> Medicine Group. 2021 June 04;2(6):450-459. doi: 10.37871/jbres1259.
Impact of a Clinical Pathway for Hospital Management of Community-Acquired Pneumonia: A Retrospective Cohort Study
Jose Lorca Barchín1, Philip Erick Wikman-Jorgensen2,3*, Laíz Bello4 and Reyes Pascual1,3,4
2Internal Medicine Service, University Hospital San Juan de Alicante, Spain
3Foundation for the Promotion of Biomedical and Healthcare Research (FISABIO), Spain
4University Miguel Hernández de Elche, Spain
- Community-acquired pneumonia
- Clinical variability
- Clinical pathway
- Impact
- Spain
Abstract
Introduction: Community-acquired pneumonia is a prevalent disease that is managed in heterogeneous ways. Clinical pathways have been proposed as one way to mitigate this variability, but few implementation experiences have been published. The primary objective of this study is to analyse the effects of implementing a standardised clinical pathway for community-acquired pneumonia on length of hospital stay.
Methods: Retrospective cohort study comparing two equivalent time periods with and without a clinical pathway. We described patient characteristics in both periods and compared mean length of hospital stay, mortality, rate of complications, and readmissions within 30 days.
Results: A total of 170 patients were included across both periods. Mean length of hospital stay in patients treated before implementation of the clinical pathway was 6.05 days versus 5.43 days afterward (p = 0.28). The segmented regression analysis showed a change in slope for the length of hospital stay (0.04) following implementation of the clinical pathway. The proportion of patients hospitalised for more than 6 days was 37.5% in the first period, compared to 29.6% in the second (p = 0.088). Multivariable analysis showed that nonadherence to the clinical pathway was associated with a hospital stay of longer than 6 days (p = 0.048). Mortality dropped from 10.5% to 4.7% after the clinical pathway was established (p = 0.12). The proportion of patients readmitted within 30 days due to CAP was 8.8% before the establishment of the clinical pathway and 0% afterwards (p = 0.006).
Conclusion: A clinical pathway for managing community-acquired pneumonia was associated with a reduction in length of hospital stay and readmittance. There was a trend towards mortality reduction.
Introduction
Community-Acquired Pneumonia (CAP) is a serious public health problem worldwide due to its high prevalence and mortality. Different studies estimate that there are around 2 to 10 cases per 1000 adults per year, with the highest incidence in people aged 85 years or older [1-4]. Prospective studies have reported that CAP mortality ranges from 2% to 6% in outpatients and from 15% to 22% in hospitalised patients; again, the highest figures are seen in patients of advanced age [5-9].
The important prevalence and mortality of this disease translates to a significant economic burden for the healthcare system, with estimated direct and indirect treatment-derived costs ascending to EUR 114.8 million in 2001 [7], of which EUR 66.8 million corresponded to the population aged 65 or older. The mean direct cost for CAP treatment in hospitalised patients is EUR 1553 per admission; this cost can range from EUR 1210 to EUR 3457 depending on the patient’s characteristics [7,10-13]. Some authors argue that the length of hospital stay and the duration of intravenous antibiotic treatment are the main factors contributing to higher costs [11], making these variables key targets for interventions aiming to improve clinicians’ approach to the disease.
The great variability in the clinical management of CAP highlights the need to standardised practices [14-17]. While some of this diversity is justified by the individual characteristics of each case, the participation of a myriad of professionals causes a great deal of unjustified variability, because the criteria for admission, initial empirical antibiotic therapy, basic examinations conducted, and mean length of stay are all subject to the criteria of professionals from different medical services.
Clinical pathways have been proposed as a possible solution to mitigate this problem. These are defined as coordination tools that detail which activities and interventions to perform each day; which professional should be responsible; and how long the interventions should last. This guidance simplifies decision-making, optimises resource use, standardises and tailors the hospital stay to patient needs, and improves the quality of care. In short, clinical pathways are the operational version of clinical practice guidelines [4]. Countries that have already implemented clinical pathways for managing CAP have achieved significant improvements in survival, mean length of hospital stay, and derived healthcare costs [18,19]. However, in Spain few experiences on implementing clinical pathways for CAP have been published [16].
The primary objective of this study is to analyse the effect that implementing a clinical pathway for CAP in hospitalised patients has on mean length of stay. Secondary objectives are to assess the impact of the intervention on readmittance for CAP or other causes and on the incidence of complications (death, admission to the intensive care unit [ICU], empyema), and to estimate the degree of adherence.
Methods
This retrospective cohort study took place in the Elda General University Hospital, a tertiary centre in the interior of Alicante province (Spain). The catchment area of the hospital covers approximately 188,680 inhabitants, and the centre has 394 beds for inpatients. Our study included inpatients in the Internal Medicine wards, including the Infectious Diseases unit and the Pulmonology section, sampling was consecutive.
We compared outcomes in two defined time periods: the first from 5 February 2017 to 30 June 2017, before implementation of the clinical pathway, and the second from 5 February 2018 to 30 June 2018, after implementation was complete. Before pathway implementation there were local and national guidelines published and pneumonia management was carried out according to the treating physician´s criteria. The pathway was promoted by the Internal Medicine Department, it was designed by a group if internists, emergency room physicians and infectious disease specialists. Pneumologists were also consulted. The pathway is presented in the supplemental material. Several meetings with the different departments involved in the management of CAP (emergency, were treatment is usually started, internal medicine, infectious diseases and pneumology) to explain and promote the pathway were undertaken. The pathway was filled by paper, however electronic tools for prescription and severity assessment were introduced. Complete pathway was also published in the hospital intranet.
Patient inclusion criteria were: age of 14 years or older, with clinical signs of lower respiratory tract infection and radiological infiltrate on the chest X-ray. The exclusion criteria were: hospital admission in the previous 30 days; residence in a geriatric centre; receiving active treatment through hospital outpatient services or the home hospitalisation unit; presenting immunosuppression because of chemotherapy, biologic drugs, corticosteroids (prednisone 20 mg/day for two weeks or equivalent), methotrexate, azathioprine, mycophenolate, haemodialysis, or HIV infection with a CD4 T lymphocyte count of less than 350; suspicion of bronchoaspiration; and the presence of risk factors for Pseudomonas infection.
According to the literature reviewed, [16,20] the expected difference in mean hospital stay between patients in whom the clinical pathway was and was not applied was 1 day (Standard Deviation [SD] [2]; Confidence Interval [CI] of 95%, and statistical power of 90%), in favour of those treated under the clinical pathway. Based on these parameters, a sample size of 85 patients per group was established, for a total sample of 170.
We analysed different variables that we considered to be primary, such as mean length of hospital stay, as well as others related to epidemiological, clinical, analytical, microbiological, and radiological characteristics.
Data were collected from electronic and paper-based medical records using the clinical pathway verification form completed by the physicians. Variables were analysed using SPSS statistical software, 20.0 (IBM Corporation), and R software [21].
By means of a descriptive analysis, percentages, means and SDs were calculated for normally distributed quantitative variables, while medians and Interquartile Ranges (IQRs) were calculated for non-parametric variables. Categorical variables were compared using the chi-squared test, and quantitative variables using the student’s T test for normally distributed variables and the Mann-Whitney U for non-parametric variables. To estimate the magnitude of the associations between explanatory variables and hospital stay longer than 6 days, we fit a binary logistic regression model, estimating the Odds Ratio (OR) and 95% CI. P values of less than 0.05 were considered statistically significant. To analyse trends in length of hospital stay, a segmented regression analysis was performed using the Davies test.
The ethics committee at the Elda General University Hospital approved the study. Given its retrospective nature, it was not necessary to obtain informed consent. The study complies with the Declaration of Helsinki and with the Data Protection Act, and its performance followed the Standards for Good Clinical Practice and the tenets of Act 14/2007 on Biomedical Research [22,23].
Results
Study population
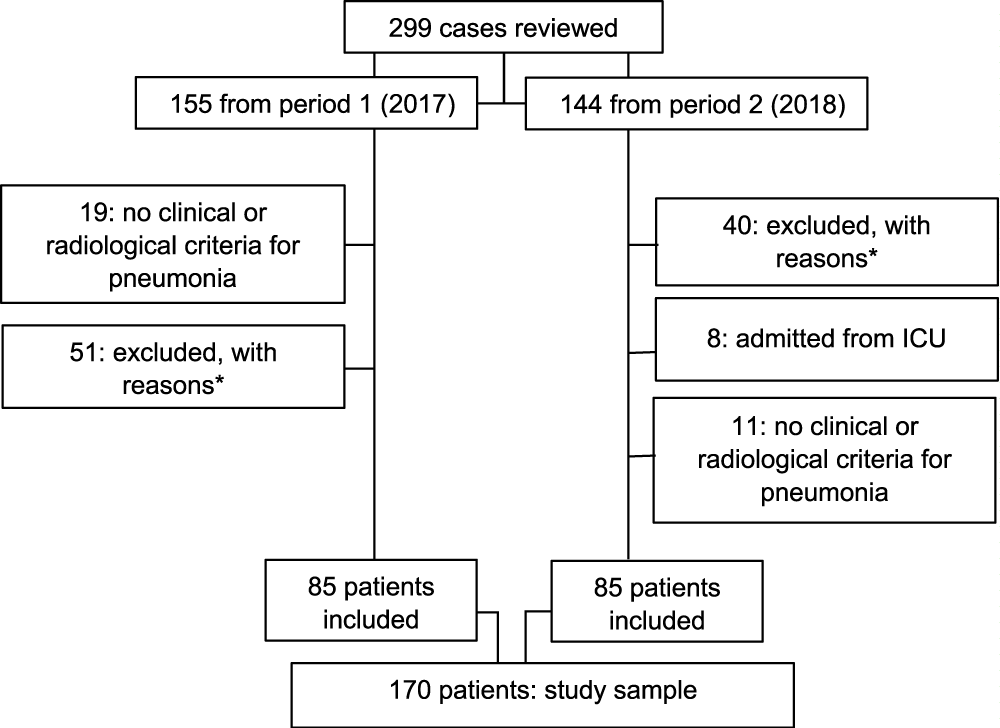
Figure 1 shows the patient selection flow chart. A total of 170 patients were included (85 in each study period); Table 1 shows their baseline clinical characteristics. There was a slight predominance of men in both periods, but no differences were observed between periods for clinical characteristics or for toxic habits. In terms of comorbidities, the group in the second period showed a higher proportion of diabetes mellitus (30.6% versus 22.4%) and nephropathy (8.2% versus 4.7%), though these differences did not reach statistical significance.
| Table 1: Baseline patient characteristics (categorical variables). | ||||
| Variables | Period 1 (2017) N = 85 n (%) |
Period 2 (2018) N = 85 n (%) |
p value (between periods) |
|
| Sex | Men | 44 (51.8) | 45 (52.9) | 0.89 |
| Women | 41 (48.2) | 40 (47.1) | ||
| Admission unit | Internal Medicine | 24 (28.2) | 24 (28.2) | 0.98 |
| Infectious Diseases | 38 (44.7) | 37 (43.5) | ||
| Pulmonology | 23 (27.1) | 24 (28.2) | ||
| Smoking | Yes | 19 (22.4) | 21 (24.7) | 0.89 |
| No | 51 (60) | 48 (56.5) | ||
| Ex-smoker | 15 (17.6) | 16 (18.8) | ||
| Alcoholism | Yes | 17 (20) | 12 (14.1) | 0.35 |
| No | 67 (78.8) | 73 (85.9) | ||
| In recovery | 1 (1.2) | 0 (0) | ||
| Other drugs | Yes | 1 (1.2) | 0 (0) | 0.36 |
| No | 83 (97.6) | 85 (100) | ||
| In recovery | 1 (1.2) | 0 (0) | ||
| Hypertension | Yes | 50 (58.8) | 49 (59.6) | 0.88 |
| No | 35 (41.2) | 36 (42.4) | ||
| Diabetes mellitus | Yes | 19 (22.4) | 26 (30.6) | 0.22 |
| No | 66 (77.6) | 59 (69.4) | ||
| Cardiopathy | Yes | 28 (32.9) | 31 (36.5) | 0.63 |
| No | 57 (67.1) | 54 (63.5) | ||
| Heart failure | Yes | 11 (12.9) | 13 (15.3) | 0.66 |
| No | 74 (87.1) | 72 (84.7) | ||
| COPD | Yes | 15 (17.6) | 18 (21.2) | 0.56 |
| No | 70 (82.4) | 67 (78.8) | ||
| Liver disease | Yes | 3 (3.5) | 2 (2.4) | 0.65 |
| No | 82 (96.5) | 83 (97.6) | ||
| Kidney disease | Yes | 4 (4.7) | 7 (8.2) | 0.35 |
| No | 81 (95.3) | 78 (91.8) | ||
| Stroke | Yes | 7 (8.2) | 10 (11.8) | 0.44 |
| No | 78 (91.8) | 75 (88.2) | ||
| COPD: Chronic Obstructive Pulmonary Disease | ||||
Characteristics of the pneumonia
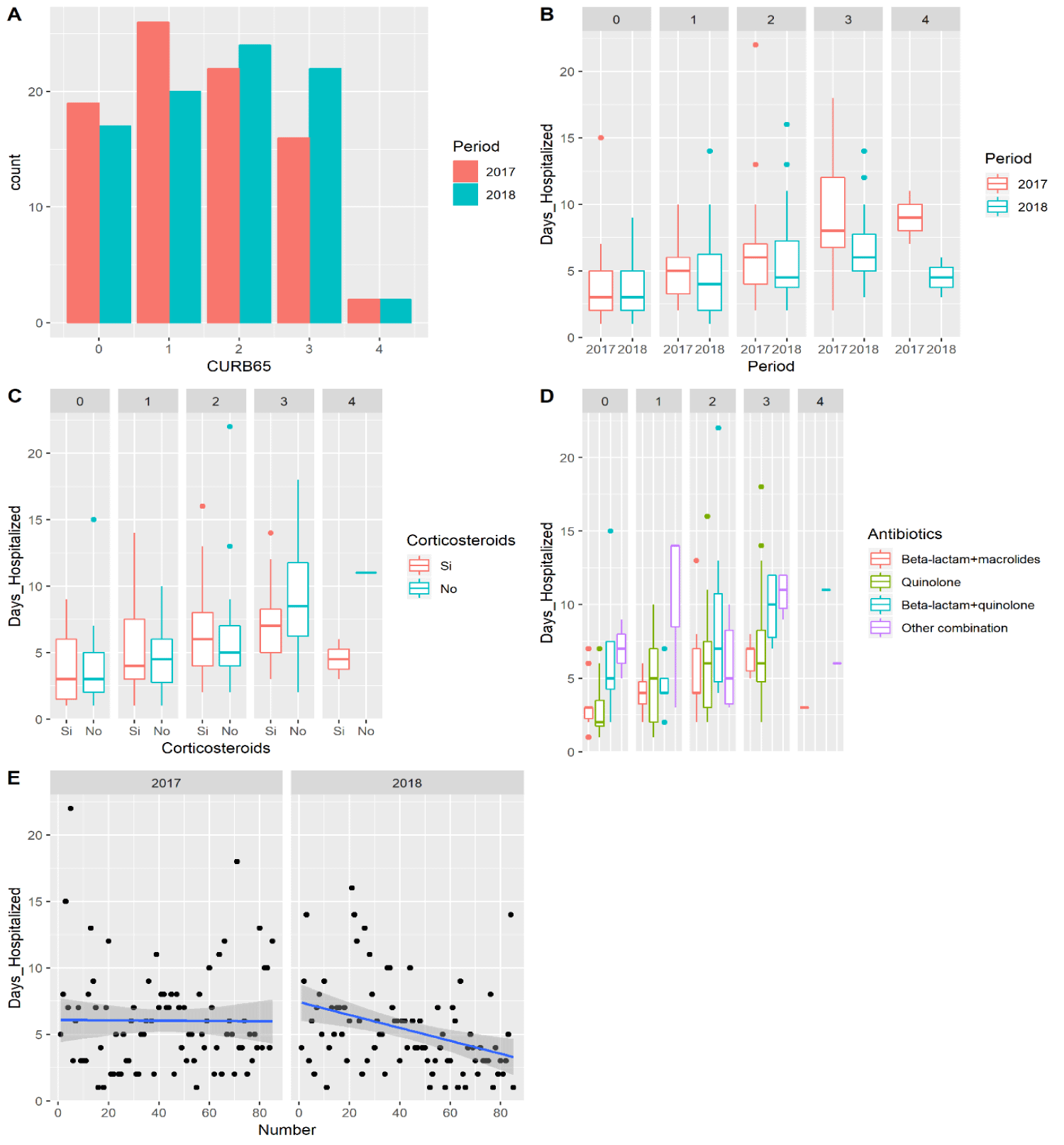
Radiologically, a significantly higher proportion of patients showed pleural effusion in the 2017 period (28.2% vs 12.9%, p = 0.014). However, the patients treated in 2018 were more likely to have multilobar or bilateral pneumonia (24.7% vs 8.2%, p = 0.013). The severity of the pneumonias leading to admission was greater in 2018. Although no significant differences were apparent in terms of vital signs on admittance (systolic Blood Pressure [BP], diastolic BP, temperature and pulse), the patients in the 2018 group did show higher levels of glycaemia (150.61 mg/dL vs 134.52 mg/dL, p = 0.003), C-reactive protein (163.50 mg/dL vs 132.91 mg/dL, p = 0.005), lactate (13.75 mmol/L vs 11.83 mmol/L, p = 0.042), and international normalised ratio (1.19 vs 1.10, p = 0.001). Moreover, they showed significantly lower partial pressure of oxygen (pO2, 59.01 mmHg versus 66.50 mmHg, p = 0.008) and sodium (137 mEq/L versus a 138 mEq/L, p = 0.03). Furthermore, in the second period there was a slightly higher percentage of patients with a CURB-65 pneumonia severity score of 2 or more (56.4% vs 47%, p = 0.14), but this result could have been due to chance (Figure 2A).
| Table 2: Complementary microbiological tests. | ||||
| Complementary tests | Period 1 (2017) N = 85 n (%) |
Period 2 (2018) N = 85 n (%) |
p value (between periods) |
|
| Legionella antigen test | Positive | 2 (2.4) | 4 (4.7) | 0.12 |
| Negative | 77 (90.6) | 80 (94.1) | ||
| Not performed | 6 (7.1) | 1 (1.2) | ||
| Pneumococcus antigen test | Positive | 7 (8.3) | 13 (15.1) | 0.020 |
| Negative | 71 (84.5) | 73 (84.9) | ||
| Not performed | 6 (7.1) | 0 (0) | ||
| Blood culture | Positive | 5 (5.9) | 1 (1.2) | 0.12 |
| Negative | 34 (40) | 27 (31.8) | ||
| Contaminated | 4 (4.7) | 9 (10.6) | ||
| Not performed | 42 (49.4) | 48 (56.5) | ||
| Sputum culture | Positive | 9 (10.6) | 12 (14.1) | 0.020 |
| Negative | 11 (12.9) | 21 (24.7) | ||
| Normal flora | 12 (14.1) | 19 (22.4) | ||
| Not performed | 53 (62.4) | 33 (38.8) | ||
| Respiratory virus PCR | Not performed | 58 (68.2) | 47 (55.3) | 0.085 |
| Negative | 26 (30.6) | 29 (34.1) | ||
| Influenza type A | 1 (1.2) | 3 (3.5) | ||
| Influenza type B | 0 (0) | 4 (4.7) | ||
| Respiratory syncytial virus | 0 (0) | 2 (2.4) | ||
| HIV | Not performed | 79 (92.9) | 73 (85.9) | 0.13 |
| Positive | 0 (0) | 0 (0) | ||
| Negative | 5 (5.9) | 12 (14.1) | ||
| Previously known infection | 1 (1.2) | 0 (0) | ||
| HBV | Not performed | 81 (95.3) | 75 (88.2) | 0.38 |
| Non-immunised | 3 (3.5) | 6 (7.1) | ||
| Vaccine-immunised | 0 (0) | 2 (2.4) | ||
| Previous infection | 1 (1.2) | 1 (1.2) | ||
| Active infection | 0 (0) | 1 (1.2) | ||
| HCV | Not performed | 80 (94.1) | 75 (88.2) | 0.052 |
| Positive | 0 (0) | 0 (0) | ||
| Negative | 3 (3.5) | 10 (11.8) | ||
| Previously known infection | 2 (2.4) | 0 (0) | ||
| PCR: Polymerase Chain Reaction; HBV: Hepatitis B Virus; HCV: Hepatitis C Virus | ||||
Implementation
Regarding the analysis of clinical pathway implementation, table 2 shows that in the second period, the centre carried out more microbiological analyses, like antigen tests for Legionella or pneumococcus; sputum cultures; respiratory virus PCR; and serology tests for HIV, HBV, and HCV. The antigen tests for pneumococcus were performed in all patients in the 2018 period, and 15.1% yielded positive results, compared to 8.3% in the previous period (p = 0.020). The performance of sputum cultures also increased; 14.1% of the cases in 2018 tested positive (p = 0.020). The two periods also showed a change in the PCR respiratory viral panels ordered, with a non-significant increase in the second period leading to slightly more diagnoses of infections caused by influenza viruses A and B and respiratory syncytial virus. Orders for Legionella antigen tests saw a significant increase in 2018 (98.8% vs 92.8%, p = 0.117), although their positivity did not (Tables 3 & 4).
| Table 3: Radiological characteristics, treatment, and complications. | ||||
| Variable | Period 1 (2017) N = 85 n (%) |
Period 2 (2018) N = 85 n (%) |
p value (between periods) |
|
| Pleural effusion | Yes | 24 (28.2) | 11 (12.9) | 0.014 |
| No | 61 (71.8) | 74 (87.1) | ||
| Radiological involvement | Unilobar | 70 (82.4) | 59 (69.4.) | 0.013 |
| Bilobar | 8 (9.4) | 5 (5.9) | ||
| Multilobar or bilateral | 7 (8.2) | 21 (24.7) | ||
| Antibiotic treatment | Beta-lactam + macrolide | 7 (8.2) | 30 (35.3) | 0.001 |
| Quinolone | 52 (61.2) | 39 (45.9) | ||
| Beta-lactam + quinolone | 18 (21.2) | 6 (7.1) | ||
| Other combination | 8 (9.4) | 10 (11.8) | ||
| Corticotherapy | Yes | 21 (24.7) | 71 (83.5 ) | 0.001 |
| No | 64 (75.3) | 14 (16.5) | ||
| Readmittance for CAP | Yes | 7 (8.8) | 0 (0) | 0.006 |
| No | 73 (91.2) | 81 (100) | ||
| Readmittance for other reason | Yes | 3 (3.8) | 4 (4.9) | 0.71 |
| No | 77 (96.2) | 77 (95.1) | ||
| In-hospital mortality | Yes, due to CAP | 5 (5.9) | 4 (4.7) | 0.73 |
| No | 80 (94.1) | 81 (95.3) | ||
| Outpatient mortality | Yes, due to CAP | 1 (1.2) | 0 (0) | 0.13 |
| Yes, due to other cause | 3 (3.8) | 0 (0) | ||
| No | 76 (95) | 81 (100) | ||
| Empyema | Yes | 0 (0) | 1 (1.2) | 0.32 |
| No | 85 (100) | 75 (98.8) | ||
| ICU admission | Yes | 1 (1.2) | 2 (2.4) | 0.56 |
| No | 84 (98.8) | 83 (97.6) | ||
| CAP: Community-Acquired Pneumonia; ICU: Intensive Care Unit | ||||
| Table 4: Differences between quantitative variables between the two study periods. | |||
| Variables | Period 1 (2017) N = 85 |
Period 2 (2018) N = 85 | p value |
| Systolic blood pressure, mmHg, Mean ± SD | 123.99 ± 24.64 | 129.56 ± 26.30 | 0.80 |
| Diastolic blood pressure, mmHg, Mean ± SD | 70.08 ± 17.43 | 73.00 ± 14.38 | 0.54 |
| Temperature °C, Mean ± SD | 37.49 ± 1.09 | 37.21 ± 1.05 | 0.27 |
| Pulse, beats per minute, Mean ± SD | 92.91 ± 20.02 | 96.58 ±17.51 | 0.41 |
| Glycaemia, mg/dL, Mean ± SD | 134.52 ± 48.24 | 150.61 ± 60.64 | 0.003 |
| Urea, mg/dL, Mean ± SD | 47.56 ± 27.07 | 47.27 ± 23.81 | 0.28 |
| Creatine, mg/dL, Mean ± SD | 1.16 ± 0.42 | 2.24 ± 9.97 | 0.27 |
| Sodium mEq/L, Median (IQR) | 138 (137-141) | 137 (135-140) | 0.03 |
| Glutamic oxaloacetic transaminase IU/L, Mean ± SD | 30.37 ± 35.54 | 29.35 ± 19.41 | 0.82 |
| Glutamine-pyruvate transaminase, IU/L, Mean ± SD | 28.95 ± 22.87 | 29.59 ± 20.26 | 0.99 |
| C-reactive protein, mg/dL, Mean ± SD | 132.91 ± 93.00 | 163.50 ± 111.83 | 0.005 |
| Procalcitonin, ng/mL, Mean ± SD | 2.00 ± 5.70 | 2.70 ± 6.18 | 0.32 |
| pH, Mean ± SD | 7.44 ± 0.03 | 7.39 ± 0.36 | 0.17 |
| pCO2, mmHg, Mean ± SD | 35.32 ± 6.23 | 35.82 ± 8.20 | 0.57 |
| pO2, mmHg, Median (IQR) | 66.50 (56.80-73.90) | 59.01 (50.95-68.10) | 0.008 |
| HCO3-, Mean ± SD | 24.16 ± 3.10 | 24.04 ± 4.38 | 0.61 |
| Lactate, mmol/L, Mean ± SD | 11.83 ± 6.59 | 13.75 ± 7.71 | 0.042 |
| Haematocrit (%), Mean ± SD | 38.68 ± 5.27 | 37.89 ± 5.78 | 0.57 |
| Platelets/mm3, Mean ± SD | 242.858 ± 112.607 | 225.258 ± 91.104 | 0.16 |
| International normalised ratio, Median (IQR) | 1.10 (1.01-1.21) | 1.19 (1.09-1.31) | 0.001 |
| pCO2: partial pressure of Carbon Dioxide, pO2: partial pressure of Oxygen. Values are expressed as mean ± standard deviation or as median (interquartile range). | |||
In terms of treatment, there was a significant increase in the use of corticosteroids between periods, in line with recommendations in the clinical pathway (83.5% vs 24.7%, p = 0.001), as well as a reduction in the prescription of respiratory quinolones (levofloxacin) (61.2% vs 45.9%, p = 0.001), in favour of a beta-lactam (amoxicillin-clavulanate or ceftriaxone) plus macrolide (azithromycin) (8.2% vs 35.3%, p = 0.001).
We defined adherence to the clinical pathway recommendations according to whether the patient received antibiotics as indicated by the pathway and corticosteroids. Adherence stood at 67.1%.
Mean length of hospital stay and complications
Mean length of hospital stay in patients treated before implementation of the clinical pathway was 6.05 days versus 5.43 days afterward (p = 0.28). In an exploratory analysis, we observed that after stratifying patients according to the severity of the pneumonia, there was a benefit in patients with a higher CURB-65 score, which was especially pronounced in patients with a score of 3 or 4 (Figure 2B). The routine use of corticosteroids seemed to benefit only the patients with a CURB-65 of 3 or more (Figure 2C), and the beta-lactam plus macrolide combination seemed to be the antibiotic treatment associated with the shortest hospital stay (Figure 2D). Multivariable analysis (Table 5) showed that a hospital stay of longer than 6 days was significantly associated with non-adherence to the clinical pathway (OR 2.73, 95% CI 1.03-7.78, p = 0.048), a CURB-65 score of 2 or more (OR 3.97, 95% CI 1.58-10.72, p = 0.004), and lactate levels of more than 11 mmol/L (OR 2.95; 95% CI 1.17-7.90, p = 0.024). On the other hand, a hospital stay of less than 6 days was associated with the absence of chronic obstructive pulmonary disease (OR 0.21, 95% CI 0.06-0.64, p = 0.008) or pleural effusion (OR 0.25, 95% CI 0.08-0.71, p = 0.011) and a percentage of haematocrit of more than 36% (OR 0.32, 95% CI 0.12-0.80, p = 0.016).
| Table 5: Multivariable binary logistic regression: factors associated with hospital stay longer than 6 days. | |||
| Variable | Odds ratio | 95% CI | p-value |
| CURB-65 ≥ 2 | 3.97 | 1.58-10.72 | 0.004 |
| Non-adherence to clinical pathway | 2.73 | 1.03-7.78 | 0.048 |
| Absence of COPD | 0.21 | 0.06-0.64 | 0.008 |
| Lactate > 11 mmol/L | 2.95 | 1.17-7.90 | 0.024 |
| Haematocrit > 36% | 0.32 | 0.12-0.80 | 0.016 |
| Absence of pleural effusion | 0.25 | 0.08-0.71 | 0.011 |
| CI: Confidence Interval; COPD: Chronic Obstructive Pulmonary Disease | |||
The segmented regression analysis showed a change in slope for the length of hospital stay (0.04) following implementation of the clinical pathway (Figure 2E). Moreover, readmittances due to CAP also declined (8.8% vs 0%, p = 0.006), with no increase in admissions for other causes (3.8% vs 4.9%, p = 0.5). There was also a decrease in the rate of pleural effusion after implementation of the clinical pathway (28.2% to 12.9%, p = 0.014). In addition, mortality was cut in half, from 10.5% to 4.7%, although this difference did not reach statistical significance (p = 0.12).
Discussion
The implementation of a clinical pathway for community-acquired pneumonia was associated with reduced length of hospital stay, readmissions rate, and incidence of pleural effusion, as well as a non-significant decrease in mortality. It is unknown what role the corticosteroids and the increased use of azithromycin – with its anti-inflammatory effect – could have played in these results [24,25]. Treatment with corticosteroids has recently been analysed and its routine use was discouraged due to the absence of clear evidence for improved outcomes. Investigators highlighted the need to identify specific patient profiles who could benefit from its use [26]. In our study, beneficial effects were seen only in severe pneumonias, with CURB-65 scores of 3 or more. Recently the use of corticosteroids in severe pneumonias has been shown to be a cost-effective strategy [27].
The difficulties in observing significant differences in mean hospital stay could be at least partly attributable to the greater disease severity in the patients from the 2018 period. Severity is defined by radiological findings of a higher percentage of patients with multilobar o bilateral involvement and by analytical parameters like higher serum levels of C-reactive protein and lactate and lower hypoxia. Moreover, and despite not reaching statistical significance, there were more patients with a CURB-65 score of 2 or more in the 2018 group.
Some results clearly favoured implementation of the clinical pathway. There was a statistically significant reduction in readmissions due to CAP between the 2017 and 2018 study periods, despite the more serious disease profile in the latter group. Previous studies have not observed this benefit [19,28]. However, the clinical pathways were different, and it is possible that some specific elements of our pathway could have played a role in reducing readmissions.
Another of our specific objectives was to assess the differences related to the development of complications (death, admission to the ICU, empyema) in patients from both periods. Although we did not observe differences in the incidence of empyema or in-hospital mortality due to CAP, there was a significant decrease in the development of pleural effusion. Moreover, although the CAPs in the second period were more severe, there was no increase in admissions to the ICU.
Recently, Lloyd, et al. [29] found that a package of interventions, which were supported by evidence of their efficacy when implemented alone, failed to produce benefits when applied together. This study differed from ours in several ways (population, intervention, etc.), but the greater coherence and temporal structure of our clinical pathway was probably responsible for the better outcomes achieved relative to their intervention package.
One of the main limitations of the study is its retrospective nature, which conditioned the completeness of data for some variables of interest. Although it was difficult to establish the exact degree of adherence among clinicians, indirect data suggest that the clinical pathway was at least partially followed. First of all, the fact that the pneumonias in the second period were more severe could indicate that emergency services made more use of the pneumonia severity indexes like CURB-65 and Fine/PSI, which were recommended in the clinical pathway. Moreover, an increase in the use of microbiological diagnostic tests, such as antigen tests for Legionella or pneumococcus, sputum cultures, respiratory virus PCR (during flu season), and HIV, HBV, and HCV serology (according to the patient profile), together suggest greater adherence to the diagnostic guidance in the clinical pathway. There were similar indications at the treatment level, with increased use of parenteral corticosteroids and a more evident tendency to prescribe beta-lactams plus macrolides rather than quinolones. However, despite implementing the necessary electronic tools (calculators for the CURB-65 and Fine scales, treatment protocols within the e-prescription programme, and publication on the hospital intranet) and holding several multidisciplinary sessions to disseminate the clinical pathway, adherence was estimated at just 67.1% of the cases. This modest uptake could stem from different factors, which should be analysed in another study, but we believe that therapeutic inertia and lack of work culture in the use of these tools could play an important role.
Although a multicentre study would be desirable, we believe that our results are generalisable to other hospitals of similar characteristics. In short, our results suggest that implementation of a clinical pathway is beneficial to both patients and health professionals, standardising diagnostic and therapeutic management of the CAP patients. Nevertheless, more randomised and prospective studies, designed specifically to assess the impact of clinical pathways and their individual components, are necessary.
Acknowledgements
To the Internal Medicine Society of the Valencian Community that partially funded the study.
What is Known
- There is controversy whether clinical pathways improve outcomes of hospitalized community acquired pneumonia.
- There is controversy regarding the use of corticosteroids in pneumonia cases.
What this Article Ads
- Suggests that clinical pathways improve outcomes of community acquired pneumonia.
- Suggests subgroups of patients that could benefit from corticosteroids.
References
- Montull B, Menéndez R, Torres A, Méndez R. Predictors of severe sepsis among patients hospitalized for community-acquired pneumonia. MLO Med Lab Obs. 2016 Mar;48(3):8, 10-2; quiz 14. PMID: 27116802.
- Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012 Jan;67(1):71-9. doi: 10.1136/thx.2009.129502. Epub 2010 Aug 20. PMID: 20729232.
- Rello J. Demographics, guidelines, and clinical experience in severe community-acquired pneumonia. Crit Care. 2008;12 Suppl 6(Suppl 6):S2. doi: 10.1186/cc7025. PMID: 19105795; PMCID: PMC2607112.
- Welte T, Köhnlein T. Global and local epidemiology of community-acquired pneumonia: the experience of the CAPNETZ Network. Semin Respir Crit Care Med. 2009 Apr;30(2):127-35. doi: 10.1055/s-0029-1202941. Epub 2009 Mar 18. PMID: 19296412.
- Vila-Corcoles A, Ochoa-Gondar O, Rodriguez-Blanco T, Raga-Luria X, Gomez-Bertomeu F; EPIVAC Study Group. Epidemiology of community-acquired pneumonia in older adults: a population-based study. Respir Med. 2009 Feb;103(2):309-16. doi: 10.1016/j.rmed.2008.08.006. Epub 2008 Sep 18. PMID: 18804355.
- Ochoa-Gondar O, Vila-Córcoles A, de Diego C, Arija V, Maxenchs M, Grive M, Martin E, Pinyol JL; EVAN-65 Study Group. The burden of community-acquired pneumonia in the elderly: the Spanish EVAN-65 study. BMC Public Health. 2008 Jun 27;8:222. doi: 10.1186/1471-2458-8-222. PMID: 18582392; PMCID: PMC2522374.
- Monge V, San-Martín VM, González A. The burden of community-acquired pneumonia in Spain. Eur J Public Health. 2001 Dec;11(4):362-4. doi: 10.1093/eurpub/11.4.362. PMID: 11766474.
- Ewig S, Ruiz M, Torres A, Marco F, Martinez JA, Sanchez M, Mensa J. Pneumonia acquired in the community through drug-resistant Streptococcus pneumoniae. Am J Respir Crit Care Med. 1999 Jun;159(6):1835-42. doi: 10.1164/ajrccm.159.6.9808049. PMID: 10351928.
- Rello J, Quintana E, Ausina V, Net A, Prats G. A three-year study of severe community-acquired pneumonia with emphasis on outcome. Chest. 1993 Jan;103(1):232-5. doi: 10.1378/chest.103.1.232. PMID: 8417885.
- Bartolomé M, Almirall J, Morera J, Pera G, Ortún V, Bassa J, Bolíbar I, Balanzó X, Verdaguer A; Maresme Community-Acquired Pneumonia Study Group (GEMPAC). A population-based study of the costs of care for community-acquired pneumonia. Eur Respir J. 2004 Apr;23(4):610-6. doi: 10.1183/09031936.04.00076704. PMID: 15083763.
- Fernández Alvarez R, Gullón Blanco JA, Rubinos Cuadrado G, Jiménez Sosa A, Hernández García C, Medina Gonzálvez A, González Martín I. Neumonía adquirida en la comunidad: influencia de la duración de la antibioterapia intravenosa en la estancia hospitalaria y relación coste/efectividad [Community-acquired pneumonia: influence of the duration of intravenous antibiotic therapy on hospital stay and the cost-benefit ratio]. Arch Bronconeumol. 2001 Oct;37(9):366-70. Spanish. doi: 10.1016/s0300-2896(01)78816-3. PMID: 11674935.
- González-Moraleja J, Sesma P, González C, López ME, García JF, Alvarez-Sala JL. Cuál es el coste de las neumonías que ingresamos inadecuadamente? [What is the cost of inappropriate admission of pneumonia patients?]. Arch Bronconeumol. 1999 Jul-Aug;35(7):312-6. Spanish. doi: 10.1016/s0300-2896(15)30067-3. PMID: 10439127.
- Antunes C, Pereira M, Rodrigues L, Organista D, Cysneiros A, Paula F, Nunes B, Barbosa P, Bárbara C, Escoval A, Diniz A, Froes F. Hospitalization direct cost of adults with community-acquired pneumonia in Portugal from 2000 to 2009. Pulmonology. 2020 Sep-Oct;26(5):264-267. doi: 10.1016/j.pulmoe.2020.02.013. Epub 2020 May 29. PMID: 32482604.
- Julián-Jiménez A, González-Castillo J, Candel González FJ. ¿Cuándo, dónde y cómo ingresar al paciente con neumonía adquirida en la comunidad? Rev Clínica Española. 2013;213(2):99-107. doi:10.1016/j.rce.2012.02.006
- Julián-Jiménez A, Timón-Zapata J, Laserna-Mendieta EJ, Pineda-Tenor D. El uso rutinario del Pneumonia Severity Index junto a otros criterios mejora el manejo de la neumonía adquirida en la comunidad en el servicio de urgencias [The routine use of the Pneumonia Severity Index together with other criteria improves the management of community acquired pneumonia]. Enferm Infecc Microbiol Clin. 2013 Mar;31(3):195-6. Spanish. doi: 10.1016/j.eimc.2012.09.006. Epub 2012 Oct 16. PMID: 23084605.
- Muñoz LÁS, Cabeza MÁT, Herrero JC, et al. Desarrollo y proceso de mejora de la vía clínica ‘neumonía adquirida en la comunidad con ingreso hospitalario’ en un hospital de ámbito comarcal. Rev Calid Asist. 2006;21(6):299-310. doi: 10.1016/S1134-282X(06)70799-0
- Capelastegui A, España PP, Quintana JM, Gorordo I, Gallardo MS, Idoiaga I, Bilbao A. Management of community-acquired pneumonia and secular trends at different hospitals. Respir Med. 2005 Mar;99(3):268-78. doi: 10.1016/j.rmed.2004.08.010. PMID: 15733501.
- Frei CR, Bell AM, Traugott KA, Jaso TC, Daniels KR, Mortensen EM, Restrepo MI, Oramasionwu CU, Ruiz AD, Mylchreest WR, Sikirica V, Raut MR, Fisher A, Schein JR. A clinical pathway for community-acquired pneumonia: an observational cohort study. BMC Infect Dis. 2011 Jul 6;11:188. doi: 10.1186/1471-2334-11-188. PMID: 21733161; PMCID: PMC3142517.
- Meehan TP, Weingarten SR, Holmboe ES, Mathur D, Wang Y, Petrillo MK, Tu GS, Fine JM. A statewide initiative to improve the care of hospitalized pneumonia patients: The Connecticut Pneumonia Pathway Project. Am J Med. 2001 Aug 15;111(3):203-10. doi: 10.1016/s0002-9343(01)00803-8. PMID: 11530031.
- Marrie TJ, Lau CY, Wheeler SL, Wong CJ, Vandervoort MK, Feagan BG. A controlled trial of a critical pathway for treatment of community-acquired pneumonia. CAPITAL Study Investigators. Community-Acquired Pneumonia Intervention Trial Assessing Levofloxacin. JAMA. 2000 Feb 9;283(6):749-55. doi: 10.1001/jama.283.6.749. PMID: 10683053.
- R Core Team. R: A language and environment for statistical computing. 2019.
- Palomo López P, Redondo Mena C. Legislación vigente y Ética en Investigación Clínica. Rev Int Ciencias Podol. 2012;6(2):81-93. doi: 10.5209/rev_ricp.2012.v6.n2.39317
- Ley 14/2007, de 3 de julio, de Investigación Biomédica (BOE, 4 de julio de 2007) [Law 14/2007, 3 July, on biomedical research (BOE, 4 July 2007)]. Rev Derecho Genoma Hum. 2007 Jan-Jun;(26):283-325. Spanish. PMID: 18201045.
- Chen LP, Chen JH, Chen Y, Wu C, Yang XH. Efficacy and safety of glucocorticoids in the treatment of community-acquired pneumonia: A meta-analysis of randomized controlled trials. World J Emerg Med. 2015;6(3):172-8. doi: 10.5847/wjem.j.1920-8642.2015.03.002. PMID: 26401176; PMCID: PMC4566005.
- Briel M, Spoorenberg SMC, Snijders D, Torres A, Fernandez-Serrano S, Meduri GU, Gabarrús A, Blum CA, Confalonieri M, Kasenda B, Siemieniuk RAC, Boersma W, Bos WJW, Christ-Crain M; Ovidius Study Group; Capisce Study Group; STEP Study Group. Corticosteroids in Patients Hospitalized With Community-Acquired Pneumonia: Systematic Review and Individual Patient Data Metaanalysis. Clin Infect Dis. 2018 Jan 18;66(3):346-354. doi: 10.1093/cid/cix801. PMID: 29020323.
- Metlay JP, Waterer GW, Long AC, et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. doi:10.1164/rccm.201908-1581ST
- Pliakos EE, Andreatos N, Tansarli GS, Ziakas PD, Mylonakis E. The Cost-Effectiveness of Corticosteroids for the Treatment of Community-Acquired Pneumonia. Chest. 2019;155(4):787-794. doi:10.1016/j.chest.2018.11.001
- Carratalà J, Garcia-Vidal C, Ortega L, et al. Effect of a 3-step critical pathway to reduce duration of intravenous antibiotic therapy and length of stay in community-acquired pneumonia: A randomized controlled trial. Arch Intern Med. 2012;172(12):922-928. doi:10.1001/archinternmed.2012.1690
- Lloyd M, Karahalios A, Janus E, Skinner EH, Haines T, De Silva A, Lowe S, Shackell M, Ko S, Desmond L, Karunajeewa H; Improving Evidence-Based Treatment Gaps and Outcomes in Community-Acquired Pneumonia (IMPROVE-GAP) Implementation Team at Western Health. Effectiveness of a Bundled Intervention Including Adjunctive Corticosteroids on Outcomes of Hospitalized Patients With Community-Acquired Pneumonia: A Stepped-Wedge Randomized Clinical Trial. JAMA Intern Med. 2019 Aug 1;179(8):1052-1060. doi: 10.1001/jamainternmed.2019.1438. PMID: 31282921; PMCID: PMC6618815.
- Prina E, Ranzani OT, Torres A. Community-acquired pneumonia. Lancet. 2015 Sep 12;386(9998):1097-108. doi: 10.1016/S0140-6736(15)60733-4. Epub 2015 Aug 12. PMID: 26277247; PMCID: PMC7173092.
- National Institute for Health and Care Excellence. Pneumonia in adults : diagnosis and management. December, 2014. https://bit.ly/3fHafHz
- Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, Lewis SA, Macfarlane JT. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003 May;58(5):377-82. doi: 10.1136/thorax.58.5.377. PMID: 12728155; PMCID: PMC1746657.
- Lee JS, Giesler DL, Gellad WF, Fine MJ. Antibiotic Therapy for Adults Hospitalized With Community-Acquired Pneumonia: A Systematic Review. JAMA. 2016 Feb 9;315(6):593-602. doi: 10.1001/jama.2016.0115. PMID: 26864413. Lee JS, Giesler DL, Gellad WF, Fine MJ. Antibiotic Therapy for Adults Hospitalized With Community-Acquired Pneumonia: A Systematic Review. JAMA. 2016 Feb 9;315(6):593-602. doi: 10.1001/jama.2016.0115. PMID: 26864413.
- Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher DM, Niederman MS, Torres A, Whitney CG; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007 Mar 1;44 Suppl 2(Suppl 2):S27-72. doi: 10.1086/511159. PMID: 17278083; PMCID: PMC7107997.
- Horita N, Otsuka T, Haranaga S, Namkoong H, Miki M, Miyashita N, Higa F, Takahashi H, Yoshida M, Kohno S, Kaneko T. Adjunctive Systemic Corticosteroids for Hospitalized Community-Acquired Pneumonia: Systematic Review and Meta-Analysis 2015 Update. Sci Rep. 2015 Sep 16;5:14061. doi: 10.1038/srep14061. PMID: 26374694; PMCID: PMC4571641.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.