krüttgen, et al. [13] reported that the sensitivity was 95% or higher in samples with Ct < 30, but then decreased to 45% and 22% in those with Ct between 30-35 and > 35, respectively. In meta-analysis on RAT by Lee, et al. [14], sensitivity increased with low Ct value. In present study the sensitivity increased to 78% in the case of a high viral load; thus, supporting previous reports [7,12]. In short, the results of our study thus certify that the clinical performance of Roche SARS-CoV-2 RAT was good in nasopharyngeal swabs with Ct values < 20, which made it a reliable screening test in patients with high viral load [6]. Less sensitive lateral flow or RAT required a higher viral load to record a positive result, only identify people during their most infectious period.
In this study, the presentation of false negative cases with Ct value less than 20 was asymptomatic hypoxia (n = 10); probably late in the course of disease. It confirmed the fact that RAT was negative in late course of disease [6]. Moreover, the patients on oxygen therapy either nasal canular or mask were found to be less co-operative during nasopharyngeal swab procedure; this logistic point was important in emergency setting, and, it also highlighted the need for either repeating RAT or RT-PCR for confirmation of diagnosis. Anosmia was the only presenting symptom in two cases in this category and their clinical course was late [31], supporting the previous facts; performance of RAT was affected by the dynamics of SARS-CoV-2 viral load in early pre-symptomatic and later stages of viral shedding [28]. According to [32], anosmia symptom was usually late; 5 days after onset of other symptoms. Gopaul, et al. [33] suggested to consider clinical and radiological features in interpretation of RAT results especially in negative cases. Review paper on RAT advised that studies on RAT should be prospective and interpretation should include symptoms and timing-symptom onset days [16]. In one review report, RAT testing in the first week from symptom onset resulted in substantially higher sensitivity [6]. Thus, antigen tests were recommended for individuals with symptoms during the first 5 to 7 days of infection; RAT done in very early phase and one week after symptom onset would give negative results.
The number of false-positives was 120 in this study. Several possible reasons were errors in RAT test operation, detection of inactive or residual SARS-CoV-2 at low density in clinical specimens, cross-contamination and cross-reactions with other substances in clinical samples: endogenous (eg. blood) or exogenous (e.g. nasal spray ions, or chemicals that affect the pH of the test cassette). RAT positivity did not exclude other infection, or co-infection with coronaviruses other than SARS-CoV-2, as many test kits were designed to detect highly conserved proteins. Highly sensitive tests may detect inactive virus, or virus at low density in clinical specimens. LFIAs may be susceptible to temperature fluctuations, humidity, and positioning of the cassette during the testing procedure.
The current recommendations from the World Health Organization (WHO) concerning the minimum antigen-detection performance are these kits having the diagnostic a sensitivity and specificity of ≥ 80% and ≥ 97%, respectively and a few reports showed that RAT met WHO criteria [34]. In this study, the Roche SARS-CoV-2 RAT had nearly 80% sensitivity in in specimens with Ct values < 20 and 74% specificity; not completely match the WHO desired clinical performance. In hospital setting, clinical impression of the physicians would compensate the weakness of RAT to get early diagnosis and treatment. In public health setting, contact tracing, screening of healthy carriers and early diagnosis of clusters would be useful. RAT was so far quick screening and detection of COVID-19 cases among high-risk groups and in high-congregate environments (such as prisons and long-term care facilities), it was the best logistic tests at present especially in developing country like Myanmar. Therefore, rapid tests provided opportunities for early detection and isolation; the results must be integrated into wider strategies to control transmission (holistic public health approach) [35].
In this study, the specificity was 74.1% whereas it was nearly 100% in results of manufacturer Roche [8]. The use of RAT in public health intervention was mainly to control transmission: early detection of cases, contact tracing, population-wide testing. It was also helpful to safeguard health care workers by early case detection and isolation. In addition, RAT could identify clusters or outbreaks in specific settings: training center, camps, monasteries and jails. Highly specific RAT were useful for screening in high incidence settings. In this study area, a large training hospital- tertiary center, screening with RAT could not delay urgent procedures- coronary artery stenting, CABG, urgent hemodialysis and emergency surgery.
There were several limitations in this study. Firstly, the accuracy Roche RAT was lower than the value of manufacturer and other findings owing to differences in prevalence and patient population. Secondly, shortage of health care worker during peak of epidemic caused low physical performance skills, leading to errors in RAT results. Thirdly, analytical reactivity (sensitivity) to RAT with variants of SARS-CoV-2 should be tested. Fourthly, RAT positivity did not exclude other infection, or co-infection with coronaviruses other than SARS-CoV-2. Fifthly, work over load of health care worker was difficult to solved being developing country. Finally, hook effect should be considered in false negative cases and follow up was necessary.
Conclusion
The accuracy of the SARS-CoV-2 Roche RAT in diagnosing SARS-CoV-2 infections was neither the same as nor superior to both RT-PCR and manufacturer’s data. The sensitivity was fairly good in those cases with low Cycle threshold values of less than 20. Clinical opinion of physicians on call was paramount important especially in interpretation of RAT test results. Those with late symptoms like anosmia and some cases with dyspnoea where the RAT results were likely to be false negative, the use of RAT should be reappraised. Awareness of human errors on RAT should not be ignored if the operator has volume overload particularly in the peak of epidemics.
Recommendation
The clinician should not use RAT in patients presenting with late symptoms to avoid false negative results. For preparation of fourth wave in the future, human resources should be increased to avoid physical exhaustion of health care workers; minimizing the human errors.
Ethical Consideration
The study was approved by Hospital Research and Ethics Committee of No(1) Defence Services General Hospital, Yangon. Informed consent was obtained from each patients/ healthy subject.
Acknowledgement
The authors would like to thank all the candidates for giving informed consent to this study. The authors also acknowledged Prof Ko Ko Lwin, Prof Kyaw Zay Ya, Prof Myint Zaw for administrative support and Professor Tin Moe Mya for laboratory support.
References
- WHO. WHO Coronavirus (COVID-19) Dashboard. 2021. https://bit.ly/3m4naXv
- Mattiuzzi C, Henry BM, Lippi G. Making sense of rapid antigen testing in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) diagnostics. Diagnosis (Berl). 2020 Nov 26:dx-2020-0131. doi: 10.1515/dx-2020-0131. Epub ahead of print. PMID: 33554523.
- Jegerlehner S, Suter-Riniker F, Jent P, Bittel P, Nagler M. Diagnostic accuracy of a SARS-CoV-2 rapid antigen test in real-life clinical settings. Int J Infect Dis. 2021 Aug;109:118-122. doi: 10.1016/j.ijid.2021.07.010. Epub 2021 Jul 7. PMID: 34242764; PMCID: PMC8260496.
- Saeed U, Uppal SR, Piracha ZZ, Rasheed A, Aftab Z, Zaheer H, Uppal R. Evaluation of SARS-CoV-2 antigen-based rapid diagnostic kits in Pakistan: formulation of COVID-19 national testing strategy. Virol J. 2021 Feb 13;18(1):34. doi: 10.1186/s12985-021-01505-3. PMID: 33581714; PMCID: PMC7881305.
- Takeuchi Y, Akashi Y, Kato D, Kuwahara M, Muramatsu S, Ueda A, Notake S, Nakamura K, Ishikawa H, Suzuki H. Diagnostic performance and characteristics of anterior nasal collection for the SARS-CoV-2 antigen test: a prospective study. Sci Rep. 2021 May 18;11(1):10519. doi: 10.1038/s41598-021-90026-8. PMID: 34006975; PMCID: PMC8131686.
- Brümmer LE, Katzenschlager S, Gaeddert M, Erdmann C, Schmitz S, Bota M, Grilli M, Larmann J, Weigand MA, Pollock NR, Macé A, Carmona S, Ongarello S, Sacks JA, Denkinger CM. Accuracy of novel antigen rapid diagnostics for SARS-CoV-2: A living systematic review and meta-analysis. PLoS Med. 2021 Aug 12;18(8):e1003735. doi: 10.1371/journal.pmed.1003735. Erratum in: PLoS Med. 2021 Oct 13;18(10):e1003825. PMID: 34383750; PMCID: PMC8389849.
- Carcione D, IntraJ, Riggio D, Sabella S, Rondelli L, Barbieri S, Leoni V, Biondi ML. Evaluation of a rapid diagnostic test for detection of SARS-CoV-2 antigen in nasopharyngeal swabs. Microbiologia Medica. 2021;36. doi:10.4081/mm.2021.9623
- Salvagno GL, Gianfilippi G, Bragantini D, Henry BM, Lippi G. Clinical assessment of the Roche SARS-CoV-2 rapid antigen test. Diagnosis (Berl). 2021 Jan 18;8(3):322-326. doi: 10.1515/dx-2020-0154. PMID: 33554511.
- Mosi L, Sylverken AA, Oyebola K, Badu K, Dukhi N, Goonoo N, Mante PK, Zahouli J, Amankwaa EF, Tolba MF, Fagbamigbe AF, de Souza DK, Matoke-Muhia D. Correlating WHO COVID-19 interim guideline 2020.5 and testing capacity, accuracy, and logistical challenges in Africa. Pan Afr Med J. 2021 May 31;39:89. doi: 10.11604/pamj.2021.39.89.27522. PMID: 34466191; PMCID: PMC8379409.
- Bujang MA, Adnan TH. Requirements for Minimum Sample Size for Sensitivity and Specificity Analysis. J Clin Diagn Res. 2016 Oct;10(10):YE01-YE06. doi: 10.7860/JCDR/2016/18129.8744. Epub 2016 Oct 1. PMID: 27891446; PMCID: PMC5121784.
- Lambert-Niclot S, Cuffel A, Le Pape S, Vauloup-Fellous C, Morand-Joubert L, Roque-Afonso AM, Le Goff J, Delaugerre C. Evaluation of a Rapid Diagnostic Assay for Detection of SARS-CoV-2 Antigen in Nasopharyngeal Swabs. J Clin Microbiol. 2020 Jul 23;58(8):e00977-20. doi: 10.1128/JCM.00977-20. PMID: 32404480; PMCID: PMC7383555.
- Abdulrahman A, Mustafa F, AlAwadhi AI, Alansari Q, AlAlawi B, AlQahtani M. Comparison of SARS-COV-2 nasal antigen test to nasopharyngeal RT-PCR in mildly symptomatic patients. MedRxiv. 2020. doi: 10.1101/2020.11.10.20228973
- Krüttgen A, Cornelissen CG, Dreher M, Hornef MW, Imöhl M, Kleines M. Comparison of the SARS-CoV-2 Rapid antigen test to the real star Sars-CoV-2 RT PCR kit. J Virol Methods. 2021 Feb;288:114024. doi: 10.1016/j.jviromet.2020.114024. Epub 2020 Nov 20. PMID: 33227341; PMCID: PMC7678421.
- Lee J, Song JU, Shim SR. Comparing the diagnostic accuracy of rapid antigen detection tests to real time polymerase chain reaction in the diagnosis of SARS-CoV-2 infection: A systematic review and meta-analysis. J Clin Virol. 2021 Sep 16;144:104985. doi: 10.1016/j.jcv.2021.104985. Epub ahead of print. PMID: 34560340; PMCID: PMC8444381.
- Wyllie AL, Fournier J, Casanovas-Massana A, Campbell M, Tokuyama M, Vijayakumar P, Warren JL, Geng B, Muenker MC, Moore AJ, Vogels CBF, Petrone ME, Ott IM, Lu P, Venkataraman A, Lu-Culligan A, Klein J, Earnest R, Simonov M, Datta R, Handoko R, Naushad N, Sewanan LR, Valdez J, White EB, Lapidus S, Kalinich CC, Jiang X, Kim DJ, Kudo E, Linehan M, Mao T, Moriyama M, Oh JE, Park A, Silva J, Song E, Takahashi T, Taura M, Weizman OE, Wong P, Yang Y, Bermejo S, Odio CD, Omer SB, Dela Cruz CS, Farhadian S, Martinello RA, Iwasaki A, Grubaugh ND, Ko AI. Saliva or Nasopharyngeal Swab Specimens for Detection of SARS-CoV-2. N Engl J Med. 2020 Sep 24;383(13):1283-1286. doi: 10.1056/NEJMc2016359. Epub 2020 Aug 28. PMID: 32857487; PMCID: PMC7484747.
- Dinnes J, Deeks JJ, Adriano A, Berhane S, Davenport C, Dittrich S, Emperador D, Takwoingi Y, Cunningham J, Beese S, Dretzke J, Ferrante di Ruffano L, Harris IM, Price MJ, Taylor-Phillips S, Hooft L, Leeflang MM, Spijker R, Van den Bruel A; Cochrane COVID-19 Diagnostic Test Accuracy Group. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2020 Aug 26;8(8):CD013705. doi: 10.1002/14651858.CD013705. Update in: Cochrane Database Syst Rev. 2021 Mar 24;3:CD013705. PMID: 32845525; PMCID: PMC8078202.
- Pollock NR, Tran K, Jacobs JR, Cranston AE, Smith S, O'Kane CY, Roady TJ, Moran A, Scarry A, Carroll M, Volinsky L, Perez G, Patel P, Gabriel S, Lennon NJ, Madoff LC, Brown C, Smole SC. Performance and Operational Evaluation of the Access Bio CareStart Rapid Antigen Test in a High-Throughput Drive-Through Community Testing Site in Massachusetts. Open Forum Infect Dis. 2021 May 26;8(7):ofab243. doi: 10.1093/ofid/ofab243. PMID: 34250188; PMCID: PMC8244626.
- Osmanodja B, Budde K, Zickler D, Naik MG, Hofmann J, Gertler M, Hülso C, Rössig H, Horn P, Seybold J, Lunow S, Bothmann M, Barrera-Pesek A, Mayrdorfer M. Diagnostic accuracy of a novel SARS-CoV-2 antigen-detecting rapid diagnostic test from standardized self-collected anterior nasal swabs. MedRxiv. 2021. doi: 10.3390/jcm10102099
- Lindner AK, Nikolai O, Kausch F, Wintel M, Hommes F, Gertler M, Krüger LJ, Gaeddert M, Tobian F, Lainati F, Köppel L, Seybold J, Corman VM, Drosten C, Hofmann J, Sacks JA, Mockenhaupt FP, Denkinger CM. Head-to-head comparison of SARS-CoV-2 antigen-detecting rapid test with self-collected nasal swab versus professional-collected nasopharyngeal swab. Eur Respir J. 2021 Apr 15;57(4):2003961. doi: 10.1183/13993003.03961-2020. PMID: 33303544; PMCID: PMC7736752.
- Whelehan DF, Algeo N, Brown DA. Leadership through crisis: Fighting the fatigue pandemic in healthcare during COVID-19. BMJ Leader. 2021;5(2):108. doi: 10.1136/leader-2020-000419
- Salari N, Khazaie H, Hosseinian-Far A, Khaledi-Paveh B, Kazeminia M, Mohammadi M, Shohaimi S, Daneshkhah A, Eskandari S. The prevalence of stress, anxiety and depression within front-line healthcare workers caring for COVID-19 patients: a systematic review and meta-regression. Hum Resour Health. 2020 Dec 17;18(1):100. doi: 10.1186/s12960-020-00544-1. PMID: 33334335; PMCID: PMC7745176.
- Sasangohar F, Jones SL, Masud FN, Vahidy FS, Kash BA. Provider Burnout and Fatigue During the COVID-19 Pandemic: Lessons Learned From a High-Volume Intensive Care Unit. Anesth Analg. 2020 Jul;131(1):106-111. doi: 10.1213/ANE.0000000000004866. PMID: 32282389; PMCID: PMC7173087.
- Weenink JW, Kool RB, Hesselink G, Bartels RH, Westert GP. Prevention of and dealing with poor performance: an interview study about how professional associations aim to support healthcare professionals. Int J Qual Health Care. 2017 Oct 1;29(6):838-844. doi: 10.1093/intqhc/mzx114. PMID: 29024984.
- Vizheh M, Qorbani M, Arzaghi SM, Muhidin S, Javanmard Z, Esmaeili M. The mental health of healthcare workers in the COVID-19 pandemic: A systematic review. Journal of Diabetes & Metabolic Disorders. 2020;19(2):1967–1978. doi: 10.1007/s40200-020-00643-9
- Khandker SS, Nik Hashim NHH, Deris ZZ, Shueb RH, Islam MA. Diagnostic Accuracy of Rapid Antigen Test Kits for Detecting SARS-CoV-2: A Systematic Review and Meta-Analysis of 17,171 Suspected COVID-19 Patients. J Clin Med. 2021 Aug 8;10(16):3493. doi: 10.3390/jcm10163493. PMID: 34441789; PMCID: PMC8397079.
- Yin N, Debuysschere C, Decroly M, Bouazza FZ, Collot V, Martin C, Ponthieux F, Dahma H, Gilbert M, Wautier M, Duterme C, De Vos N, Delforge ML, Malinverni S, Cotton F, Bartiaux M, Hallin M. SARS-CoV-2 Diagnostic Tests: Algorithm and Field Evaluation From the Near Patient Testing to the Automated Diagnostic Platform. Front Med (Lausanne). 2021 Apr 6;8:650581. doi: 10.3389/fmed.2021.650581. PMID: 33889587; PMCID: PMC8055843.
- Kanji JN, Zelyas N, MacDonald C, Pabbaraju K, Khan MN, Prasad A, Hu J, Diggle M, Berenger BM, Tipples G. False negative rate of COVID-19 PCR testing: a discordant testing analysis. Virol J. 2021 Jan 9;18(1):13. doi: 10.1186/s12985-021-01489-0. PMID: 33422083; PMCID: PMC7794619.
- Schuit E, Veldhuijzen IK, Venekamp RP, van den Bijllaardt W, Pas SD, Lodder EB, Molenkamp R, GeurtsvanKessel CH, Velzing J, Huisman RC, Brouwer L, Boelsums TL, Sips GJ, Benschop KSM, Hooft L, van de Wijgert JHHM, van den Hof S, Moons KGM. Diagnostic accuracy of rapid antigen tests in asymptomatic and presymptomatic close contacts of individuals with confirmed SARS-CoV-2 infection: cross sectional study. BMJ. 2021 Jul 27;374:n1676. doi: 10.1136/bmj.n1676. PMID: 34315770; PMCID: PMC8314145.
- Mouliou DS, Gourgoulianis KI. False-positive and false-negative COVID-19 cases: respiratory prevention and management strategies, vaccination, and further perspectives. Expert Rev Respir Med. 2021 Aug;15(8):993-1002. doi: 10.1080/17476348.2021.1917389. Epub 2021 Apr 25. PMID: 33896332; PMCID: PMC8074645.
- Nalumansi A, Lutalo T, Kayiwa J, Watera C, Balinandi S, Kiconco J, Nakaseegu J, Olara D, Odwilo E, Serwanga J, Kikaire B, Ssemwanga D, Nabadda S, Ssewanyana I, Atwine D, Mwebesa H, Bosa HK, Nsereko C, Cotten M, Downing R, Lutwama J, Kaleebu P. Field evaluation of the performance of a SARS-CoV-2 antigen rapid diagnostic test in Uganda using nasopharyngeal samples. Int J Infect Dis. 2021 Mar;104:282-286. doi: 10.1016/j.ijid.2020.10.073. Epub 2020 Oct 30. PMID: 33130198; PMCID: PMC7836828.
- Santos REA, da Silva MG, do Monte Silva MCB, Barbosa DAM, Gomes ALDV, Galindo LCM, da Silva Aragão R, Ferraz-Pereira KN. Onset and duration of symptoms of loss of smell/taste in patients with COVID-19: A systematic review. Am J Otolaryngol. 2021 Mar-Apr;42(2):102889. doi: 10.1016/j.amjoto.2020.102889. Epub 2021 Jan 6. PMID: 33445036; PMCID: PMC7833280.
- Klopfenstein T, Zahra H, Kadiane-Oussou NJ, Lepiller Q, Royer PY, Toko L, Gendrin V, Zayet S. New loss of smell and taste: Uncommon symptoms in COVID-19 patients on Nord Franche-Comte cluster, France. Int J Infect Dis. 2020 Nov;100:117-122. doi: 10.1016/j.ijid.2020.08.012. Epub 2020 Aug 7. PMID: 32771635; PMCID: PMC7410813.
- Gopaul R, Davis J, Gangai L, Goetz L. Practical Diagnostic Accuracy of Nasopharyngeal Swab Testing for Novel Coronavirus Disease 2019 (COVID-19). West J Emerg Med. 2020 Sep 28;21(6):1-4. doi: 10.5811/westjem.2020.8.48420. PMID: 33052811; PMCID: PMC7673872.
- Berger A, Nsoga MTN, Perez-Rodriguez FJ, Aad YA, Sattonnet-Roche P, Gayet-Ageron A, Jaksic C, Torriani G, Boehm E, Kronig I, Sacks JA, de Vos M, Bausch FJ, Chappuis F, Renzoni A, Kaiser L, Schibler M, Eckerle I. Diagnostic accuracy of two commercial SARS-CoV-2 antigen-detecting rapid tests at the point of care in community-based testing centers. PLoS One. 2021 Mar 31;16(3):e0248921. doi: 10.1371/journal.pone.0248921. PMID: 33788882; PMCID: PMC8011749.
- Crozier A, Rajan S, Buchan I, McKee M. Put to the test: use of rapid testing technologies for covid-19. BMJ. 2021 Feb 3;372:n208. doi: 10.1136/bmj.n208. PMID: 33536228.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.