> Medicine Group. 2021 May 20;2(5):339-342. doi: 10.37871/jbres1238.
Neutrophil to Lymphocyte Ratio Reflects the Proteolytic Activity of the Abdominal Aortic Aneurysm Wall
Vasile Aurelian Sasarman1,2,4, Octavian Andercou2,3*, Schjoth Bruno4, Manuel Chira1,2, Alexandru Oprea1,2, Catalin Trifan1,2 and Dan Bindea1,2
2University of Medicine and Pharmacy, Iuliu Hatieganu, Cluj-Napoca
3Department of Vascular Surgery, Cluj-Napoca, Romania
4Regional Hospital Center Metz-Thionville, Hôpital de Mercy1, Metz, France
Abstract
Background: This study aims to evaluate the local proteolytic activity from the level of Abdominal Aortic Aneurysm (AAA) wall and correlate the obtained values with the preoperative values of NLRs (Neutrophil-Lymphocyte Ratio), evaluating a possible association between the two variables and, implicitly, between the local proteolysis process and the systemic inflammatory response of those patients diagnosed with AAA.
Methods: The current study is monocentric, observational, and prospective, taking place at the Department of Cardiovascular Surgery, Cluj-Napoca, Romania. Patients undergoing elective or emergency classical surgery for unruptured AAA or ruptured AAA were included in the study. During classical surgery, samples from the infrarenal aortic aneurysmal wall were collected in a standardized manner, from the central part of the anterior wall from uAAA and rAAA and were analyzed by gel zymography.
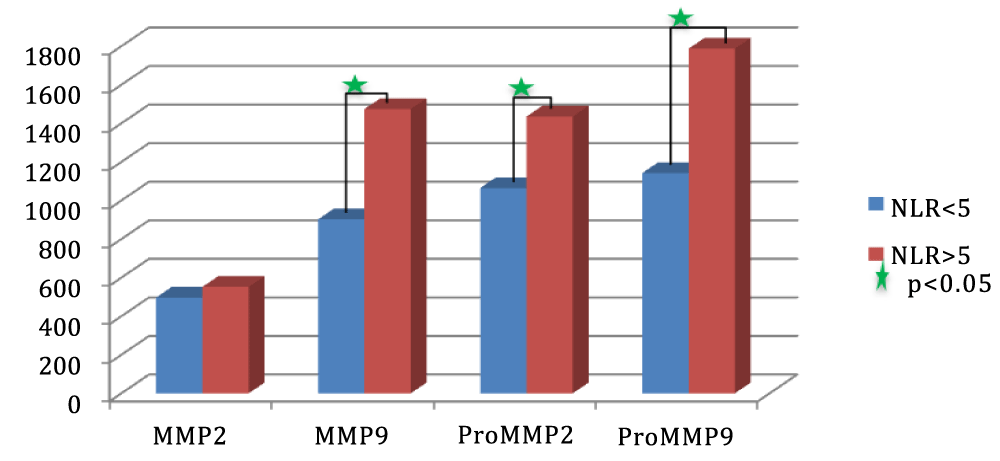
Results: The concentration of MMP2 was similar in the ruptured/non-ruptured group, without any statistical significance. In the MMP-9 case, we obtained a mean of 821.35 U arb/µg at the level of unruptured aneurysmal wall and 1411.57 U arb/µg at the level of the ruptured aneurysmal wall. According to the ANOVA test, there is a significant difference between the two categories of aneurysms. The same correlation was observed regarding both the zymogen category, pro-MMP-2, as well as pro-MMP-9: they expressed significant higher quantities of inactive enzymes in rAAA.
We splitted the study population into two categories: patients who presented preoperative NLR values < 5 and > 5. MMP-2 collagenase levels did not register statistical differences between the two groups, p = 0.3236. High levels of MMP-9 are positively associated with increased values of NLR, the NLR<5 group had an MMP-9 mean of 902.41(473.71) U arb/µg, statistically lower than the MMP-9 mean indicated in the NLR>5 group, 1474(521.21) U arb/µg. Similarly, MMP-2 and MMP-9 zymogens were found in statistically higher quantities (p < 0.05) in the NLR>5 group of patients.
Conclusions: This is the first study that analyzes a possible correlation between the local proteolytic activity at the site of the dilated aneurysmal aortic wall and circulating levels of NLR. Following the results obtained, we conclude that the group of patients presenting with NLR>5 preoperatively, as in the rAAA group, significantly greater levels of MMP-9 and inactive proenzymes were identified. Local metalloproteinase MM9 activity is proportional to the systemic inflammatory activity. Concomitantly, we hypothesize that the increased sensitivity of NLR as a prognostic marker in AAA pathology, which is ensured and confirmed by its strong association with local proteolytic activity, directly implied in the evolution of the disease.
Introduction
The Extracellular Matrix (ECM) provides structural support, regulates growth factor bioavailability and cytokine activity, influencing cellular function and behavior that modulates physiologic or pathologic remodeling of the aorta [1]. The disbalance of Metalloproteinases (MMPs) can stimulate proteolytic activity at the ECM level, leading to tissue degeneration. In addition, MMPs activate a variety of non-matriculated elements, including cytokines and chemokines, influencing the inflammatory processes [2]. Most MMPs are capable of activating precursors to other MMPs, creating an enzymatic cascade capable of amplifying the proteolytic activity of MMPs [3].
Inflammatory infiltrate is the primary source of local proteolytic activity through overexpression of MMP-9 [4,5]. MMP-2 is physiologically expressed by mesenchymal cells in the middle layer of the aortic wall. These two gelatinases, largely expressed in AAA, are considered the primary proteolytic enzymes responsible for the degradation of the ECM [6,7].
The purpose of this study is to evaluate the local proteolytic activity at the level of Abdominal Aortic Aneurysm (AAA) wall and correlate the obtained values with the preoperative values of NLRs (Neutrophil-Lymphocyte Ratio), evaluating a possible association between the two variables and, implicitly, between the local proteolysis process and the systemic inflammatory response of those patients diagnosed with AAA.
Materials and Methods
The current study is monocentric, observational, and prospective, taking place in the Department of Cardiovascular Surgery, Cluj-Napoca, Romania. Patients undergoing elective or emergency classical surgery for uAAA or rAAA were included in the study. Inclusion criteria in the study were: AAA size greater than 5.5 cm in men and 5 cm in women, symptomatic aneurysms, expansion rate of the aneurysm greater than 5 mm in 6 months, and/or rupture of it. We excluded patients whose clinical or paraclinical data were incomplete, a coexisting diagnosis of acute/subacute bacterial infection, patients needing preoperative resuscitation maneuvers, and who benefited from endovascular treatment.
Biological samples were taken the day before the surgical intervention for patients with uAAA and/or immediately preoperatively for those with rAAA. NLR was calculated by dividing the absolute number of neutrophils by the number of lymphocytes. During classical surgery, samples from the infrarenal aortic aneurysmal wall were collected in a standardized manner, from the central part of the anterior and were analyzed by gel zymography. Expressing metalloprotease activity is done through densitometry, depending on the volume curve, which is expressed as arbitrary units per total amount of protein.
Statistical differences were evaluated by Chi-square calculation, a value greater than 5, and a mid p lower than 0.05 were considered statistically significant. Means were compared with the help of the parametric test ANOVA, when the Bartlett p value was greater than 0.05, along with the nonparametric test Kruskal-Wallis when lower. For both tests, a p lower than 0.05 was considered statistically significant.
Results
Population study
In the final analysis, sixty-four patients were diagnosed with AAA, 31 with rAAA, and 33 with uAAA, who benefitted from elective or emergency surgical treatment of AAA. All patients were of Caucasian race and 92.18% of the population were men. Mean age of patients in the study was 67.57 ± 7.6 years old. Mean size of the transverse diameter of the aneurysms was 6.67cm, measured by abdominal ultrasonography or CT angiography, with a mean of 6.41cm for the category of patients presenting with uAAA and 6.94cm for the category of patients presenting with rAAA.
Levels of MMP-2, MMP-9 and their zymogens
The concentration of MMP2 was similar in the ruptured/non-ruptured group, without any statistical significance. In the MMP-9 case, we obtained a significant difference between the two categories, with a mean of 821.35 U arb/µg at the level of uAAA wall and 1411.57 U arb/µg at the level of rAAA wall. The same correlation was observed regarding both the zymogen category, pro-MMP-2, as well as the pro-MMP-9 as they expressed significant higher quantities of inactive enzymes in rAAA. The MMP-9/MMP-2 ratio in the rAAA category was 2.498, significantly higher compared to the uAAA category, in which it reached 1.751, p < 0.05.
Levels of MMP-2, MMP-9 and their homologue zymogens depend on the NLR
We splitted the study population into two categories: patients who presented preoperative NLR values smaller than 5 and higher than 5. For each category we calculated: mean, quartiles, SD, and mode values for MMP-2, MMP-9, and proenzymes obtained through zymographic analysis of human aortic tissue samples (Table 1) (Figure 1).
| Table 1: Quantitative results of metalloproteinases MMP-2, MMP-9, and their homologue zymogens from patients with preoperative NLR levels < 5 and > 5. | ||||||||
| MMP | Mean | Var | Std Dev | Median | 75% | Max | Mode | |
| MMP-2 | NLR<5 | 497.0256 | 60486.973 | 245.941 | 532 | 678 | 998 | 667 |
| NLR>5 | 552.12 | 24881.526 | 157.7388 | 562 | 667 | 787 | 403 | |
| MMP-9 | NLR<5 | 902.4103 | 224401.72 | 473.7106 | 862 | 1128 | 2191 | 411 |
| NLR>5 | 1474 | 271664.25 | 521.2142 | 1310 | 1754 | 2556 | 710 | |
| ProMMP-2 | NLR<5 | 1064.4615 | 331452.25 | 575.7189 | 1018 | 1345 | 2242 | 1018 |
| NLR>5 | 1435.16 | 235039.89 | 484.8091 | 1373 | 1739 | 2886 | 611 | |
| proMMP-9 | NLR<5 | 1141.9744 | 308831.0256 | 555.7257 | 1023 | 1458 | 2494 | 774 |
| NLR>5 | 1787.12 | 283395.5267 | 532.3491 | 1734 | 2129 | 2889 | 1383 | |
From the statistical analysis, we obtained the following results: MMP-2 collagenase levels did not register statistical differences between the two groups, p = 0.3236. High levels of MMP-9 are positively associated with increased values of NLR. The NLR<5 group had an MMP-9 mean of 902.41(473.71) U arb/µg, statistically lower than the MMP-9 mean indicated in the NLR>5 group, 1474(521.21) U arb/µg. Similarly, MMP-2 and MMP-9 zymogens were found in statistically higher quantities (p < 0.05) in the NLR>5 group of patients.
Discussion
In the current study, the MMP-9/MMP-2 ratio in the rAAA category was 2.498 U arb/µg, significantly greater than 1.751 U arb/µg in the uAAA category with p < 0.05. This was a consequence of the fact that MMP-9 levels increased significantly in the rAAA group, and MMP-2 levels were relatively constant.
MMP-2 is constitutively expressed by resident cells of the aortic wall and, unlike MMP-9, its synthesis is not influenced by inflammatory cells. Additional reports confirm increased levels of this metalloproteinase in small and medium AAAs, launching the hypothesis that it has an important role in the incipient phases of aneurysmal pathology [8,9].
In contrast, the enzymatic activity of MMP-9 measured at the rAAA wall was significantly greater than that registered in intact aneurysms. Immunohistologic studies demonstrate that the intensified activity of MMP-9 is caused mainly by inflammatory cells: macrophages and neutrophils, as well as mesenchymal cells to a smaller degree. In this regard, it was demonstrated that circulating inflammatory cell activity is regulated by modulators such as: cytokines (TNF-α and IL-1β), chemokines, and growth factors which promote MMP secretion through Mitogen-Activated Protein Kinase Pathways (MAPKs) [10-12].
The importance of inflammatory infiltrate in AAA pathology is confirmed by the correlation between Neutrophil Gelatinase-Associated Lipocalin (NGAL) and AAA progression. This is a protein expressed normally by neutrophils during inflammatory processes. Through the association between NGAL and MMP-9, the metalloproteinase degradation is inhibited, and its enzymatic activity is prolonged, which results in intensified proteolytic degrading processes [13-18].
This is the first study that analyzes a possible correlation between the proteolytic activity and circulating levels of NLR in patients diagnosed with AAA.
We observed a significant increase in MMP-9 amount in patients with preoperative NLR>5. This result confirms the interdependence of increased MMP-9 activity and the presence of an inflammatory infiltrate. Similarly, MMP-2 and MMP-9 zymogens were found at statistically significant increased levels (p < 0.05) in the group of patients with preoperative NLR>5.
Many studies concluded that NLR>5 preoperatively represents an increased risk of postoperative mortality and morbidity in the case of AAA. In our past study, the mean NLR value in the rAAA category was almost 3 times greater than that of uAAA. In the rAAA case, 77.6% of patients presented with preoperative NLR>5, compared to the uAAA group in which only 32.5% had preoperative NLR>5 (OR: 5.085; 95% CI: 3.0025–8.6145, p < 0000.1) [19-21].
Conclusion
Following the results of the current study, we conclude that rAAAs are associated with exacerbated proteolytic activity through the overexpression of MMP-9, as well as MMP-2 and MMP-9 zymogens. In the group of patients presenting with NLR>5 preoperatively, as in the rAAA group, significantly greater levels of MMP-9 and inactive proenzymes were identified, compared to those in the NLR<5 group. Local metalloproteinase MM9 activity is proportional to the systemic inflammatory activity. Concomitantly, we hypothesize that the proven stability and viability of NLR as a prognostic marker is ensured and confirmed by its strong association with local proteolytic activity, directly implied in the evolution of aneurysmal pathology.
References
- Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med. 2014 Nov 27;371(22):2101-8. doi: 10.1056/NEJMcp1401430. PMID: 25427112.
- Maguire EM, Pearce SWA, Xiao R, Oo AY, Xiao Q. Matrix Metalloproteinase in Abdominal Aortic Aneurysm and Aortic Dissection. Pharmaceuticals (Basel). 2019 Aug 6;12(3):118. doi: 10.3390/ph12030118. PMID: 31390798; PMCID: PMC6789891.
- Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002 Sep;110(5):625-32. doi: 10.1172/JCI15334. PMID: 12208863; PMCID: PMC151106.
- Patel MI, Melrose J, Ghosh P, Appleberg M. Increased synthesis of matrix metalloproteinases by aortic smooth muscle cells is implicated in the etiopathogenesis of abdominal aortic aneurysms. J Vasc Surg. 1996 Jul;24(1):82-92. doi: 10.1016/s0741-5214(96)70148-9. PMID: 8691532.
- Sasaguri Y, Murahashi N, Sugama K, Kato S, Hiraoka K, Satoh T, Isomoto H, Morimatsu M. Development-related changes in matrix metalloproteinase expression in human aortic smooth muscle cells. Lab Invest. 1994 Aug;71(2):261-9. PMID: 8078305.
- Wilson WR, Anderton M, Schwalbe EC, Jones JL, Furness PN, Bell PR, Thompson MM. Matrix metalloproteinase-8 and -9 are increased at the site of abdominal aortic aneurysm rupture. Circulation. 2006 Jan 24;113(3):438-45. doi: 10.1161/CIRCULATIONAHA.105.551572. PMID: 16432074.
- Molnar A, Săcui D, Scridon T. Risk factors influencing the surgical outcome in 138 consecutive patients with infrarenal aortic aneurysm: experience at the Cluj-Napoca Cardiovascular Surgery Center. Chirurgia (Bucur). 2014 Mar-Apr;109(2):223-8. PMID: 24742415.
- Lipka D, Boratyński J. Metaloproteinazy MMP. Struktura i funkcja [Metalloproteinases. Structure and function]. Postepy Hig Med Dosw (Online). 2008 Jul 3;62:328-36. Polish. PMID: 18614970.
- Davis V, Persidskaia R, Baca-Regen L, Itoh Y, Nagase H, Persidsky Y, Ghorpade A, Baxter BT. Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 1998 Oct;18(10):1625-33. doi: 10.1161/01.atv.18.10.1625. PMID: 9763536.
- McMillan WD, Tamarina NA, Cipollone M, Johnson DA, Parker MA, Pearce WH. Size matters: the relationship between MMP-9 expression and aortic diameter. Circulation. 1997 Oct 7;96(7):2228-32. doi: 10.1161/01.cir.96.7.2228. PMID: 9337194.
- Petersen E, Gineitis A, Wågberg F, Angquist KA. Activity of matrix metalloproteinase-2 and -9 in abdominal aortic aneurysms. Relation to size and rupture. Eur J Vasc Endovasc Surg. 2000 Nov;20(5):457-61. doi: 10.1053/ejvs.2000.1211. PMID: 11112465.
- Manicone AM, McGuire JK. Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol. 2008 Feb;19(1):34-41. doi: 10.1016/j.semcdb.2007.07.003. Epub 2007 Jul 10. PMID: 17707664; PMCID: PMC2235912.
- Brophy CM, Reilly JM, Smith GJ, Tilson MD. The role of inflammation in nonspecific abdominal aortic aneurysm disease. Ann Vasc Surg. 1991 May;5(3):229-33. doi: 10.1007/BF02329378. PMID: 2064915.
- Kjeldsen L, Cowland JB, Borregaard N. Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochim Biophys Acta. 2000 Oct 18;1482(1-2):272-83. doi: 10.1016/s0167-4838(00)00152-7. PMID: 11058768.
- Anchidim O, Nemes A, Molnar A. Are cardiovascular rehabilitation programs implemented in young patients with acute coronary syndromes following revascularisation procedures?. Balneo Research Journal. May 2020;11(3):133-140.
- Fontaine V, Jacob MP, Houard X, Rossignol P, Plissonnier D, Angles-Cano E, Michel JB. Involvement of the mural thrombus as a site of protease release and activation in human aortic aneurysms. Am J Pathol. 2002 Nov;161(5):1701-10. doi: 10.1016/S0002-9440(10)64447-1. PMID: 12414517; PMCID: PMC1850780.
- Ramos-Mozo P, Madrigal-Matute J, Vega de Ceniga M, Blanco-Colio LM, Meilhac O, Feldman L, Michel JB, Clancy P, Golledge J, Norman PE, Egido J, Martin-Ventura JL. Increased plasma levels of NGAL, a marker of neutrophil activation, in patients with abdominal aortic aneurysm. Atherosclerosis. 2012 Feb;220(2):552-6. doi: 10.1016/j.atherosclerosis.2011.11.023. Epub 2011 Nov 25. PMID: 22169111.
- Bartoli MA, Parodi FE, Chu J, Pagano MB, Mao D, Baxter BT, Buckley C, Ennis TL, Thompson RW. Localized administration of doxycycline suppresses aortic dilatation in an experimental mouse model of abdominal aortic aneurysm. Ann Vasc Surg. 2006 Mar;20(2):228-36. doi: 10.1007/s10016-006-9017-z. Epub 2006 Mar 30. PMID: 16572291.
- Appleton ND, Bailey DM, Morris-Stiff G, Lewis MH. Neutrophil to lymphocyte ratio predicts perioperative mortality following open elective repair of abdominal aortic aneurysms. Vasc Endovascular Surg. 2014 May;48(4):311-6. doi: 10.1177/1538574413519713. Epub 2014 Jan 24. PMID: 24464606.
- Aurelian SV, Adrian M, Andercou O, Bruno S, Alexandru O, Catalin T, Dan B. Neutrophil-to-Lymphocyte Ratio: A Comparative Study of Rupture to Nonruptured Infrarenal Abdominal Aortic Aneurysm. Ann Vasc Surg. 2019 Jul;58:270-275. doi: 10.1016/j.avsg.2018.11.026. Epub 2019 Feb 13. PMID: 30769065.
- Kordzadeh A, Malietzis G, Browne T, Prionidis I, Panayiotopoulos YP. Neutrophil to lymphocyte ratio (NLR) of five predicts 30-day morbidity in ruptured abdominal aortic aneurysms (rAAA): a retrospective cohort study. Int J Surg. 2015 Mar;15:45-8. doi: 10.1016/j.ijsu.2015.01.013. Epub 2015 Jan 30. PMID: 25641718.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.