> Medicine Group. 2021 June 02;2(6):431-438. doi: 10.37871/jbres1255.
Withanolide D of Ashwagandha improves Apoptosis in the Bone Marrow of Leukemic Murine Model
Sayantan Ghosh and Sujata Law*
- Leukemia
- Apoptosis
- Withnolide D
- Bone marrow
- Ashwagandha
Abstract
Background: Leukemia is one of the most occurring haematological pathologies in the world which develops due to the impairment in the hematopoietic machinery. Cellular death via apoptosis is severely impaired in this pathophysiological condition and leads to the progression of the disease.
Objective: We have tried to unearth the efficacy of Withanolide D, a steroidal lactone derived from Withania somnifera or Ashwagandha on some of the apoptotic machinery components, i.e. TERT, BCL2 and PUMA in the experimental leukemic mice.
Materials and Methods: LD50 and EC50 values of Withanolide D were estimated. Three groups of animals were taken for experimental purpose i.e. Group I = Leukemic, (L); Group II = Control, (C); Group III = Leukemic treated with Withanolide D, (L + WD). Group III received Withanolide D via oral route and other two groups received equal volume of distilled water. Various cytological, immunofluorescence and flow cytometric studies were taken into consideration post administration.
Result: Leukemic group showed increased cellular proliferation and decreased cellular death as compared to control. Post Withanolide D administration TERT, BCL2 and PUMA expression started to shift towards normal status. This shift in the expressional values of increased apoptosis rate and decreased cellular proliferation revealed by cytological, immunofluorescence studies and flow cytometric investigations.
Conclusion: As Withanolide D decreased the suppression of apoptosis and impaired the progression of the disease, so, we can conclude that Withanolide D of Ashwagandha may hold a promise towards a new therapeutic strategy in leukemia.
Abbreviations
PUMA: p53 Upregulated Modulator of Apoptosis; BCL2: B-Cell Lymphoma 2; TERT: Telomerase Reverse Transcriptase; HPLC: High Performance Liquid Chromatography; ENU: N-Ethyl-N-Nitrosourea; WD: Withanolide D; LD50: Lethal Dose 50; EC50: Effective Concentration 50; MFI: Mean Fluorescence Intensity
Introduction
Deregulated haematopoiesis is the basis of Leukaemia where hematopoietic machinery gives rise to abnormal cell lineages, the hallmark of leukemic pathophysiology [1-4]. In the battle against this dreadful disease many strategies, therapeutic regimen, were formulated and implemented which bears various side effects and other complications so we turned towards Mother Nature in search of more effective, natural alternatives.
Withania somnifera or Ashwagandha is one of the most ancient and widely used herbs in traditional Indian Ayurvedic medicine mainly found in Indian subcontinent along with many parts of south Asia [5,6]. In Ayurvedic system this plant is used in various diseases and disorders. It is extensively studied for its biologically active constituents and contains several pharmacologically important steroidal lactones i.e. Withanolides, Glycowithanolides, Withaferins etc. Various studies on Withanolides portrayed their effect on inhibition of metastasis, angiogenesis, cell cycle arrest, cytotoxicity, apoptosis, cytoskeletal destabilization etc [7-9].
In leukemic pathophysiology suppression of apoptotic mechanism leads to progression of the disease. BCL2 or B-cell lymphoma 2 is ubiquitously expressed in all leukocytes. This protein has a pivotal role in cellular survival, cellular proliferation and activation. In leukemic condition BCL2 plays a very important role in the survival and progression of the disease by suppressing the drug induced apoptosis [10-16].
On the other hand PUMA (p53 Upregulated Modulator of Apoptosis), a member of the BH3-only protein family is widely known as potent mediator of apoptosis. A wide variety of stimuli, such as infection, toxins, cytokine withdrawal, deregulated oncogene expression, growth factor etc induces PUMA mediated apoptosis [17-20].
Another key molecule is TERT (Telomerase Reverse Transcriptase), whose expression increases in apoptosis resistant cells. Various cancer cells have been noted with high level of telomerase activity that helped in the maintenance of immortal state and decreased apoptosis rate [21-23].
In our present study we tried to unveil the efficacy of Withanolide D on the apoptotic machinery particularly focusing on PUMA, BCL2 and TERT in experimental leukemic mice which may open up a new avenue in the therapeutic modality of leukemia.
Materials and Methods
Disease induction
Three groups of animals were taken into consideration for experimental purposes.: Group-I: Litter pups of 10-14 days weighing 4-5 gms were given intra-peritoneal injection of ENU, a potent carcinogen, at a dose of 80 mg/kg of body weight for developing leukemia (N = 60) by 6-8 months time period as confirmed by peripheral blood hemogram [24]. Group-II: Control group of mice (N = 18) received equal volume of saline in similar condition. Group III: Post development of Leukaemia these animals received Withanolide D (N = 30) by oral route for 30 days.
HPLC
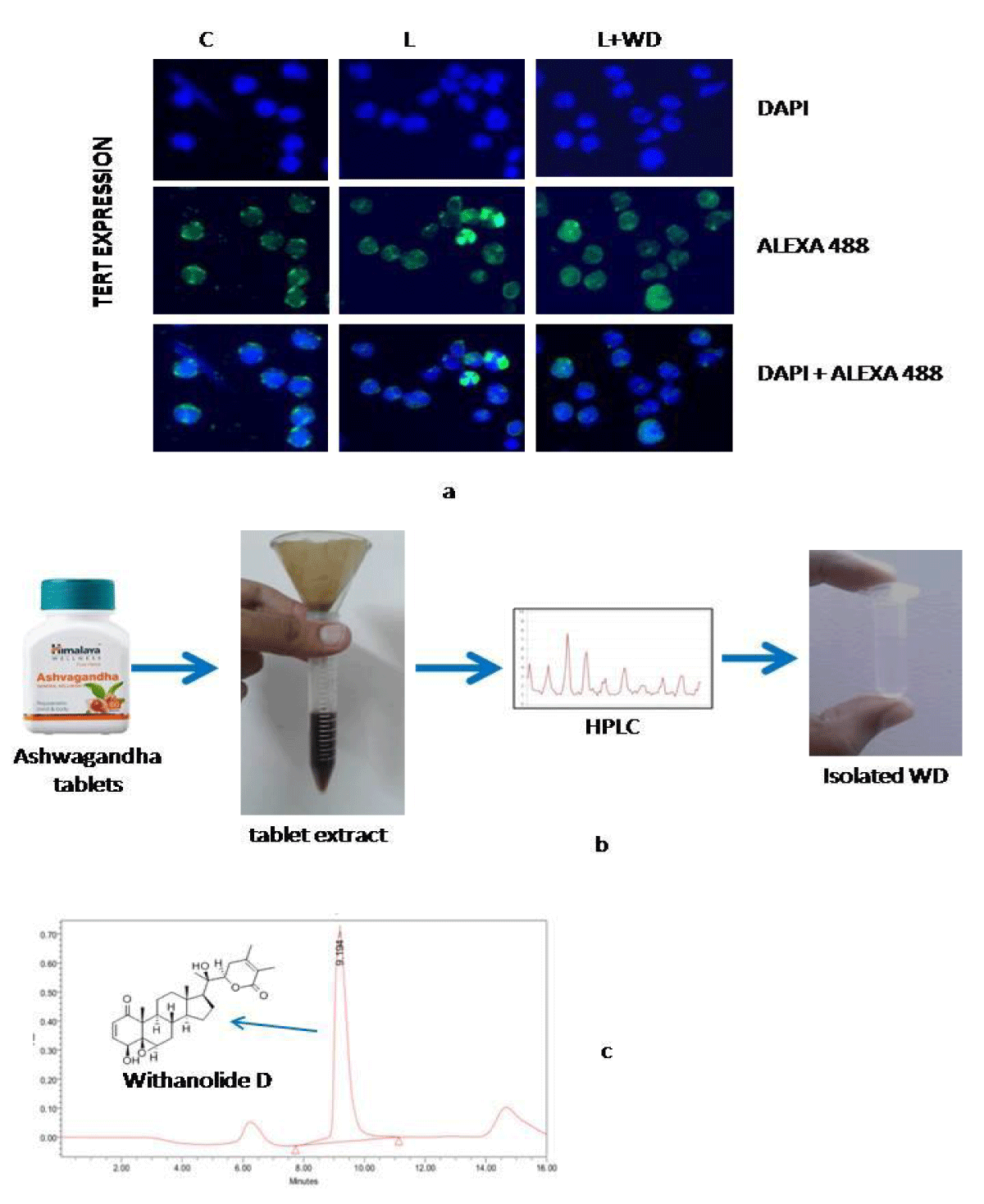
20 grams of Ashwagandha powder was dissolved in 100ml of distilled water over night at 4 degree Celsius, followed by filtration under sterile condition. Flitrate was treated as 100%. This filtrate was filtered again through 0.22micron filter membrane and used for High Performance Liquid chromatography. Withanolide D (WD) was separated and collected from Ashwagandha whole extract.
Estimation of LD50 and administration of Withanolide D
To determine the LD50 value isolated Withanolide D was administered via oral route in the leukemic mice at a dose of 50, 60, 70, 75 and 80 mg/kg of bodyweight. It was found that at the dosimetry of 75 mg/kg of bodyweight 50 percent of all the experimental animals died.
To denote EC50 value half of LD50 value was taken into consideration. Withanolide D was administered at a dose of 27.5, 37.5 and 47.5 mg/kg of bodyweight. 50% of experimental animals showed change in their physiological, haematological and cytological parameters at a dose 37.5 mg/kg bodyweight.
So, Withanolide D was administered via oral route for 30days at a dose of 37.5 mg/kg of bodyweight.
Dye exclusion method
Bone marrow cells were flushed out from the femurs of experimental animals. Single cells were prepared from the extracted marrows portions. Single cell suspension was divided into two parts, one part was used for flow cytometric analysis and other for the dye exclusion method. Single cells were incubated with 0.04% Trypan blue (Sigma, USA) stain at 1:1 ratio for 10 minutes at room temperature. Post incubation cells were charged into hemocytometer (Rohem, India) for evaluation.
Immunocytochemistry
We have performed immunocytochemical studies on PFA-fixed bone marrow cells, using monoclonal antibody against PUMA, BCL2, TERT (Cell Signaling, USA) tagged with Alexa 488 (Invitrogen, USA) and DAPI to stain nucleus (Sigma, USA) for evaluating the expression pattern with and without the treatment.
Flow cytometry
Cells from the control and treated groups were fixed at room temperature in 1.5% PFA and pelleted. Fixed cells were permeabilised by resuspension with vortexing in 500 μl of chilled 90% methanol and incubated at 4°C for 15 min to 20 min. Thereafter, cells were washed twice in FACS fluid (PBS containing 1% BSA) and were divided into different sorting tubes with 1.5 x 106 cells per 100 μl of fresh FACS fluid. 2 μl, anti-PUMA antibody and anti-BCL2 antibody (Cell Signaling, USA) were then added into respective sorting tubes and incubated for 30 min at 37°C. This was followed by the addition of secondary antibody Alexa 488 (Invitrogen, USA) to each primary antibody containing tubes and incubated further for 30 min at 37°C. Finally cells were washed by centrifugation and resuspended in FACS fluid for acquisition and analysis by BD FACS Calibur (Becton-Dickinson, USA) using CellQuest Pro software (v9.1 Becton-Dickinson, USA).
Statistical evaluation
Intergroup comparison was evaluated by student’s t test and for multiple comparisons one way Analysis of Variance (ANOVA) was used. All data are presented as mean ± standard deviation. Each experiment was performed three times for statistical significance. Significance was defined as p < 0.01.
Results
Dosimetry and estimation of LD50 and EC50 values
Aqueous solution of Withanolide D was administered in experimental leukemic animal groups in order to estimate the LD50 value. LD50 refers to a dose at which 50% of all test subjects die. Previous studies have estimated LD50 value of Withanolide D in normal Swiss albino mice but LD50 value of Withanolide D in leukemic mice was unavailable. Withanolide D was administered at a dosimetry of 50 mg/kg, 60 mg/kg, 70 mg/kg, and 80 mg/kg of bodyweight via oral route. 100% mortality rate was noted at 80mg/kg of bodyweight dose and at 70 mg/kg of bodyweight 33.33% mortality rate was observed. A fraction between 70 and 80 mg/kg bodyweight, i.e. 75 mg/kg bodyweight was administered and 50% of all test animals died (Table 1).
| Table 1: LD50 value of Withanolide D. | ||||||
| Withanolide D mg/kg | Dead | Survived | Total | Rate | % Mortality | % Survival |
| 80 | 4 | 0 | 6 | 6/0 | 100 | 0 |
| 75 | 3 | 3 | 6 | 3/3 | 50 | 50 |
| 70 | 2 | 4 | 6 | 2/4 | 33.33 | 66.66 |
| 60 | 1 | 5 | 6 | 1/5 | 16.66 | 83.33 |
| 50 | 0 | 6 | 6 | 0/6 | 0 | 100 |
Similarly effective concentration or EC50 value was evaluated. Experimental animals were divided into 3 groups and they received an aqueous solution of Withanolide D via oral route. Three different doses of Withanolide D i.e. 27.5 mg/kg, 37.5 mg/kg and 47.5 mg/kg of bodyweight was administered. At a dose of 37.5 mg/kg of bodyweight Withanolide D showed maximum effect in 50% of the experimental animals (Table 2).
| Table 2: EC50 value of Withanolide D. | ||||
| Withanolide D mg/kg | Total | Activity observed | Rate | % |
| 27.5 | 6 | 1 | 1/6 | 16.67 |
| 37.5 | 6 | 3 | 3/6 | 50 |
| 47.5 | 6 | 6 | 6/0 | 100 |
Summarily, at a dose of 75 mg/kg of bodyweight, Withanolide D showed severe toxicity in leukemic mice and 50 percent of animals died. The estimated Effective concentration of Withanolide D was 37.5 mg/kg of bodyweight.
Assessment of cellular death by dye exclusion method
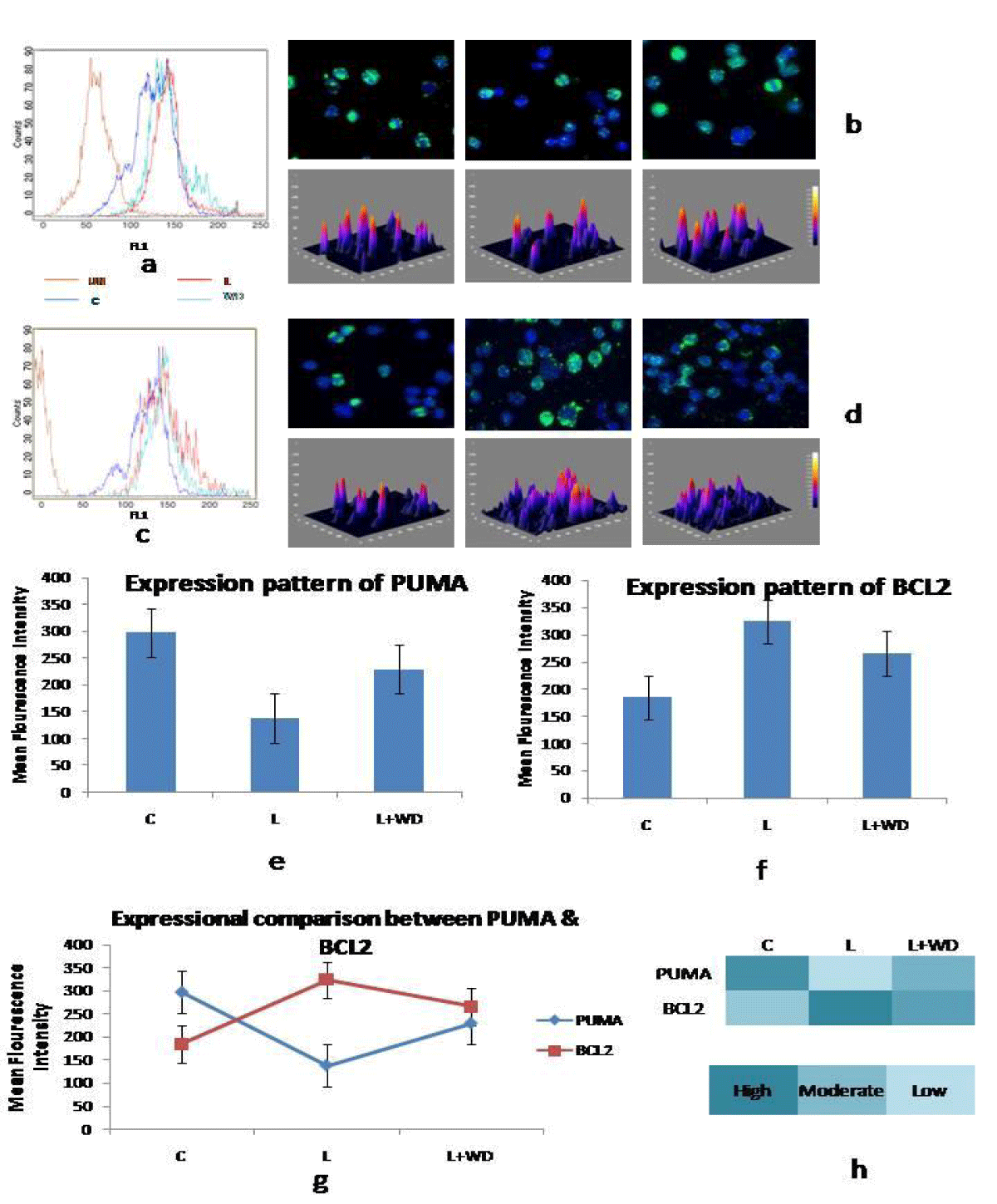
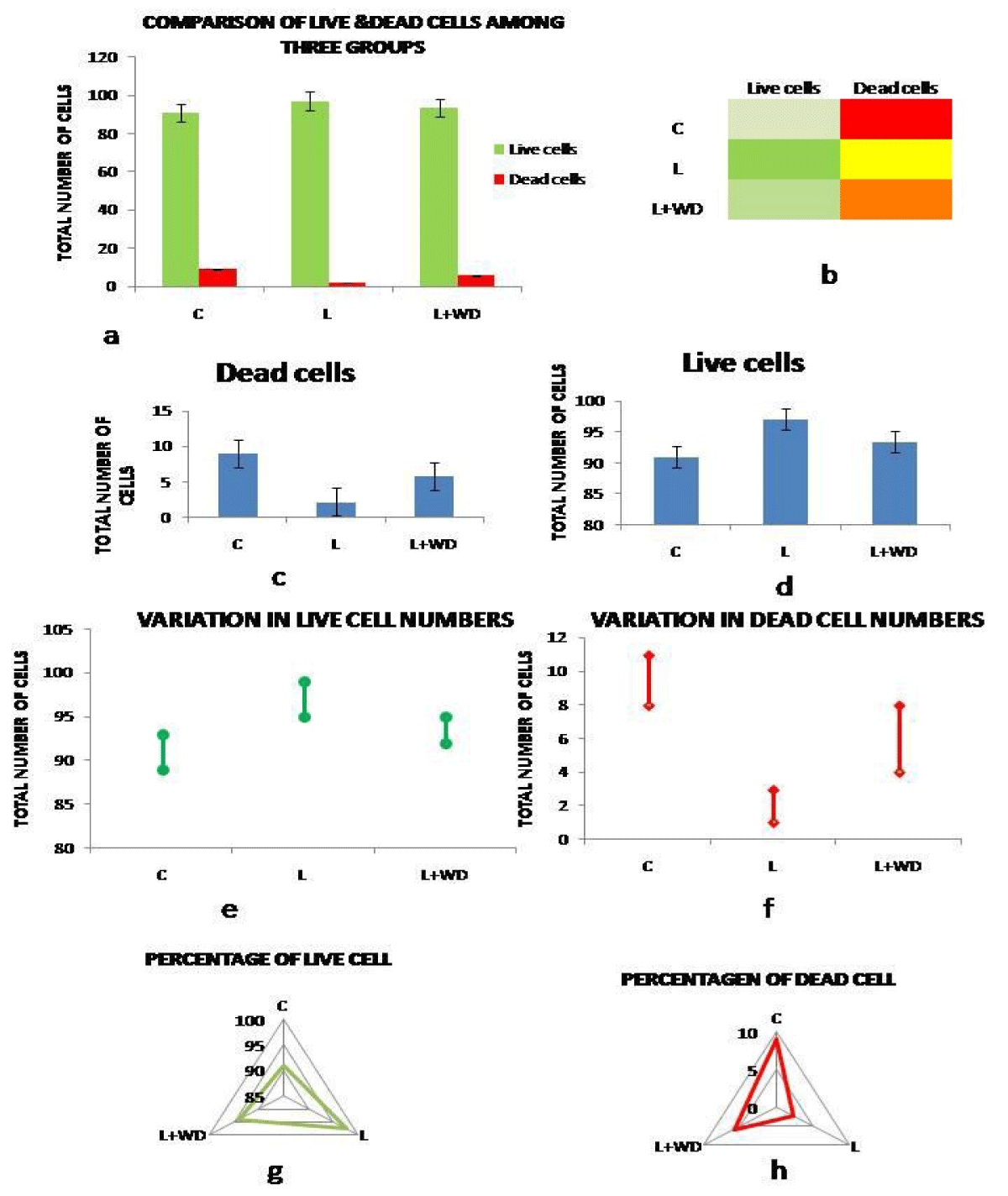
Post Withanolide D administration bone marrow cells were assessed for the cellular death ratio. In Control(C) group (Group II) percentage of live cell was 91 ± 0.85% and dead cell was 9 ± 1.04%. On the other hand leukemic (L) group (Group I) showed increased (p = 0.01) survivability with a percentage of 97.78 ± 1.12% and decreased mortality, 2.22 ± 0.95%. Post WD administration the percentages of live cells have decreased to 94.15 ± 1.33% and percentage dead cells increased to 5.85 ± 0.63% in group III (L+WD). Cytochemical and immunocytochemical methods revealed augmented cellular death in the treated group compared to the leukemic group (Figure 2 a-h).
Withanolide D increases pro apoptotic activity
PUMA has been widely related to pro-apoptotic function. In our study expressional level of PUMA decreased significantly in the leukemic group (MFI 138.09 ± 1.32) as compared to control (MFI 297.16 ± 2.01). Another key component is BCL2 and its expression level in the leukemic group notably increased (MFI 323.66 ± 1.36) as compared to the control group (MFI 184.95 ± 2.42). Simultaneous decline in the expressional level of PUMA and increase in BCL2 expression clearly indicated towards deregulation in apoptotic machinery and survival of the abnormal cells.
On the other hand when the leukemic animals were treated with Withanolide D the expression levels of PUMA (MFI 229.66 ± 2.11) was markedly increased & BCL2 (MFI 265.33 ± 1.98) significantly decreased (p = 0.01) than that of their leukemic counterpart. Administration of Withanolide D released the suppression on the apoptotic machinery and with this release rate of apoptosis increased. Increased apoptosis rate not only hindered expansion of abnormal cells but also helped bone marrow micro environment to overcome the catastrophe caused by leukemia (Figure 1 a-h).
Expressional change of TERT post Withanolide D administration
In our immuocytochemical studies it was noted that the expressional value of TERT in leukemic condition was increased than that of control. In concurrence to our findings other studies have showed augmented level of TERT in most tumor cells. Increased TERT levels are often associated with the proliferative capacity of somatic cells and in leukaemic condition this proliferative phenomenon helped in the progression of abnormal leukemic cells by supporting the anti apoptotic function.
Post Withanolide D administration expression of TERT shifted towards normal value and triggered the apoptosis machinery as decreased telomerase activity has been directly associated with increased vulnerability to apoptosis (Figure 4a).
Discussion
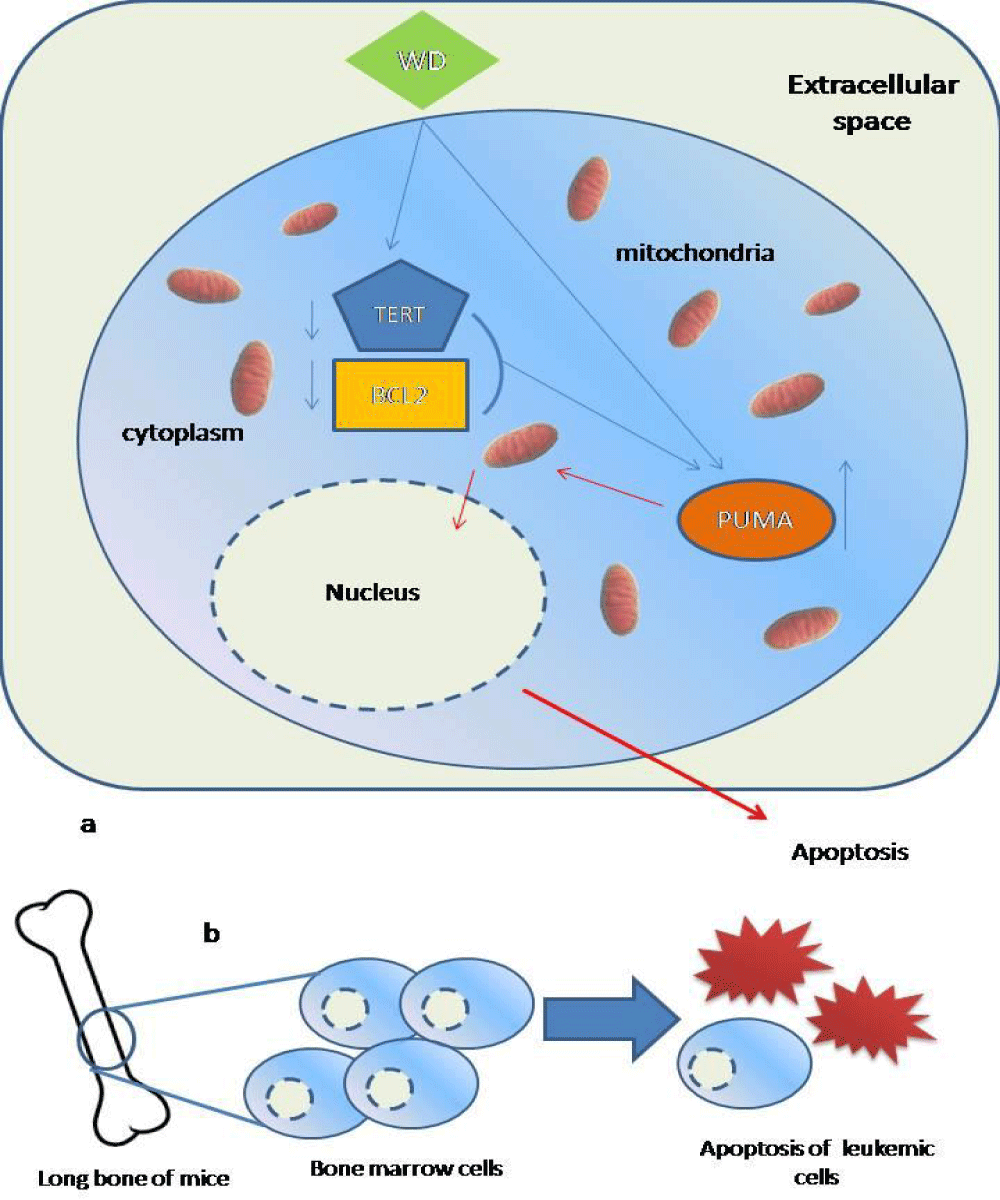
Fine balance between cell proliferation and death maintains the healthy environment in an organism and any imbalance leads to abnormality and ultimately to diseased condition. In our leukemic model the balance between cellular proliferation and death was severely hindered by abnormal cellular proliferation and decreased cellular death or apoptosis. Apoptosis is an intricately tuned mechanism comprises of many key components of extrinsic and intrinsic pathway and in our experimental endeavour we have taken three representative key components TERT, BCL2 and PUMA regarding the associated particular anti-apoptotic and pro-apoptotic functionality. In leukemic model augmented levels of TERT jammed the apoptotic machinery and helped in the survival of the abnormal cells. Increased abnormal cells led to progression of the disease. Not only increase in the anti apoptotic and proliferative mechanism helped leukaemic progression but also pro apoptotic system is involved. PUMA is a very potent activator of apoptosis. Augmented TERT and BCL2 levels and decreased PUMA expression tipped the apoptotic system off balance. As a result abnormal cells were proliferated in large numbers. Withanolide D, when administered into the leukemic system started to interact with the key components, i.e., TERT, BCL2 and PUMA. Expressional decline of TERT and BCL2 indicated increased vulnerability to apoptosis in the abnormal hematopoietic cells. Simultaneously increased PUMA expression also set the apoptotic system in motion. The two way pressure on the apoptotic machinery lead to cellular death. Increased death rate and decline in the proliferative action significantly decreased the disease progression (Figure 3).
As Withanolide D showed promising result, so, we can conclude that Withanolide D of Ashwagandha may hold a promise to a cheaper, safer and easily available alternative for the therapeutic management of leukemia.
Limitations of the study
There are many signalling molecules involved in the intrinsic and extrinsic pathway of apoptotic machinery in Leukaemia which could have given us a more elaborate view of the scenario but we have taken some representative key molecules only, for the initial study, to progress surely in future, in a broader aspect.
Acknowledgement
We would like to thank Ministry of AYUSH for funding the project (Grant no Z-28015/16/2016-HPC (EMR)-AYUSH-A). We sincerely thank, Director of Calcutta School of Tropical Medicine. We would also like to thank Dr. Pralay Majumder, Presidency University for allowing us to use the fluorescence imaging facility and Dr. Ramdhan Majhi, Indian Institute of Chemical Biology (IICB) for providing Withanolide D.
References
- Law S, Maiti D, Palit A, Majumder D, Basu K, Chaudhuri S, Chaudhuri S. Facilitation of functional compartmentalization of bone marrow cells in leukemic mice by biological response modifiers: an immunotherapeutic approach. Immunol Lett. 2001 Apr 2;76(3):145-52. doi: 10.1016/s0165-2478(00)00317-5. PMID: 11306141.
- Law S, Begum B, Chaudhuri S. Pluripotent bone marrow cells in leukemic mice elicit enhanced immune reactivity following sheep erythrocyte administration in-vivo. A possible S-LFA3 interactive immunotherapy. J Exp Clin Cancer Res. 2003 Jun;22(2):213-21. PMID: 12866571.
- Sujata L, Chaudhuri S. Stem cell niche, the microenvironment and immunological crosstalk. Cell Mol Immunol. 2008 Apr;5(2):107-12. doi: 10.1038/cmi.2008.13. PMID: 18445340; PMCID: PMC4651246.
- Law S, Chaudhuri S. Stem cell niche failure concerns bone marrow failure--a diagnostic and therapeutic consideration. J Stem Cells. 2011;6(2):67-73. PMID: 22997847.
- Henrich CJ, Brooks AD, Erickson KL, Thomas CL, Bokesch HR, Tewary P, Thompson CR, Pompei RJ, Gustafson KR, McMahon JB, Sayers TJ. Withanolide E sensitizes renal carcinoma cells to TRAIL-induced apoptosis by increasing cFLIP degradation. Cell Death Dis. 2015 Feb 26;6(2):e1666. doi: 10.1038/cddis.2015.38. PMID: 25719250; PMCID: PMC4669816.
- Issa ME, Wijeratne EMK, Gunatilaka AAL, Cuendet M. Withanolide D Exhibits Similar Cytostatic Effect in Drug-Resistant and Drug-Sensitive Multiple Myeloma Cells. Front Pharmacol. 2017 Sep 8;8:610. doi: 10.3389/fphar.2017.00610. PMID: 28943850; PMCID: PMC5596074.
- Ichikawa H, Takada Y, Shishodia S, Jayaprakasam B, Nair MG, Aggarwal BB. Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-kappaB (NF-kappaB) activation and NF-kappaB-regulated gene expression. Mol Cancer Ther. 2006 Jun;5(6):1434-45. doi: 10.1158/1535-7163.MCT-06-0096. PMID: 16818501.
- Lacombe J, Cretignier T, Meli L, Wijeratne EMK, Veuthey JL, Cuendet M, Gunatilaka AAL, Zenhausern F. Withanolide D Enhances Radiosensitivity of Human Cancer Cells by Inhibiting DNA Damage Non-homologous End Joining Repair Pathway. Front Oncol. 2020 Jan 8;9:1468. doi: 10.3389/fonc.2019.01468. PMID: 31970089; PMCID: PMC6960174.
- Wijeratne EMK, Oliveira MCF, Mafezoli J, Xu YM, Minguzzi S, Batista PHJ, Pessoa ODL, Whitesell L, Gunatilaka AAL (2018) Withaferin A and Withanolide D Analogues with Dual Heat-Shock-Inducing and Cytotoxic Activities: Semisynthesis and Biological Evaluation. Journal of Natural Products, 81(4), 825–837. https://doi.org/10.1021/acs.jnatprod.7b00918
- Ruvolo PP, Deng X, May WS. Phosphorylation of Bcl2 and regulation of apoptosis. Leukemia. 2001 Apr;15(4):515-22. doi: 10.1038/sj.leu.2402090. PMID: 11368354.
- Zinkel S, Gross A, Yang E. BCL2 family in DNA damage and cell cycle control. Cell Death Differ. 2006 Aug;13(8):1351-9. doi: 10.1038/sj.cdd.4401987. Epub 2006 Jun 9. PMID: 16763616.
- Coxon A, Rieu P, Barkalow FJ, Askari S, Sharpe AH, von Andrian UH, Arnaout MA, Mayadas TN. A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity. 1996 Dec;5(6):653-66. doi: 10.1016/s1074-7613(00)80278-2. PMID: 8986723.
- Ding Y, Yang Z, Zhang W, Xu Y, Wang Y, Hu M, Ma F, Long H, Tao N, Qin Z. Eugenol triggers BCL2 + Gr1 + myeloid-derived suppressor cell apoptosis via endogenous apoptosis pathway. RSC Advances. 2018; 8(7): 3833-3838. doi: 10.1039/C7RA13499A
- El Kebir D, József L, Pan W, Filep JG. Myeloperoxidase delays neutrophil apoptosis through CD11b/CD18 integrins and prolongs inflammation. Circ Res. 2008 Aug 15;103(4):352-9. doi: 10.1161/01.RES.0000326772.76822.7a. Epub 2008 Jul 10. PMID: 18617697.
- Graf M, Reif S, Kröll T, Hecht K, Nuessler V, Schmetzer H. Expression of MAC-1 (CD11b) in acute myeloid leukemia (AML) is associated with an unfavorable prognosis. Am J Hematol. 2006 Apr;81(4):227-35. doi: 10.1002/ajh.20526. PMID: 16550517.
- Xu S, Li X, Zhang J, Chen J. Prognostic Value of BCL2 Expression Level for Acute Myeloid Leukemia Patients: A Meta-Analysis. PLOS ONE. 2015;10(8): e0135981. doi: 10.1371/journal.pone.0135981
- Guirguis AA, Slape CI, Failla LM, Saw J, Tremblay CS, Powell DR, Rossello F, Wei A, Strasser A, Curtis DJ. PUMA promotes apoptosis of hematopoietic progenitors driving leukemic progression in a mouse model of myelodysplasia. Cell Death Differ. 2016 Jun;23(6):1049-59. doi: 10.1038/cdd.2015.159. Epub 2016 Jan 8. PMID: 26742432; PMCID: PMC4987724.
- Guirguis AA, Slape CI, Failla LM, Saw J, Tremblay CS, Powell DR, Rossello F, Wei A, Strasser A, Curtis DJ. PUMA promotes apoptosis of hematopoietic progenitors driving leukemic progression in a mouse model of myelodysplasia. Cell Death Differ. 2016 Jun;23(6):1049-59. doi: 10.1038/cdd.2015.159. Epub 2016 Jan 8. PMID: 26742432; PMCID: PMC4987724.
- Zhang F, Li Y, Tang Z, Kumar A, Lee C, Zhang L, Zhu C, Klotzsche-von Ameln A, Wang B, Gao Z, Zhang S, Langer HF, Hou X, Jensen L, Ma W, Wong W, Chavakis T, Liu Y, Cao Y, Li X. Proliferative and survival effects of PUMA promote angiogenesis. Cell Rep. 2012 Nov 29;2(5):1272-85. doi: 10.1016/j.celrep.2012.09.023. Epub 2012 Nov 1. PMID: 23122957.
- Zhang LN, Li JY, Xu W. A review of the role of Puma, Noxa and Bim in the tumorigenesis, therapy and drug resistance of chronic lymphocytic leukemia. Cancer Gene Ther. 2013 Jan;20(1):1-7. doi: 10.1038/cgt.2012.84. Epub 2012 Nov 23. PMID: 23175245.
- Vinayagamurthy S, Ganguly A, Chowdhury S. Extra-telomeric impact of telomeres: Emerging molecular connections in pluripotency or stemness. J Biol Chem. 2020 Jul 24;295(30):10245-10254. doi: 10.1074/jbc.REV119.009710. Epub 2020 May 22. PMID: 32444498; PMCID: PMC7383370.
- Mattson MP, Zhang P, Fu W. Roles for TERT and Telomerase in Cell Differentiation and Apoptosis. 17.
- Shen Y, Xi F, Li H, Luo Y, Chen C, Wang L. Telomerase reverse transcriptase suppression inhibits cell proliferation and promotes cell apoptosis in hepatocellular cancer. IUBMB Life. 2018 Jul;70(7):642-648. doi: 10.1002/iub.1758. Epub 2018 Apr 29. PMID: 29707886.
- Druckrey H, Preussmann R, Ivankovic S, Schmähl D. Organotrope carcinogene Wirkungen bei 65 verschiedenen N-Nitroso-Verbindungen an BD-Ratten [Organotropic carcinogenic effects of 65 various N-nitroso- compounds on BD rats]. Z Krebsforsch. 1967;69(2):103-201. German. PMID: 4230610.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.