> Medicine Group. 2021 July 28;2(7):624-631. doi: 10.37871/jbres1288.
Malaria Transfusional Transmission: Epidemiological Review, Screening Protocols and Prevention Mechanisms
Márcia Maria Ferreira-Silva1,2*, Aline Menezes Carlos2 and Glaucia Aparecida Domingos Resende2
2Department of Hematology and Hemotherapy, Federal University of Triangulo Mineiro, MG, Brazil
Abstract
Malaria is a neglected tropical disease, whose main form of transmission occurs through the bite of the female Anopheles mosquito infected by the parasite Plasmodium sp. Its clinical symptoms range from asymptomatic cases to more severe and fatal conditions. Added to this natural transmission mechanism, many studies report that Malaria is one of the main infectious diseases transmitted by transfusion. There are reports of prevalence among blood donors in the five continents, with the highest number of cases in Africa, Asia and South America, regions of high endemicity. Factors such as the high prevalence rate of asymptomatic malaria carriers, as well as deficient regulation in the screening of blood donors and an ineffective hemovigilance policy make the risk of Transfusion-Transmitted Malaria (TTM) worse, exposing millions of people possible contamination by transfusion, especially in underdeveloped countries. Patients with underlying diseases or immunosuppressed who require polytransfusions are the most susceptible to TTM. After an eventual transfusion of bags contaminated by Plasmodium sp, these patients can develop the most severe form of the disease, presenting high-risk clinical complications that can culminate in fatal outcomes. In view of the facts and aiming at greater transfusion safety, it is observed that stricter regulatory policies aimed at preventing TTM are needed; such policies will be more comprehensive if coordinated by the World Health Organization (WHO) and more effective if they are adequate to the reality of endemic and non-endemic countries. In blood banks, control measures should focus mainly on broad serological coverage with high performance tests, in addition to active hemovigilance programs and encouragement of research and implementation of methods of inactivation of pathogens in blood component bags. Given the above, this study was carried out with the aim of providing knowledge of the current panorama of the prevalence of malaria among blood donors and of documented cases of TTM around the world, as well as demonstrating the disease tracking methodologies in use in different countries, and present possibilities for adopting mechanisms that allow better control of the transfusional transmission of malaria in blood banks.
Research Methods
Online searches were conducted of all available articles on the prevalence of malaria among blood donors and documented cases of transfusion transmission in endemic and non-endemic countries, published until July 2021. Full articles of major relevance, written in English, Spanish and Portuguese were consulted. The searched databases were Pubmed, Scopus and LILACS using combinations of the following search terms: ‘Malaria’, “blood transfusion”, “Plasmodium”, “blood donors”. In addition to these databases, official government health websites and WHO data were consulted with a view to the possibility of presenting the most current epidemiological data.
Malaria’s Epidemiology
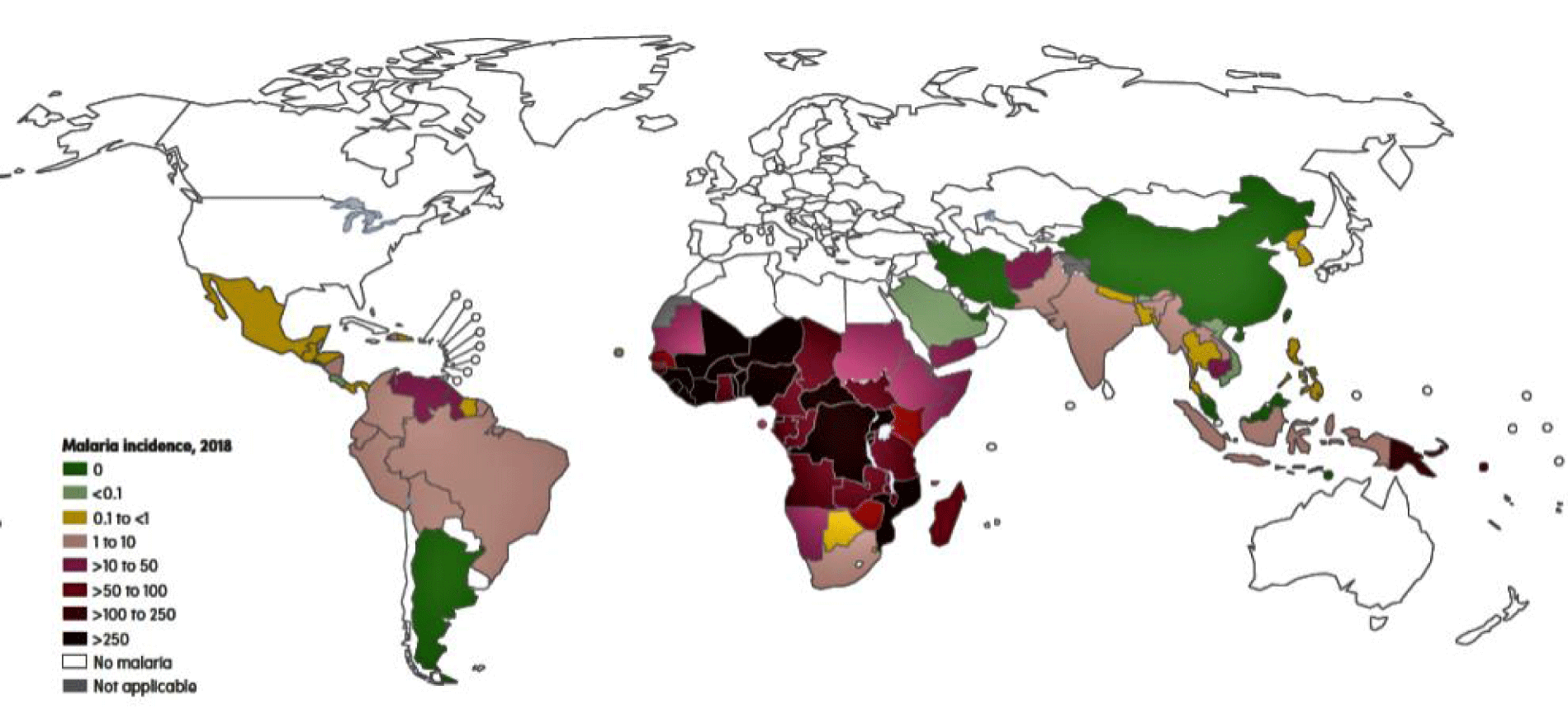
Malaria is a neglected tropical disease, caused by parasites of the Plasmodium genus, which are classified as: P. falciparum, P. vivax, P. malariae and P. ovale. In 2019, there were an estimated 229 million cases of malaria in 90 countries and territories. Malaria deaths in 2019 reached more than 436,000 [1] (PAHO Report, 2020/last World Malaria Report Released in December 2020). The disease is widely distributed, mainly in the tropical regions of the globe, and is still one of the most important public health problems worldwide. The most affected countries are the Africans, located south of the Sahara desert (responsible for more than 90% of cases), those of Southeast Asia and Latin America, particularly those that make up the Amazon region (Figure 1).
The African continent is the most affected by the disease, with 10 countries standing out in the incidence of malaria: Burkina, Faso, Congo, Cameroon, Ghana, Mali, Mozambique, Niger, Nigeria, Uganda and Tanzania. In these countries, there was an increase of 3.5 million cases between 2016 and 2017. Altogether there were 200 million accumulated cases of the disease in Africa in 2018 [2].
According to the World Health Organization (WHO), Asia ranks second in malaria incidence (7% of cases), after Africa (90%). Malaria is endemic in 19 countries in Asia, where 2.31 billion people or 62% of the total population of these countries are at risk of contracting the disease. In 2011, over 2,000 deaths were reported, with India, Indonesia, Myanmar and Pakistan accounting for more than 85% of reported (confirmed) cases and deaths on the continent [3,4].
WHO data indicate a drop in the number of cases in 2019, but this drop was less pronounced than initials estimates. In that same year, 94% of all cases occurred on the African continent, 3% in Southeast Asia, 2% in the Eastern Mediterranean and less than 1% in the Western Pacific and the Americas [2,5]. Malaria has killed more than 450,000 people, with 97% of these deaths occurring in Africa and Asia. Mortality has increased in the Americas and in the Western Mediterranean region, which also encompasses some Northeast African countries, such as Egypt, Sudan and Libya [4,6].
In 2017, there were 2,161 cases of malaria in the United States; this was the highest number in 45 years, probably associated with the increase in tourist and domestic travel. Of these cases, 86% were from Africa, with almost 67% of these coming from West Africa. Plasmodium falciparum was responsible for the majority of infections (70.5%), followed by Plasmodium vivax (10.0%), Plasmodium ovale (5.5%) and Plasmodium malariae (2.6%) [7]. In Brazil, about 99.6% of cases were identified in the Amazon region, which is considered an endemic area [8].
Although mosquito bites are the main transmission mechanism, studies have reported that malaria can be efficiently transmitted by transfusion of cellular blood components and organ transplantation and is undoubtedly responsible for most cases of transfusion-transmitted parasitic diseases in the world. Literature data on Transfusion-Transmitted Malaria (TTM) have shown that in immunosuppressed recipients, infections can be fatal if not diagnosed and treated in a timely manner. The real incidence of transfusional malaria is underreported in endemic areas. The main reason is that a large part of the affected population has asymptomatic parasitemia, and that in poorer regions blood screening is not done effectively, making it difficult to clarify whether the transmission actually occurred after the transfusion [9].
Clinical Forms
The clinical picture of Malaria is variable, depending on the origin of the individual, the species of infecting plasmodium, the use of prophylactic medication and the host’s immune response. Symptoms usually appear within 4 weeks after contact with the vector in P. falciparum infection, whereas in P. vivax and P. ovale infection they may appear after several months due to the prolonged liver tissue phase (hypnozoites) [10]. However, most infections will be symptomatic within a year of contact, regardless of the infecting species. Headache, feeling cold, arthralgia, anemia, splenomegaly and hepatomegaly are common. Severe malaria usually occurs when erythrocyte parasitism is greater than or equal to 5%, manifests with extreme prostration, lowered level of consciousness, pulmonary edema or Acute Respiratory Distress Syndrome (ARDS) [11], seizures, cardiovascular shock, abnormal bleeding, jaundice , hemoglobinuria, or severe anemia [12,13], with a mortality rate exceeding 20%, even with optimized treatment [10].
One of the most serious complications is the involvement of the central nervous system - the Cerebral Malaria -, resulting from the occlusion of the cerebral microvasculature, with altered mental status, seizures and focal neurological deficits; also with high mortality (15-25%), and neurological sequelae in survivors [14].
Although residents of endemic areas are less prone to severe Malaria, they (especially children) may have complications from chronic anemia, massive splenomegaly, causing abdominal pain and dysfunction of the bone marrow and immune system (hyperreactive malarial splenonegalia, and lymphomas), and nephrotic syndrome due to glomerular deposition of immune complexes.
Malaria and Blood Transfusion
Malaria was one of the first diseases recognized as transmissible by blood transfusion [15]. The first case of transfusional malaria was described in 1911 [16] and even today, malaria remains one of the parasitic diseases of greatest concern and incidence in hemotherapy. Transmission of the parasite by transfusion can represent an important risk factor, as they can cause severe malaria with a high mortality rate, especially in immunocompromised patientes and thhose with coeisting diseases [17].
Chronic infection can persist in humans for a long period of time: up to two years or more for P. falciparum, up to seven years for P. vivax, and even lifelong for P. malariae. Therefore, an asymptomatic individual who is unaware of their serological and/or parasitological status can transmit the parasites during blood donation [18]. Typically, the concentration of parasites is low in asymptomatic individuals; however, they can cause severe infection in immunocompromised or debilitated blood recipients.
The Plasmodium sp can survive for more than a week in blood components stored at room temperature or refrigerated at 4°C. Viable P. falciparum has already been identified in blood stored for 19 days [17,19]. Studies show greater resistance and consequently greater lethality in infections transmitted by Plasmodium falciparum, followed by Plasmodium malariae, although there are also fatal cases of transfusional contamination by Palsmodium vivax [17].
As it is an intraerythrocyte parasite, Plasmodium transmission occurs primarily through components that contain red blood cells. However, there are case reports after transfusion of platelet concentrate, leukocyte concentrate and, very rarely, by cryoprecipitates, probably due to the contamination of these blood components by red blood cells. There are also rare reports of transmission by transfusion of frozen red cell concentrate [17]. On the other hand, there are no cases transmitted by transfusion of fresh frozen plasma or blood products. The recipient transfused with a blood component that has red cells parasitized with schizont forms of Plasmodium will receive these red cells in the bloodstream, which can rupture in less than 48 hours, depending on the maturity at which the schizonts were in the donor. Therefore, depending on the amount of transfused parasitized red blood cells, the onset of symptoms may be similar to the natural cycle of the disease (around 15 days). On the other hand, as there is no liver cycle in transfusional malaria, as there are no sporozoites involved, the incubation period may be longer than in the natural cycle if the concentration of transfused parasitized red cells is very small, as the erythrocyte cycle will have to be repeated for more often, compared to natural infection, so that the parasitic concentration is such that it causes the symptoms [20]. An important difference between natural infection and TTM is that in the natural one there is an asymptomatic (pre-erythrocytic) initial phase that allows the activation of cells of innate immunity against malaria parasites. This early stage has advantages for both the parasite and the host: innate immunity gives the host time to develop more specific protective immunity; meanwhile, the parasites trigger the escape mechanism against the host’s immune response. Transfusions of contaminated blood cause the release of plasmodiums directly into the recipient’s bloodstream, triggering high-risk and potentially fatal complications [21]. In fact, the incubation period for transfusional malaria can range from 8 to 36 days for P. falciparum (median 16 days), 11 to 42 days for P. vivax (median 17 days), and 8 to 90 days (48 days) for P. Malariae [10]. This long period is one of the difficulties for the diagnostic suspicion, which can lead to a delay in the specific treatment and to serious and fatal cases.
There is no consensus on the minimum infective dose of Plasmodium to cause transfusional malaria. However, it is estimated that an individual with very low parasitemia (eg, with 0.001 parasite/μL or 1.0 parasite/mL), who donated a bag of whole blood, would produce a packed red cell bag (250 mL) with a dose of 250 parasites, which would be enough to cause infection in the recipient. Experimental evidence suggests that only 10 infected erythrocytes may be sufficient to transmit the infection; thus, even a small inoculum is potentially infectious [17].
The risk of malaria transmission through transfusion is little known and probably underestimated, especially in endemic countries due to poor or non-existent hemovigilance, difficulties related to the traceability of blood components or the difficulty in distinguishing induced cases from cases of natural infection. Worldwide, more than 3,000 cases of Transfusion-Transmitted Malaria (TTM) have been reported [22-24].
On the other hand, in the United States, where there is no endemic malaria, the average number of reported cases is three cases per year, with an average incidence of 0.25 cases per million transfusions. Between 2000 and 2017, 11 cases of TTM were reported in the US and were related to asymptomatic infected donors who had already resided in malaria-endemic countries or who had a previous diagnosis of malaria; none of these were associated with travelers residing in non-endemic countries [7,25].
On the American Continent, a compilation of studies carried out between 1971 and 2016 showed that the highest percentage of tranfusional malaria was in Mexico (50.7%), followed by 40.3% in the United States of America (USA) and 6.6% in Brazil, demonstrating that malaria in Latin America remains endemic [9].
In France, between 1990 and 2006, the risk of occurrence was 7.5 cases per million of transfusions. However, with the increase in international travel, the exposure of individuals from non-disruptive countries to endemic areas becomes more frequent, with a consequent increase in the prevalence of infected donors and the risk of transfusional transmission [26]. In several European countries, cases of TTM have also been reported. In Spain there was an outbreak in 1971 and three other cases were reported by 2002, and most cases were caused by plasmodium falciparum [27].
In endemic countries, the incidence is estimated to exceed 50 cases per million transfusions. In Brazil, according to data from the Health Surveillance Notification System (Notivisa), since the National System of Hemovigilance (NHS) began in 2002, only four cases of transfusional malaria have been reported, three in 2006, in the state of Rondônia, and one in 2007, in the state of Amazonas. All reported cases were caused by P. vivax and all progressed to death (National Health Surveillance Agency, 2009). The three cases that occurred in the state of Rondônia were neonates, transfused with fractionated packed red blood cells from a single donor. It is estimated that, in the Brazilian Amazon region alone, around 300,000 transfusions are carried out per year, which would result in 15 cases per year, strongly suggesting a high rate of underreporting [28].
The World Health Organization recommends that testing for malaria be carried out for all donors, however despite the great importance of strict TTM control, such measure has not been implemented by hemotherapy services in the sub-Saharan Africa region. In this region, malaria infection is endemic and there is a high proportion of asymptomatic carriers. Screening for the parasite was implemented only in some regions, being considered very inefficient, which can generate a high risk of malaria transmission via blood transfusion. There is little information on how African countries incorporate the World Health Organization (WHO) recommendations on TTM into their policies. As in Sub-Saharan Africa, in Ghana the blood transfusion policy does not mention the screening of blood from positive donors for malaria or the prophylactic treatment given to recipients [29,30].
In Ghana, a country in West Africa, which has one of the highest rates of endemicity on the continent, studies report a parasitemia in blood donors of approximately 50% and the incidence of transmission through contaminated blood ranging between 14-28%. The difficulty in describing the real incidence of transfusional malaria in endemic countries, such as Africa, is due to the lack of reports on the implementation of donor screening policies and also that not all malaria-positive blood recipients will develop the disease [29,31].
In more populous Asian countries such as China and India, TTM case reports and detection may also be underreported. Although many malaria research papers have been published in China, transfusion trania has been largely neglected [32]. Although China is moving towards the eradication of malaria, recent assessments have revealed that cases from the most endemic regions remain a major challenge for the definitive elimination of malaria, and, consequently, the number of TTM cases has been increasing over time. A total of 12 TTM cases were reported in China between 2013 and 2018 [33]. All recipients and donors were diagnosed using rapid diagnostic testing and peripheral blood smears. Nine recipients (75.0%) were infected by Plasmodium falciparum, two (16.7%) by Plasmodium vivax and one (8.3%) by Plasmodium ovale [32].
Oceania appears to be the continent least affected by malaria. A study from Australia showed the lowest prevalence rate among blood donors (0.1%) [34]. Although malaria is not endemic in Australia, there are between 500 and 900 cases of imported malaria annually, constituting a continuous risk of TTM [35]. The last documented case of Australian TTM was a fatal case associated with P. falciparum and occurred in 1991 [36], this being the first transfusion transmission reported since 1960. Since approximately one million donations are collected annually in the Australia, the estimated incidence of TTM since 1991 is less than one (1) in 15 million.
Control Measures (Blood Transfusion Transmitted Malaria Prevention Mechanisms)
The control of transfusional malaria is done by excluding individuals at risk and by laboratory screening of collected blood. Clinical and epidemiological screening can exclude a significant number of uninfected individuals, compromising the blood supply, and should deserve different approaches in different regions (endemic and non-endemic) and in different situations. The laboratorial screening of collected blood is carried out through the investigation of the intra-erythrocytic parasite and of antigens and antibodies in serum samples. For more effective TTM control, it is important to adopt, in addition to clinical and laboratory screening with high sensitivity and specificity methodologies, hemovigilance policies, and new technologies for the inactivation of pathogens in blood component bags.
Clinical and epidemiological screening for the prevention of transfusional malaria
Fever is the most characteristic symptom of malaria. The measurement of body temperature and investigation of fever in the days preceding the donation are mandatory requirements in any blood therapy service, as they prevent the transmission of various viral, bacterial and parasitic diseases. Individuals febrile at the time of donation or with a report of fever in the days prior to donation are considered unfit. Thus, individuals with the oligosymptomatic form of malaria, if well evaluated, may be excluded by this clinical exclusion criterion .
However, asymptomatic individuals, both in the incubation period, and chronic carriers of protozoa - which correspond to the large contingent of infected individuals who donate blood, represent a significant challenge for hemotherapy, with regard to donor selection criteria.
In non-endemic countries, the clinical and epidemiological selection criteria to prevent donation from individuals at risk of Plasmodium infection are highly sensitive, but not very specific. In general, donation candidates are excluded based on reports of displacement to risk areas and clinical history of previous malaria infection [37]. In the United States, asymptomatic individuals who traveled to endemic regions and lived in endemic regions for three years are prohibited from donating blood for 12 months. Individuals who got sick from malaria are unable to donate blood for three years after the end of treatment and absence of symptoms; no laboratory screening tests for malaria are performed [7].
In Europe, asymptomatic individuals with displacements to endemic regions are prevented from donating blood for six months. Those who lived in endemic regions within the first five years of life are unable to donate for three years after the last visit to an endemic area. Individuals with a history of malaria or with symptoms of malaria after moving to an endemic area are prevented from donating blood for three years after treatment and/or absence of symptoms. In South America, countries with endemic areas in their territory adopt different selection criteria for endemic and non-endemic áreas [21,37].
Laboratory screening for the prevention of transfusion-transmitted malaria
Laboratory screening for malaria among blood donors is a challenge to be faced by hemotherapy services, especially in endemic areas. Indirect detection methods of infection (antibodies) against Plasmodium are indicated for countries where malaria is not endemic. They have been used in Europe, New Zealand and Australia as a complement to clinical and epidemiological screening, with the aim of reducing the downtime of donor candidates exposed to areas at risk for malaria transmission and reducing the disposal of healthy blood [37]. In endemic areas, the effectiveness of the use of serological tests is questioned. The immune response to plasmodia is a complex phenomenon. The immune system presents varied responses in both the pre-erythrocytic and erythrocytic stages and involves a variety of classes and subclasses of immunoglobulins, as well as a large number of cytokines. Furthermore, anti-Plasmodium antibodies can remain circulating for decades, even in the absence of infection. In endemic areas, depending on the prevalence of positivity in the population, the use of antibody detection methods in laboratory screening of blood donors can lead to shortages. Furthermore, indirect detection tests do not allow detection of antibodies in the incubation period or even in the first days of disease symptoms, which would result in tests with false negative results [17,38]. Given the limitation of serological tests, for endemic areas it is recommended that laboratory screening for malaria be carried out using methods of direct or indirect detection of the parasite. The methods of direct visualization of Plasmodium, traditionally used to detect malaria cases, are microscopic examinations of blood slides stained with Giemsa or Giemsa with methylene blue (Walker) or Wright’s solution. Slides can be prepared as a smear or thick drop. It is also common to use acridine orange, which can be applied on slides or in capillary tubes, called QBC® tests. Among indirect detection tests, rapid immunochromatographic tests have been widely used. These tests detect plasmodial antigens circulating in the blood of those infected. The most used antigens are Histidine-Rich Protein (HRP-2), plasmodial Lactic Dehydrogenase (pLDH) and aldolase. Nucleic Acid Detection Tests (NAT) are still not common for the diagnosis of malaria [17], but they have been used in epidemiological studies of asymptomatic carriers and suggested as relevant in the laboratory screening of blood donors. Still, in endemic countries that perform laboratory screening for malaria in blood donors, the thick drop is undoubtedly the most used test. Surveillance of transfusional malaria is a challenge in areas of active transmission, probably reflected in underreporting of cases, especially in areas of Central America and the Amazonian region. Therefore, transfusional malaria can only be distinguished from naturally transmitted cases by genotyping to demonstrate that the parasite in the transfusion recipient is identical to the unit of transfused blood. A limitation of this methodology is that it cannot detect low levels of Plasmodium clones in the transfused blood. Therefore, the association of different approaches needs to be established to ensure the safety of blood transfusions through a screening with a diagnostic strategy that is sensitive enough to detect low levels of parasitemia, taking into account availability and costs [8].
Brazilian regulations for the prevention of transfusion-transmitted malaria
In Brazil, the sanitary requirements for the selection of donors began to be established in 1965 with Law No. 4,701/1965. The first donor selection criteria, based on the risk of infection by Plasmodium, were established in 1969 by an Ordinance of the extinct National Commission on Hemotherapy. In 1988, with the publication of Law No. 7.649/1988, malaria was included in the list of diseases that should obligatorily be considered in the screening of donors. Nevertheless, it was only after Mercosur Resolution No. 42/2000 that the MS, through the Collegiate Board Resolution (RDC) of the National Health Surveillance Agency (Anvisa) No. 343/2002 [39] included in the technical regulations of hemotherapy mandatory screening for malaria. Another substantial change instituted by RDC Anvisa n° 343/2002 was the one that established that risk ranges should be used as a criterion for excluding candidates for donation from endemic areas. The criteria currently established through the Ordinance of the Ministry of Health, Brazil (ORDINANCE No. 158, OF FEBRUARY 4, 2016), which redefine the technical regulation of hemotherapy procedures, to prevent the donation of individuals at risk of infection by Plasmodim sp, establish that the inability of a candidate to donate blood must occur using, as a reference criterion, the Annual Parasitic Incidence (IPA) of the Municipality. In endemic areas with an epidemiological history of malaria, the candidate will be considered unsuitable when who has had malaria in the 12 (twelve) months prior to the donation or with fever or suspected malaria in the last 30 (thirty) days and who have moved or come from a high-risk area (IPA greater than 49.9) for less than 30 (thirty) days. In non-endemic areas for malaria, the candidate who has moved or comes from municipalities located in endemic areas for less than 30 days will be considered unsuitable [40].
Alternative methods for preventing transfusion-transmitted malaria
There are several alternative strategies already tried or recommended in different parts of the world for the prevention of transfusional malaria. Among them, the universal prophylaxis of receptors and the addition of chemical substances or irradiation capable of killing plasmodium stand out. Universal prophylaxis of recipients, or minimally for those at high risk for malaria, has been recommended by several authors and by the WHO for the holoendemic and hyperendemic countries of sub-Saharan Africa, where the prevalence of positive donors for malaria é em média 25% [41]. In these areas, it is understood that the damage caused by blood shortages outweighs the risks associated with malaria prophylaxis in patients. On the other hand, the costs of this measure for the hemotherapeutic system can be prohibitive for some countries in this situation. Some malaria transmission control strategies have considered adding substances such as gentian violet to blood bags or medications [42]. This measure has been explored for some time by several researchers with the intention of eliminating plasmodia. The addition of Gentian violet had its effectiveness proven as a chemical agent capable of eliminating Plasmodium from infected red blood cells. However, due to its mitochondrial toxicity in rats and its carcinogenic potential in mice, its use is not recommended even for bloods products. The addition of drugs used for the prophylaxis or treatment of malaria is also suggested to eliminate the parasite from blood bags. There are, however, concerns that should be highlighted, such as the resistance of P. falciparum to drugs and the consequent increase in the lethal dose necessary to achieve the same efficacy that, at the same time, must be safe for the blood recipient. Added to this is the fact that the drug has no action against P. Vivax [43], which is the predominant species in the Americas. Due to the potential toxicity of additives such as gentian violet and drugs and in blood bags, new technologies are available to selectively inactivate pathogens without damaging cells or plasma; a combination of riboflavin as a photosensitizer with an ultraviolet light illumination device (Mirasol System for Whole Blood; Terumo BCT, Lakewood, Colo.) has been shown to substantially reduce P. falciparum infectivity in whole blood samples without changing quality parameters cell phone [44]; this inactivation technology may represent a promising option for TTM control in the future. In Brazil, neither pre-transfusion prophylaxis for malaria nor the addition of substances or irradiation of the bags were adopted as techniques to prevent transfusion malaria. The entire Brazilian sanitary regulation for the prevention of transfusional malaria is based on donor selection techniques, that is, on clinical and epidemiological screening, and on laboratory screening, through laboratory tests for direct detection of Plasmodium or its antigens. Thus, the great expectation for the definitive control of the TTM lies in the effective fulfillment of the goals proposed by the WHO for the eradication of malaria in the world, foreseen for the year 2030. These actions will directly impact on the number of cases of transfusional transmission of malaria, and include [45]:
• Reduction in the incidence of malaria cases by at least 90%;
• Reduced malaria mortality rates by at least 90%;
• Elimination of malaria in at least 35 countries;
• Preventing the resurgence of malaria in all malaria-free countries.
Such goals will be successful if there is better targeting of interventions, new tools to combat the vector and greater public and private financing, which are necessary to change the global trajectory of the disease and reach the internationally agreed goals [2].
Conclusions
The compilation of data for this study allowed us to demonstrate some conditions related to the epidemiology and prevalence of malaria among blood donors, as well as the current status of TTM in the world [46]. Are they;
• Although malaria is highly endemic in Africa, Southeast Asia and Latin America, there are many reports of seropositivity for Plasmodium sp. in blood donors from countries considered non-endemic;
• Malaria is one of the infectious diseases with the highest rate of transfusion transmission, with cases of TTM being documented in countries on five continents;
• Asymptomatic individuals represent a significant challenge for hemotherapy, with regard to donor selection criteria.
• TTM can trigger an aggravated clinical picture in polytransfused or immunodepressed patients, and may have fatal outcomes in these patients;
• The real number of TTM appears to be underreported, especially in countries where blood bank screening and hemovigilance regulatory policies are deficient.
In general, it is concluded that, to effectively combat TTM, stricter regulatory policies are needed; such policies will be more comprehensive if coordinated by the World Health Organization (WHO) and more effective if they are adequate to the reality of endemic and non-endemic countries. For more effective TTM control, it is important to adopt, in addition to clinical and laboratory screening - with high sensitivity and specificity methodologies, hemovigilance policies, and new technologies for the inactivation of pathogens in blood component bags.
References
- OPAS – Organização Pan Americana de Saúde. Relatório Malária, 2020. (Relatório OPAS, 2020). https://bit.ly/3iY24qU
- WHO. World Malaria Report 2019. World Health Organization: Geneva; 2019. https://bit.ly/3l6pwoC
- Battle KE, Lucas TCD, Nguyen M, Howes RE, Nandi AK, Twohig KA, Pfeffer DA, Cameron E, Rao PC, Casey D, Gibson HS, Rozier JA, Dalrymple U, Keddie SH, Collins EL, Harris JR, Guerra CA, Thorn MP, Bisanzio D, Fullman N, Huynh CK, Kulikoff X, Kutz MJ, Lopez AD, Mokdad AH, Naghavi M, Nguyen G, Shackelford KA, Vos T, Wang H, Lim SS, Murray CJL, Price RN, Baird JK, Smith DL, Bhatt S, Weiss DJ, Hay SI, Gething PW. Mapping the global endemicity and clinical burden of Plasmodium vivax, 2000-17: a spatial and temporal modelling study. Lancet. 2019 Jul 27;394(10195):332-343. doi: 10.1016/S0140-6736(19)31096-7. Epub 2019 Jun 19. PMID: 31229233; PMCID: PMC6675736.
- Wongsrichanalai C, Kurdova-Mintcheva R, Palmer K. Current Malaria Situation in Asia-Oceania. Methods Mol Biol. 2019;2013:45-56. doi: 10.1007/978-1-4939-9550-9_3. PMID: 31267492.
- Pradines B, Robert MG. Situation du paludisme dans le monde [Current situation of malaria in the world]. Rev Prat. 2019 Feb;69(2):146-149. French. PMID: 30983211.
- Nema S, Ghanghoria P, Bharti PK. Malaria Elimination in India: Bridging the Gap Between Control and Elimination. Indian Pediatr. 2020 Jul 15;57(7):613-617. doi: 10.1007/s13312-020-1888-5. PMID: 32727937; PMCID: PMC7387259.
- Mace KE, Lucchi NW, Tan KR. Malaria Surveillance - United States, 2017. MMWR Surveill Summ. 2021 Mar 19;70(2):1-35. doi: 10.15585/mmwr.ss7002a1. PMID: 33735166; PMCID: PMC8017932.
- Aschar M, Levi JE, Farinas MLRN, Montebello SC, Mendrone-Junior A, Di Santi SM. The hidden Plasmodium malariae in blood donors: a risk coming from areas of low transmission of malaria. Rev Inst Med Trop Sao Paulo. 2020 Dec 18;62:e100. doi: 10.1590/S1678-9946202062100. PMID: 33331519; PMCID: PMC7748032.
- Alho RM, Machado KV, Val FF, Fraiji NA, Alexandre MA, Melo GC, Recht J, Siqueira AM, Monteiro WM, Lacerda MV. Alternative transmission routes in the malaria elimination era: an overview of transfusion-transmitted malaria in the Americas. Malar J. 2017 Feb 15;16(1):78. doi: 10.1186/s12936-017-1726-y. PMID: 28202065; PMCID: PMC5312538.
- CDC – Center for disease control and Prevention. January 04, 2019. https://bit.ly/3l5qdhW
- Pukrittayakamee S, Chantra A, Vanijanonta S, White NJ. Pulmonary oedema in vivax malaria. Trans R Soc Trop Med Hyg. 1998 Jul-Aug;92(4):421-2. doi: 10.1016/s0035-9203(98)91075-6. PMID: 9850397.
- Sorabjee JS. Haematological changes in malaria. Bombay Hosp J. 1996;38:58.
- González L, Guzmán M, Carmona J, Lopera T, Blair S. Clinical and epidemiological characteristics of 291 patients hospitalized for malaria in Medellín (Colombia). Acta méd colomb. 2000;25:163-170.
- Valecha N, Bagga A, Chandra J, Sharma D. Cerebral symptoms with P. vivax malaria. Indian Pediatr. 1992 Sep;29(9):1176-8. PMID: 1452322.
- Kitchen AD, Chiodini PL. Malaria and blood transfusion. Vox Sang. 2006 Feb;90(2):77-84. doi: 10.1111/j.1423-0410.2006.00733.x. PMID: 16430664.
- Woolsey G. Transfusion for perniciosa anemia. Trans NY Surg Soci. 1911;132-133.
- Verra F, Angheben A, Martello E, Giorli G, Perandin F, Bisoffi Z. A systematic review of transfusion-transmitted malaria in non-endemic areas. Malar J. 2018 Jan 16;17(1):36. doi: 10.1186/s12936-018-2181-0. PMID: 29338786; PMCID: PMC5771189.
- da Silva-Nunes M, Moreno M, Conn JE, Gamboa D, Abeles S, Vinetz JM, Ferreira MU. Amazonian malaria: asymptomatic human reservoirs, diagnostic challenges, environmentally driven changes in mosquito vector populations, and the mandate for sustainable control strategies. Acta Trop. 2012 Mar;121(3):281-91. doi: 10.1016/j.actatropica.2011.10.001. Epub 2011 Oct 12. PMID: 22015425; PMCID: PMC3308722.
- Chattopadhyay R, Majam VF, Kumar S. Survival of Plasmodium falciparum in human blood during refrigeration. Transfusion. 2011 Mar;51(3):630-5. doi: 10.1111/j.1537-2995.2010.02872.x. Epub 2010 Sep 16. PMID: 20849405.
- Dover AS, Schultz MG. Transfusion-induced malaria. Transfusion. 1971 Nov-Dec;11(6):353-7. doi: 10.1111/j.1537-2995.1971.tb04429.x. PMID: 4944512.
- Garraud O. Mechanisms of transfusion-linked parasite infection. Transfus Clin Biol. 2006 Nov;13(5):290-7. doi: 10.1016/j.tracli.2006.11.005. Epub 2006 Dec 22. PMID: 17188542.
- Mardani A. Prevention strategies of transfusion-transmitted parasitic infections (TTPIs): Strengths and challenges of current approaches, and evaluation of the strategies implemented in Iran. Parasite Epidemiol Control. 2020 Feb 18;9:e00141. doi: 10.1016/j.parepi.2020.e00141. PMID: 32149193; PMCID: PMC7052507.
- Katz LM and Spencer BR, Travel and related health history: Malaria, prions and other transfusion-transmissible diseases. In: Eder A, Goldman M, editors. Screening Blood Donors with the Donor History Questionnaire, Bethesda, MD: AABB Press; 2019.
- Ahmadpour E, Foroutan-Rad M, Majidiani H, Moghaddam SM, Hatam-Nahavandi K, Hosseini SA, Rahimi MT, Barac A, Rubino S, Zarean M, Mathioudakis AG, Cevik M. Transfusion-Transmitted Malaria: A Systematic Review and Meta-analysis. Open Forum Infect Dis. 2019 Jun 11;6(7):ofz283. doi: 10.1093/ofid/ofz283. Erratum in: Open Forum Infect Dis. 2020 Jan 24;7(1):ofz540. PMID: 31334300; PMCID: PMC6634438.
- Maier CL, Gross PJ, Dean CL, Chonat S, Ip A, McLemore M, El Rassi F, Stowell SR, Josephson CD, Fasano RM. Transfusion-transmitted malaria masquerading as sickle cell crisis with multisystem organ failure. Transfusion. 2018 Jun;58(6):1550-1554. doi: 10.1111/trf.14566. Epub 2018 Mar 9. PMID: 29524230.
- Kendjo E, Houzé S, Mouri O, Taieb A, Gay F, Jauréguiberry S, Tantaoui I, Ndour PA, Buffet P, Piarroux M, Thellier M, Piarroux R; French Imported Malaria Study Group. Epidemiologic Trends in Malaria Incidence Among Travelers Returning to Metropolitan France, 1996-2016. JAMA Netw Open. 2019 Apr 5;2(4):e191691. doi: 10.1001/jamanetworkopen.2019.1691. PMID: 30951158; PMCID: PMC6523451.
- Velasco E, Gomez-Barroso D, Varela C, Diaz O, Cano R. Non-imported malaria in non-endemic countries: a review of cases in Spain. Malar J. 2017 Jun 29;16(1):260. doi: 10.1186/s12936-017-1915-8. PMID: 28662650; PMCID: PMC5492460.
- Kiesslich D, Araújo MA, Yurtsever SV, Torres K. Control of post-transfusion malaria in the Brazilian Amazon: proposal to modify technical standards. SUS Epidemiological Report. 1999;8:53-57.
- Allain JP, Owusu-Ofori AK, Assennato SM, Marschner S, Goodrich RP, Owusu-Ofori S. Effect of Plasmodium inactivation in whole blood on the incidence of blood transfusion-transmitted malaria in endemic regions: the African Investigation of the Mirasol System (AIMS) randomised controlled trial. Lancet. 2016 Apr 23;387(10029):1753-61. doi: 10.1016/S0140-6736(16)00581-X. PMID: 27116282.
- Antwi-Baffour S, Kyeremeh R, Amoako AP, Annison L, Tetteh JO, Seidu MA. The Incidence of Malaria Parasites in Screened Donor Blood for Transfusion. Malar Res Treat. 2019 Nov 25;2019:1457406. doi: 10.1155/2019/1457406. PMID: 31885856; PMCID: PMC6899260.
- Owusu-Ofori A, Gadzo D, Bates I. Transfusion-transmitted malaria: donor prevalence of parasitaemia and a survey of healthcare workers knowledge and practices in a district hospital in Ghana. Malar J. 2016 Apr 23;15:234. doi: 10.1186/s12936-016-1289-3. PMID: 27108087; PMCID: PMC4842271.
- Lin H. Current Situation of Transfusion-Transmitted Malaria in China. J Trop Med. 2021 Jul 8;2021:3970370. doi: 10.1155/2021/3970370. PMID: 34306101; PMCID: PMC8285173.
- Anand A, Mace KE, Townsend RL, Madison-Antenucci S, Grimm KE, Espina N, Losco P, Lucchi NW, Rivera H, Breen K, Tan KR, Arguin PM, White JL, Stramer SL. Investigation of a case of suspected transfusion-transmitted malaria. Transfusion. 2018 Sep;58(9):2115-2121. doi: 10.1111/trf.14778. Epub 2018 Sep 3. PMID: 30178476; PMCID: PMC6334839.
- Seed CR, Kee G, Wong T, Law M, Ismay S. Assessing the safety and efficacy of a test-based, targeted donor screening strategy to minimize transfusion transmitted malaria. Vox Sang. 2010 Apr;98(3 Pt 1):e182-92. doi: 10.1111/j.1423-0410.2009.01251.x. Epub 2009 Sep 8. PMID: 19744194.
- Liu C , Johansen C , Kurucz N , Whelan P. Annual Report of the National Arbovirus and Malaria Advisory Committee of the Communicable Diseases Network of Australia. 2005-2006. Commun Dis Intell. 2006;30:411-429.
- Stickland JF, Roberts AN, Williams V. Transfusion-induced malaria in Victoria. Med J Aust. 1992 Oct 5;157(7):499-500. doi: 10.5694/j.1326-5377.1992.tb137326.x. PMID: 1406405.
- O’Brien SF, Delage G, Seed CR, Pillonel J, Fabra CC, Davison K, Kitchen A, Steele WR, Leiby DA. The Epidemiology of Imported Malaria and Transfusion Policy in 5 Nonendemic Countries. Transfus Med Rev. 2015 Jul;29(3):162-71. doi: 10.1016/j.tmrv.2015.03.004. Epub 2015 Mar 26. PMID: 25933591.
- Wipasa J, Suphavilai C, Okell LC, Cook J, Corran PH, Thaikla K, et al. Anticorpos de longa duração e respostas de memória de células B aos parasitas da malária humana, Plasmodium falciparum e Plasmodium vivax . PLoS Pathog. 2010.
- ANVISA, 2002. RDC 50-2002- Ministério da Saúde, Brazil. https://bit.ly/3xka76F
- ANVISA 2016. RDC 102-2016. Ministério da Saúde, Brazil. https://bit.ly/2Vk1XgU
- Schindler T, Robaina T, Sax J, Bieri JR, Mpina M, Gondwe L, Acuche L, Garcia G, Cortes C, Maas C, Daubenberger C. Molecular monitoring of the diversity of human pathogenic malaria species in blood donations on Bioko Island, Equatorial Guinea. Malar J. 2019 Jan 15;18(1):9. doi: 10.1186/s12936-019-2639-8. PMID: 30646918; PMCID: PMC6332537.
- Yang SL, Di Santi SM, Amato Neto V, Moreira AA, Pinto PL, Boulos M, Campos R, de Sant’Ana EJ, Shiroma M. Ação, “in vitro”, da violeta de genciana sobre formas evolutivas do Plasmodium falciparum [In vitro activity of gentian violet on evolutive forms of Plasmodium falciparum]. Rev Inst Med Trop Sao Paulo. 1988 Jan-Feb;30(1):17-20. Portuguese. doi: 10.1590/s0036-46651988000100003. PMID: 3065904.
- Carlini ME, White AC Jr, Atmar RL. Vivax malaria complicated by adult respiratory distress syndrome. Clin Infect Dis. 1999 May;28(5):1182-3. doi: 10.1086/517779. PMID: 10452670.
- Owusu-Ofori S, Kusi J, Owusu-Ofori A, Freimanis G, Olver C, Martinez CR, Wilkinson S, Mundt JM, Keil SD, Goodrich RP, Allain JP. Treatment of Whole Blood With Riboflavin and UV Light: Impact on Malaria Parasite Viability and Whole Blood Storage. Shock. 2015 Aug;44 Suppl 1(Suppl 1):33-8. doi: 10.1097/SHK.0000000000000280. PMID: 25423125; PMCID: PMC4498649.
- Purdy E, Perry E, Gorlin J, Jensen K. Transfusion-transmitted malaria: unpreventable by current donor exclusion guidelines? Transfusion. 2004 Mar;44(3):464. doi: 10.1111/j.1537-2995.2003.00352.x. PMID: 14996210.
- Grande R, Petrini G, Silvani I, Simoneschi B, Marconi M, Torresani E. Immunological testing for malaria and blood donor deferral: the experience of the Ca’ Granda Polyclinic Hospital in Milan. Blood Transfus. 2011 Apr;9(2):162-6. doi: 10.2450/2011.0158-09. Epub 2011 Jan 17. PMID: 21251462; PMCID: PMC3096859.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.