> Biology Group. 2021 October 20;2(10):920-925. doi: 10.37871/jbres1332.
A Brief Review of Ideal Bio-Indicators, Bio-Markers and Determinants of Endemic of Fluoride and Fluorosis
Shanti Lal Choubisa1,2* and Anurag Choubisa3
2Former Department of Zoology, Government Meera Girls College, Udaipur, Rajasthan 313002, India
3Geetanjali Medical College and Hospital, Udaipur 313002, Rajasthan, India
- Bio-indicators
- Bio-markers
- Determinants
- Drinking water
- Fluoride
- Fluorosis
- Industrial fluoride pollution
- Severity
- Toxicosis
Abstract
Fluorosis in man and animals is the resultant of chronic exposure of Fluoride (F) for prolonged period through F contaminated drinking water and foods and industrial F pollution. However, fluoridated water and industrial F emissions are the major sources of F exposure for humans and domestic animals. Chronic F exposure not only deteriorate the health of human beings and animals but also causes diverse adverse toxic effects on hard (teeth and bones) and soft (organs) tissues. Various F induced pathological changes in teeth and bones are known as dental and skeletal fluorosis, respectively. However, skeletal fluorosis is more dangerous and highly significant since it diminishes the mobility at a very early age and develops crippling or lameness bone deformity. Thousands of people and domestic animals are suffering with fluorosis worldwide. Dental fluorosis is rampant and the commonest form of chronic F toxicosis and appears in subjects of almost all age groups. However, children and bovine calves are relatively more sensitive and highly susceptible to F toxicosis and revealed the earliest clinical sign of chronic F poisoning in the form of dental fluorosis. Hence, these are ideal bio-indicators for chronic F intoxication or fluorosis. Nevertheless, the magnitude or severity of fluorosis is much more depending on the density and rate of bio-accumulation of F. Biological samples, milk, urine, blood serum, teeth, nails, etc. are better bio-markers for F intoxication. However, urine F concentration is the best bio-marker for endemic of F and fluorosis. In this communication, ideal bio-indicators and bio-markers for endemic of F and fluorosis and diverse potential determinants influencing the severity of F toxicity (fluorosis) are considered and briefly and critically reviewed. Findings of this review are useful in making and implementation of health policy and the commencement of mitigation and control of fluorosis programme in F endemic areas where it is problematic for human and animal health.
Introduction
It is well known that excess consumption or intake or exposure of fluoride (F) for prolonged period causes a dreaded fluorosis disease in both humans and domestic animals [1-7]. Thousands of people and domestic animals (cattle, buffaloes, camels, sheep, goats, horses, donkeys, etc.) are suffering with this disease in all over the world. In present scenario, fluorosis is a worldwide health problem and several countries are facing with this endemic disease. In fact, F is available by naturally and human activities (anthropogenic) in water, air, soil, foods, various food chains and ecological webs [5]. However, the groundwater and industrial F emissions are the major sources of F exposure. In the world, the groundwater of most of the geographical areas is contaminated with F by natural geogenic processes such as weathering and leaching of F-rich minerals rocks through decomposition, dissociation, dissolution and interaction with water [8]. But in certain geographical provinces, freshwater reservoirs are also contaminated with F due to human activities and industrial F emissions. These F exposure sources are potential to develop the fluorosis disease in man and animals. If the fluorosis is the resultant of drinking of F-rich water and industrial F pollution then it is generally known as “hydrofluorosis” and “industrial or neighbourhood fluorosis”, respectively. In general, hydrofluorosis is more common and widely in occurrence while the industrial fluorosis is restricted to the particular locality or region [5,7,9,10]. From different geographical regions, fluorosis in man and animals has been extensively studied and reported by several workers [1,3]. However, research studies are limited on various kinds of bio-indicators, bio-markers and potential determinants of endemic of F and fluorosis [3]. In this communication, bio-indicators and bio-markers for endemic of F and fluorosis and diverse determinants or factors influencing the magnitude of F toxicity or endemic fluorosis are considered and briefly and critically reviewed. Findings of this review are useful in making and implementation of health policy and the commencement of mitigation and control of fluorosis programme in F endemic region where F is problematic for health of human and animal populations.
Fluorosis
It is well established, over F exposure or excessive ingestion and inhalation of F for long-time through any of F contaminated sources causes mild to severe F toxicity in the form of fluorosis [1,4]. F is not only damages teeth and bones (hard tissues) but also affects soft tissues or organs. Once F inters into body, it absorbed by digestive and respiratory tract and finally reaches to tissues or organs of the body through blood circulatory system. More than 50% absorbed F excreted through physiological processes, excretion and perspiration, while rest is retained in the body where it accumulates gradually in hard and soft tissues. However, due to its greater affinity with calcium, it accumulates maximum in the calcified or hard tissues (bone and teeth) as compared to non-calcified soft tissues (organs). The accumulation of F interfere the physiology of various biological systems which triggers the genesis of various adverse health effects in the body. These F induced toxic changes or effects are generally appeared at or above the threshold level of F (1.0 or 1.5 ppm) in drinking water [1,4]. F induced these changes are collectively referred as fluorosis. In general, these pathological changes in teeth and bones are permanent, non-reversible and visible by necked eyes. However, F induced changes in soft tissues are mostly reversible and disappeared on removing of source of F exposure [11].
Dental fluorosis
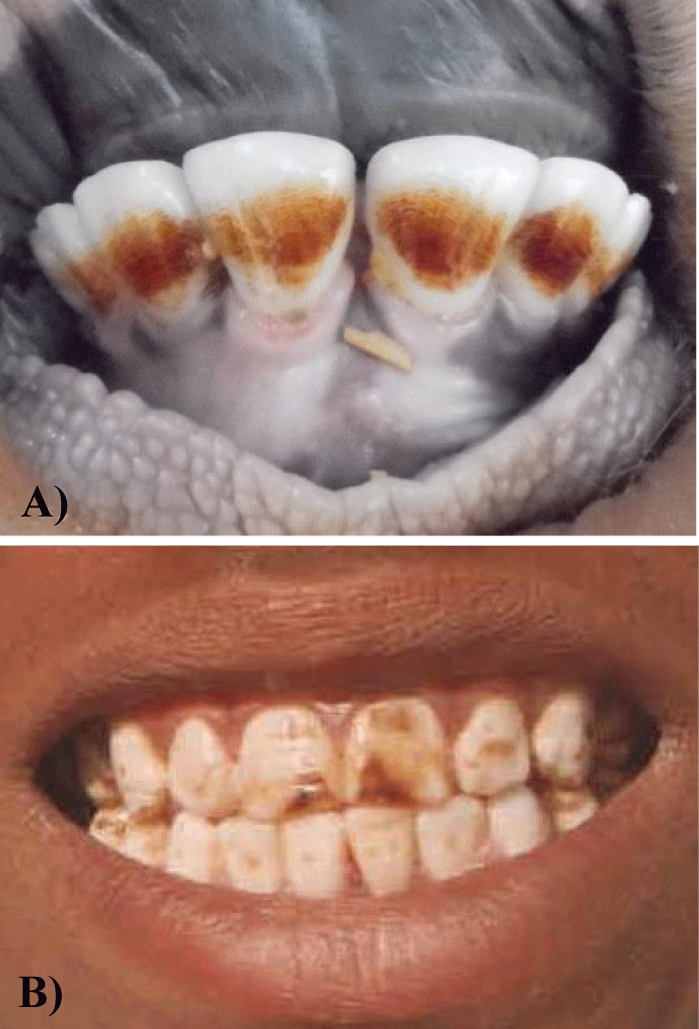
Fluoride induced dental mottling (dental fluorosis) is the earliest visible, sensitive and indexive sign of chronic F intoxication. Clinically, it is characterised by diffuse hypocalcification which is generally appeared in the form of bilateral, striated and horizontal opaque brownish pigmented streaks or patches or fine dots on teeth surface (Figures 1A & 1B). In any F endemic area, dental fluorosis is rampant and the commonest form of chronic F poisoning. As the increasing of age and F concentration in drinking water, dental fluorosis become more severe which is characterized with development of diastema (gaps) between teeth, pronounced loss of the tooth-supporting alveolar bone occurs with recession and swelling of the gingival tissues and excessive abrasion or irregular wearing of the teeth. This worst condition of dental fluorosis is more prevalent in higher age groups and at or above the 2.0 ppm F concentration in drinking water [12].
Whatsoever, dental fluorosis causes tooth loss and decay which creates a problem in mechanical digestion of food. Opaque appearance of dental fluorosis also causes aesthetic changes may trigger social constraints as well as behaviours [13]. It may also cause psychological effect and leads to low confidence in children and adolescents [14,15]. In fact, dental fluorosis is not only affecting the quality of life but also affecting the oral health in people. Recently, a study was conducted in F endemic areas of Eastern Africa revealed that the prevalence of tooth decay / loss and oral leukoplakia increased with severity of dental fluorosis. Study also revealed that increased esophageal cancer risks are found to be associated with moderate and severe dental fluorosis in subjects having more tooth loss/decay [16].
The most negative aspect of dental fluorosis in animals is that it ultimately mitigates the life-span of animals. When this entity becomes more advance and severe then due to difficulty in grazing and mastication of foods, the animals die at a young age from starving and emaciation [1,17]. Nevertheless, the death of animals at a young age has greater economic consequences for animal keepers and herd owners [4].
Skeletal fluorosis
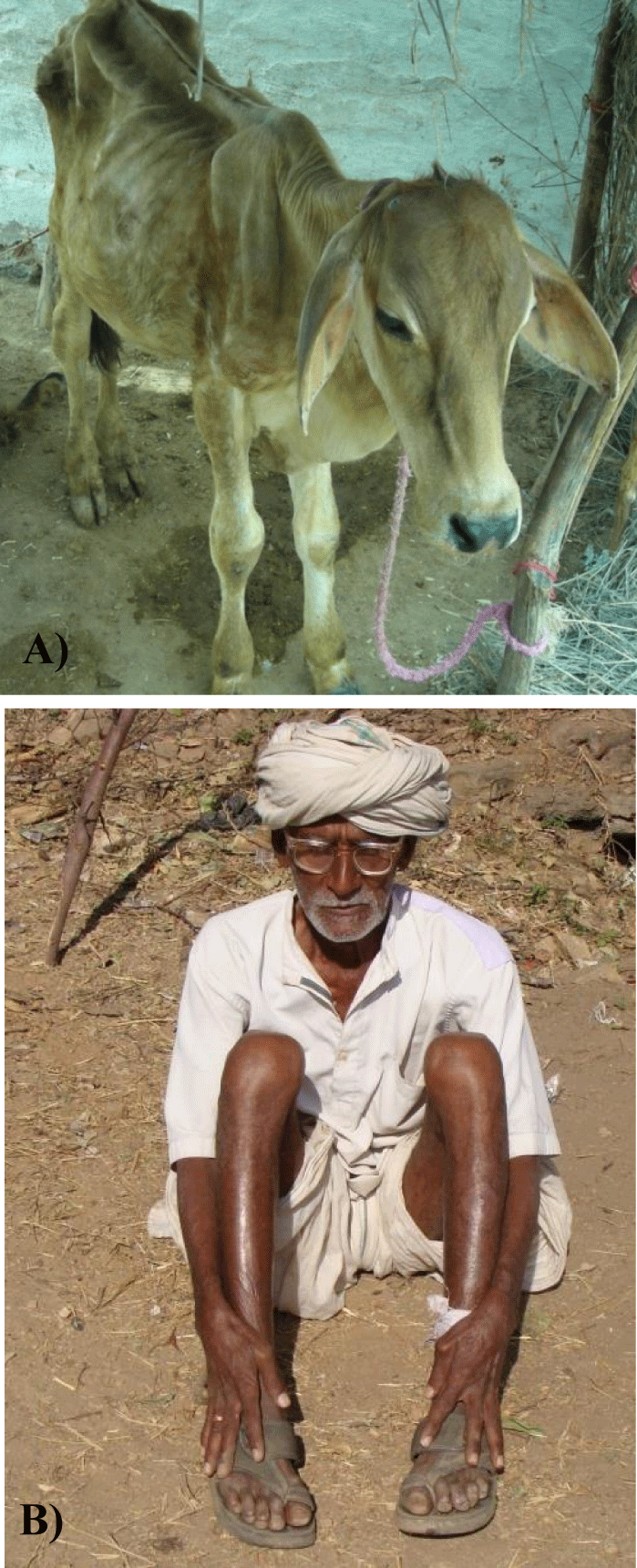
Excessive F consumption or exposure alters the equilibrium between formation and resorption of bones. This physiological process is accomplished by involvement of certain regulatory determinants and signalling pathways, thereby leading to various bone deformities which are known as skeletal fluorosis. This is very painful and more dangerous than dental fluorosis and highly significant since it diminishes the mobility at a very early age by producing gradually varying changes in bones of various body regions such as periosteal exostosis, osteosclerosis, osteoporosis and osteophytosis [18-21]. The excess accumulation of F in muscles also diminishes or restricts the various body movements and the condition leads to crippling or disabilities in humans and lameness in domestic animals (Figures 2A & 2B). As increasing of F concentration in drinking water, skeletal fluorosis becomes more severe and develops even in children or subjects of low age groups. Once F induced bone deformities developed then these are irreversible.
Non-skeletal fluorosis
Besides F induced teeth and bone deformities, the most common health complaints in man and animals of F endemic provinces are intermittent diarrhoea or constipation, abdominal pain, flatulence, urticaria, polyurea and polydipsia which have been observed and reported by several research scholars from F endemic areas [22-26]. However, in many of the F endemic areas cases of repeated abortions, sterility, reluctance to reproductive functions and erectile dysfunction in male individuals were found relatively higher than the non-endemic F areas. Indeed, gastro-intestinal discomforts, neurological and impaired reproductive dysfunctions are considered as the earlier signs of chronic F intoxication in both men and animals [27-29]. From many of the F endemic areas, low memory, learning ability, Intelligence Quotient (IQ) and cognition in school going children have also been reported [30,31]. Several studies have also revealed a significant relationship between F toxicity, dental fluorosis and neurotoxicty or impaired cognition in children [32]. In fact, F is potential to impair the function of central nervous system [33,34]. But involvement of exact mechanism at the molecular level is yet not clear.
Many studies revealed that prolonged exposure of F leads to affect various blood parameters and accelerate the haematological degeneration leading to death of erythrocytes or Red Blood Cells (RBC) and causing a mild to severe anaemia [35]. In India, in the tribal areas, especially in the state of Rajasthan, almost all drinking water sources are contaminated with F [5, 8] where genes of red cell genetic disorders such as sickle cell anaemia (Hb-SS), α- and β-thalasseamia syndromes and glucose-6-Phosphate Dehydrogenase (G-6-PD) enzyme deficiency are also endemic [36-42]. Some of these genes are lethal and causing a varying degree of anaemia in tribal individuals which is more or less depends on their status of appearance. Therefore, tribal people having any of these blood cell genetic defects are relatively more prone to F toxicosis which may cause an early death in them. However, for tits confirmation, more studies are highly suggestive in tribal dominant areas to reveal the effect of F intoxication in tribal subjects having red cell genetic disorders.
Bio-Indicators for Endemic of Fluorosis
Bio-indicators are those who are potential to reveal the presence of pollutants or contaminants by the occurrence of unique or typical symptoms or measurable responses. These are may be an organisms (animals, humans, plants, microbes, etc.) or biological responses which deliver information on alteration in the environment by changing in one of the ways: physiologically, chemically or behaviourally. Hence, these are generally used in assessing of environmental health and bio-geographical changes [43-45].
For the consideration of ideal bio-indicators for endemic of F and fluorosis in any geographical provinces or environment, these should have less resistance or have a greater susceptibility to F poisoning. Secondly, these should be given an early signs of F intoxication. Children and bovine calves are more relevant and more appropriate to consider them as bio-indicators for chronic F intoxication. Because these have less tolerance of F toxicity or relatively more susceptible to chronic F poisoning and potential to reveal the earliest clinical or pathognomic sign of F toxicosis in the form of dental fluorosis [46-49]. These are the most ideal bio-indicators because they are easily available and found almost in all the fluorosis endemic areas and they do not cause any problem to observe for evidence of dental fluorosis.
Recently, a large study was performed in mature and immature animals of different species, cattle (Bos taurus), water buffaloes (Bubalus bubalis), sheep (Ovis aries), goats (Capra hircus), horses (Equus caballus) and donkeys (Equus asinus) of such areas where almost all the drinking water sources are contaminated with F with varying amount [50]. Interesting, among these animals, immature ones were found to be more susceptible to F toxicosis (fluorosis) as they have revealed the earliest sign of chronic F poisoning which is also dental fluorosis. However, bovine calves are relatively more ideal bio-indicators for endemic of F and fluorosis compared to lames and kids [50]. These findings indicate that children and bovine calves both are most ideal bio-indicators for endemic of F and fluorosis.
Bio-markers FLUORIDE (F) and It’s Intoxication
In general, bio-markers are natural in occurrence and they are may be substance, molecule, structure, gene, process, etc. whose presence indicates the existence of living organisms. These bio-markers are often measured and evaluated to examine normal biological and pathogenic processes and used as an indicator of some biological state, physiological process, disease, etc [51]. F content in the environmental samples like forage and fodder indicates the persistence of F contamination in the environment [52,53]. However, in contrast to morbidity and mortality, F contents in biological samples such as milk, urine, blood serum, teeth, bones etc. are also better bio-markers for chronic F toxicosis in both man and animals [54-57]. For knowing the current status of chronic F poisoning, estimation of F in blood serum and urine is the most ideal and authentic way in man and animals [58]. However, among the various bio-indicators of chronic F exposure, urine F concentration is generally accepted as the best indicator of endemic of F and fluorosis or chronic F exposure because at the spot, it can be easily recollected noninvasively and systematically it reflects the burden of various F exposures such as industrial F pollution, fluoridated drinking water, F contaminated foods, etc [59,60]. But the level or concentration of F in urine is highly variable in individual to individual and region to region and is also influenced by age, climate, desert and humid environments and F concentration in drinking water or in other sources of F exposure [61].
Determinants of Fluorosis
The magnitude of chronic F intoxication (fluorosis) in man and animals living in different geographical provinces having almost similar F concentration in their drinking water sources is greatly varied. Even in the different species of animals living in the same areas, the prevalence and severity of osteo-dental fluorosis are also varied in them. Among family members, the severity of F toxicosis is also variable. Some has dental fluorosis but others members did not revealed evidence of dental mottling. This indicates that besides the F concentration in drinking water some determinants or factors are also involved to play a potential role in acceleration and influencing the chronic F toxicity. Based on the studies, these potential determinants are: the concentration of F in drinking water and its frequency and duration of F exposure, age, sex, habits, nutrition and other food constituents, chemical constituents of drinking water, Physico-chemical environmental factors as well as individual variations in susceptibility, biological response, tolerance and genetics [62-69]. However, the prevalence and severity of fluorosis are relatively higher in those people who have lower nutritional status, poor quality of foods, inadequate amount of calcium and vitamin C nutrients in diet and certain bed habits such as regular consumption of alcohol, tee, tobaccos, smoking, beetle nuts, etc. In fact, calcium and vitamin C nutrients are protective against the F intoxication [63,66,67].
In a large survey study conducted in domesticated bovine and flock animals of such geographical provinces where drinking water having low F level (1.5-1.7 ppm) [29]. Study revealed that grass eaters bovine animals were found to have relatively higher prevalence and severe form of fluorosis compared to plant eaters flock animals. This indicates that bovines are relatively more susceptible to F toxicosis compared to flocks. Such natural alleviation of F toxicity in plant eater animals living in high F endemic areas has also been reported [29]. These findings suggest that some available chemical substances in foods are responsible for making the difference and influence the chronic F toxicity in these animals. In fact, flock animals generally feed on small delicate fresh leaves, pods and small fruits of trees and shrubs which contain ample amount of calcium (Ca) and ascorbic acid (vitamin C) nutrients [29]. Both nutrients may interfere with the F metabolism and ultimately reduce the F toxicity. It is evidently cleared that either these nutrients are protective against to F toxicity in humans but whether these are protective or not in ruminant animals, more scientific studies are still needed for its further confirmation. However, authors believe that the magnitude or severity of fluorosis is much more depending on the density and rate of bio-accumulation of fluoride in the body.
Conclusions
Excess exposure of F for prolonged period causes serious fluorosis disease in man and animals. The F-rich water and industrial F emissions are the principal sources of F exposure which are highly potential for genesis of diverse adverse health effects (fluorosis). The presence of fluorosis in any geographical provinces can be predicted by having dental fluorosis in children and bovine calves as these are the most ideal bio-indicators for chronic F intoxication. In fact, children and bovine calves are highly sensitive to F toxicosis and have less tolerance to F and are easily available in fluorosis endemic areas for the observation of dental fluorosis. Biological samples, milk, urine, blood serum, teeth, bones, etc. are better bio-markers for chronic F intoxication in both man and animals. However, to know the current status of F toxicity in any geographical provinces, estimation of F in blood serum and urine is the most ideal and authentic way. However, urine F concentration is generally accepted as the best indicator of endemic of F and fluorosis. Though, the severity of fluorosis is much more influenced by several determinants such as F concentration and its frequency and duration of exposure, age, sex, habits, nutrients in foods, individual susceptibility, genetics etc. However, the severity of fluorosis is greatly influenced by the density and rate of F bio-accumulation. Interesting, in the rural areas, due to lack of knowledge, most of the people still consider fluorosis as a genetic disease because it is also found in their children and it is seen from generation to generation, whereas it develops due to exposure to F.
Acknowledgement
We are thankful to Dr. Darshana Choubisa, Associate Professor, Department Prosthodontics, Geetanjali Dental and Research Institute, Udaipur, Rajasthan 313002, India for cooperation.
References
- Adler P, Armstrong WD, Bell ME, et al. Fluorides and human health. World Health Organization Monograph Series No. 59. Geneva: World Health Organization. 1970. https://bit.ly/3auZC7y
- Choubisa SL. Endemic fluorosis in southern Rajasthan (India). Fluoride. 2001;34:61-70. https://bit.ly/3iTX5bm
- Swarup D, Dwivedi SK. Environmental pollution and effect of lead and fluoride on animal health. Indian Council of Agricultural Research, New Delhi. 2002;68-106. https://bit.ly/3iTDHLK
- Choubisa SL. A brief and critical review of endemic hydrofluorosis in Rajasthan, India. Fluoride. 2018;51:13-33.
- Choubisa SL. A brief and critical review on hydrofluorosis in diverse species of domestic animals in India. Environ Geochem Health. 2018;40:99-114. doi: 10/1007/s 10653-017-9913-x.
- Choubisa SL. Chronic fluoride exposure and its diverse adverse health effects in bovine calves in India: an epitomised review. Global J Biol Agric Health Sci. 2021;10(3):1-6. doi: 10.35248/2319-5584.21.10.107.
- Choubisa SL, Choubisa D. Status of industrial fluoride pollution and its diverse adverse health effects in man and domestic animals in India. Environ Sci Pollut Res. 2016; 23:7244-7254. doi: 10.1007/s11356-016-6319-8.
- Choubisa SL. Fluoride distribution in drinking groundwater in Rajasthan, India. Curr Sci. 2018;114:1851-1857. doi: 10.18520/cs/v114/i09/1851-1857.
- Choubisa SL. Industrial fluorosis in domestic goats (Capra hircus), Rajasthan, India. Fluoride. 2015;48:105-115.
- Choubisa SL, Choubisa D. Neighbourhood fluorosis in people residing in the vicinity of superphosphate fertilizer plants near Udaipur city of Rajasthan (India). Environ Monit Assess. 2015 Aug;187:497. doi: 10.1007/s10661-015-4723-z. Epub 2015 Jul 10. PMID: 26160742.
- Susheela AK, Mondal NK, Singh A. Exposure to fluoride in smelter workers in a primary aluminum industry in India. Int J Occup Environ Med. 2013 Apr;4(2):61-72. PMID: 23567531.
- Choubisa SL. An epidemiological study on endemic fluorosis in tribal areas of southern Rajasthan. A technical report, The Ministry of Environment and Forests, Government of India. 1996;1-56.
- Chankanka O, Levy SM, Warren JJ, Chalmers JM. A literature review of aesthetic perceptions of dental fluorosis and relationships with psychosocial aspects/oral health-related quality of life. Community Dent Oral Epidemiol. 2010 Apr;38(2):97-109. doi: 10.1111/j.1600-0528.2009.00507.x. Epub 2009 Dec 7. PMID: 20002631.
- Santos LM, Barros N, Silva W da, et al. Impact of dental fluorosis on the quality of life of children and adolescents. Revista De Odontol Da UNESP. 2014;43:326-332. doi: 10.1590/rou.2014.052.
- Nilchian F, Asgary I, Mastan F. The effect of dental fluorosis on the quality of life of female high school and precollege students of high fluoride-concentrated area. J Intl SocPrevent Commun Dentistr. 2018; 8:314-319. https://bit.ly/2YGqjmM
- Menya D, Maina SK, Kibosia C, Kigen N, Oduor M, Some F, Chumba D, Ayuo P, Middleton DRS, Osano O, Abedi-Ardekani B, Schüz J, McCormack VA. Dental fluorosis and oral health in the African Esophageal Cancer Corridor: Findings from the Kenya ESCCAPE case-control study and a pan-African perspective. Int J Cancer. 2019 Jul 1;145(1):99-109. doi: 10.1002/ijc.32086. Epub 2019 Jan 12. PMID: 30582155; PMCID: PMC6519293.
- Wang JD, Zhan CW, Chen YF, et al. A study of damage to hard tissue of goats due to industrial fluoride pollution. Fluoride. 1992;25(3):123-128. https://bit.ly/2YEySyK
- Choubisa SL. Radiological skeletal changes due to chronic fluoride intoxication in Udaipur district (Rajasthan). Poll Res. 1996;15:227-229.
- Choubisa SL, Choubisa DK, Joshi SC, Choubisa L. Fluorosis in some tribal villages of Dungarpur district of Rajasthan, India. Fluoride. 1997;30:223-228. https://bit.ly/3lFfieR
- Choubisa SL, Choubisa L, Choubisa DK. Endemic fluorosis in Rajasthan. Indian J Environ Health. 2001 Oct;43(4):177-89. PMID: 12395525.
- Choubisa SL. Toxic effects of fluoride on human bones. Adv Pharmacol Toxicol. 2012;13:9-13.
- Choubisa SL. Fluoride in drinking water and its toxicosis in tribals, Rajasthan, India. Proc Natl Acad Sci India Sect B Biol Sci. 2012; 82:325-330. doi: 10.1007/s40011-012-0047-0048.
- Choubisa SL. Status of fluorosis in animals. Proc Natl Acad Sci India Sect B Biol Sci. 2012;82(3):331-339. doi: 10.1007/s40011-012-0026-0.
- Choubisa SL, Modasiya V, Bahura CK, Sheikh Z. Toxicity of fluoride in cattle of the Indian Thar Desert, Rajasthan, India. Fluoride. 2012;45(4):371-376.
- Choubisa SL. Fluorotoxicosis in diverse species of domestic animals inhabiting areas with high fluoride in drinking waters of Rajasthan, India. Proc Natl Acad Sci India Sect B Biol Sci. 2013;83:317-321. doi: 10.1007/s40011-012-0138-6.
- Choubisa SL. Fluoride toxicosis in immature herbivorous domestic animals living in low fluoride water endemic areas of Rajasthan, India: an observational survey. Fluoride. 2013;46:19-24.
- Choubisa SL. Osteo-dental fluorosis in horses and donkeys of Rajasthan, India. Fluoride. 2010;43:5-10.
- Choubisa SL. Fluorosis in dromedary camels of Rajasthan, India. Fluoride. 2010;43:194-199.
- Choubisa SL, Mishra GV, Sheikh Z, Bhardwaj B, Mali P, Jaroli VJ. Food, fluoride, and fluorosis in domestic ruminants in the Dungarpur district of Rajasthan, India. Fluoride. 2011;44:70-76.
- Sharma JD, Sohu D, Jain P. Prevalence of neurological manifestations in a human population exposed to fluoride in drinking water. Fluoride. 2009;42:127-132.
- Singh VP, Chauhan DS, Tripathi S, et al. A correlation between serum vitamin, acetylcholinesterase activity and IQ in children with excessive endemic fluoride exposure in Rajasthan, India. Intl J Med Sci. 2013;1:12-26.
- Spittle B. Dental fluorosis as a marker for fluoride-induced cognitive impairment, (Editorial). Fluoride. 2016;49:3-4.
- Guo Z, He Y, Zhua Q, Research on the neurobehavioral function of workers occupationally exposed to fluoride. Indust Health Occupat Dis. 2001;27:346-348.
- Translated by Julian Brooke and published with the concurrence of Indust Health Occupat Dis in Fluoride. 2008;41:152-155.
- Singh VP, Chauhan DS, Tripathi S. Acetylcholinesterase activity in fluorosis adversely affects mental well-being: an experimental study in rural Rajasthan. European Acad Res. 2014;2:5857-5869.
- Pornprasert S, Wanachantararak P, Kantawong F, Chamnanprai S, Kongpan C, Pienthai N, Yanola J, Duangmano S, Prasannarong M. Excessive fluoride consumption increases haematological alteration in subjects with iron deficiency, thalassaemia, and glucose-6-phosphate dehydrogenase (G-6-PD) deficiency. Environ Geochem Health. 2017 Aug;39(4):751-758. doi: 10.1007/s10653-016-9845-x. Epub 2016 Jun 18. PMID: 27318827.
- Jain RC, Andrew AM, Choubisa SL, Acharya A, Joshi KC. Sickle cell gene in the Mina tribal population of Kherwara tehsil of Udaipur district in Rajasthan. Indian J Med Res. 1983 Oct;78:552-555. PMID: 6668011.
- Jain RC, Andrew AM, Choubisa SL. Sickle cell & thalassaemic genes in the tribal population of Rajasthan. Indian J Med Res. 1983 Dec;78:836-40. PMID: 6674173.
- Choubisa SL. Abnormal haemoglobins, thalassaemia and G-6-PD enzyme deficiency in Rajasthan (western-India). Haematologia (Budap). 1991;24(3):153-65. PMID: 1841846.
- Choubisa SL, Choubisa DK, Choubisa L. Erythrocyte genetic disorders in inhabitants of Aravali hilly environment. Indian J Anthrop Hum Genet. 2004;23:145-159.
- Choubisa SL. Sickle cell haemoglobin, thalassaemia and G-6-PD enzyme deficiency genes in Garasiya tribe inhabited malaria endemic areas of Sirohi District, Rajasthan (India). J Commun Dis. 2009 Mar;41(1):13-18. PMID: 19886170.
- Choubisa SL, Choubisa A. Status of erythrocyte genetic disorders in people of desert and humid environments, Rajasthan, India: focus on natural selection in tribals against malaria. Proc Indian Natl Sci Acad. 2021 Sept;87(3):433-445. doi: 10.1007/s43538-021-00045-2.
- Choubisa SL. Mollusc as bio-indicators for the trophic stages of lakes and lotic environments. Bull Pure Appl Sci. 1992;11:35-40.
- Choubisa SL. Snails as bio-indicators for dreaded trematodiasis diseases. J Commun Dis. 2010 Sep;42(3):223-6. Erratum in: J Commun Dis. 2010 Sep;42(3):2 p preceding 171. PMID: 22471188.
- Parmar TK, Rawtani D, Agrawal YK. Bioindicators: the natural indicator of environmental pollution. Front in life Sci. 2016;9:110-118.
- Choubisa SL, Sompura K. Dental fluorosis in tribal villages of Dungarpur district (Rajasthan). Poll Res. 1996;15:45-47.
- Choubisa SL, Sompura K, Bhatt SK, et al. Prevalence of fluorosis in some villages of Dungarpur district of Rajasthan. Indian J Environ Health. 1996;38(2):119-126.
- Choubisa SL. Chronic fluoride intoxication (fluorosis) in tribes and their domestic animals. Intl J Environ Stud. 1999;56:703-716.
- Choubisa SL. Some observations on endemic fluorosis in domestic animals in Southern Rajasthan (India). Vet Res Commun. 1999 Nov;23(7):457-65. doi: 10.1023/a:1006325710222. PMID: 10598076.
- Choubisa SL. Bovine calves as ideal bio-indicators for fluoridated drinking water and endemic osteo-dental fluorosis. Environ Monit Assess. 2014 Jul;186(7):4493-4498. doi: 10.1007/s10661-014-3713-x. Epub 2014 Mar 27. PMID: 24671615.
- WHO. International programme on chemical safety biomarkers in risk assessment: validity and validation. 2001.
- Dwivedi SK, Dey S, Swarup D. Hydrofluorosis in water buffalo (Bubalus bubalis) in India. Sci Total Environ. 1997 Nov 27;207(2-3):105-159. doi: 10.1016/s0048-9697(97)00247-7. PMID: 9447740.
- Trangadia BJ, Kaul PL, Patel BJ, Joshi DV, Kaul L. Chronic fluorosis in buffaloes: clinic-pathological studies. Indian J Vet Pathol. 2015;39:354-357.
- Samal UN, Naik BN. The fluorosis problem in tropical sheep. Fluoride. 1992;25:183-190.
- Swarup D, Dey S, Patra RC, Dwivedi SK, Lali S. Clinico- epidemiological observations of industrial bovine fluorosis in India. Indian J Anim Sci. 2001;1:1111-1115.
- Sankhala SS, Harshwal R, Paliwal P, Agarwal A. Toe nails as a biomarker of chronic fluoride exposure secondary to high water fluoride content in areas with endemic fluorosis. Fluoride. 2014;47:235-240.
- Death C, Coulson G, Kierdorf U, Kierdorf H, Morris WK, Hufschmid J. Dental fluorosis and skeletal fluoride content as biomarkers of excess fluoride exposure in marsupials. Sci Total Environ. 2015 Nov 15;533:528-41. doi: 10.1016/j.scitotenv.2015.06.054. Epub 2015 Jul 16. PMID: 26188404.
- Patra RC, Dwivedi SK, Bhardwaj B, Swarup D. Industrial fluorosis in cattle and buffalo around Udaipur, India. Sci Total Environ. 2000 May 15;253(1-3):145-50. doi: 10.1016/s0048-9697(00)00426-150. PMID: 10843338.
- Del Carmen AF, Javier FH, Aline CC. Dental fluorosis, fluoride in urine, and nutritional status in adolescent students living in the rural areas of Guanajuato, Mexico. J Int Soc Prev Community Dent. 2016 Nov-Dec;6(6):517-522. doi: 10.4103/2231-0762.195510. PMID: 28032042; PMCID: PMC5184384.
- Watanable M, Kono K, Orita Y, Dote T, Usuda K, Takahashi Y. Influence of dietary fluoride intake on urinary fluoride concentration and evaluation of corrected levels in spot urine. Proceedings of the 20th Conference of the International Society for Fluoride Research, Beijing, China, September 5-9. 1994.
- Rafique T, Ahmed I, Soomro F, Khan MH, Shirin K. Fluoride levels in urine, blood plasma and serum of people living in an endemic fluorosis area in the Thar Desert, Pakistan. J Chem Soc Pakistan. 2015;37:1223-1230.
- Choubisa SL, Choubisa L, Sompura K, Choubisa D. Fluorosis in subjects belonging to different ethnic groups of Rajasthan, India. J Commun Dis. 2007 Sep;39(3):171-177. PMID: 18697581.
- Choubisa SL, Choubisa L, Choubisa D. Osteo-dental fluorosis in relation to nutritional status, living habits and occupation in rural areas of Rajasthan, India. Fluoride. 2009;42:210-215.
- Choubisa SL, Choubisa L, Choubisa D. Osteo-dental fluorosis in relation to age and sex in tribal districts of Rajasthan, India. J Environ Sci Eng. 2010;52:199-204.
- Choubisa SL. Natural amelioration of fluoride toxicity (fluorosis) in goats and sheep. Curr Sci. 2010;99:1331-1332.
- Choubisa SL, Choubisa L, Choubisa D. Reversibility of natural dental fluorosis. Int J Pharmacol Biol Sci. 2011;5:89-93.
- Choubisa SL, Mishra GV. Fluoride toxicosis in bovines and flocks of desert environment. Int Pharmacol Biol Sci. 2013;7:35-40.
- Choubisa SL. Osteo-dental fluorosis in relation to chemical constituents of drinking waters. J Environ Sci Eng. 2012 Jan;54(1):153-158. PMID: 23741872.
- Choubisa SL. Why desert camels are least afflicted with osteo-dental fluorosis? Curr Sci. 2013;105:1671-1672.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.