> Environmental Sciences. 2021 Apr 15;2(4):244-250. doi: 10.37871/jbres1219.
Hypoxanthine Modified Polyethylene Glycol Diglycidyl Ether Gel for Ciprofloxacin Adsorption
Longfei Zhang1,2, Shuai Zhang1, Cheng Wang1, Jiaming Hu1, Wa Li1, Shanggeng Li1,3, Lirong Yang1,4, and Lin Zhang1*
2State Key Laboratory of Environment-friendly Energy Materials & School of Material Science and Engineering, Southwest University of Science and Technology, Mianyang, 621010, P. R. China
3Department of Engineering and Applied Physics, University of Science and Technology of China, Hefei, 230026, P. R. China
4School of Chemistry and Chemical Engineering, Mianyang Teachers’ College, Mianyang, 621000, P. R. China
- Hypoxanthine
- Adsorption
- Ciprofloxacin
- PEGDE
Abstract
A hypoxanthine modified polyethylene glycol diglycidyl ether gel was prepared by the ring opening polymerization of polyethylene glycol diglycidyl ether with hypoxanthine in a simple sol-gel method. The structure and composition were characterized by SEM, FT-IR, BET and XRD. The adsorption experiments of ciprofloxacin at different pH, temperature, contact time and initial concentration were studied. The results show that the gel is porous with the average pore size of 5.2 nm, the optimum adsorption pH is 5 and the saturated adsorption time is 240 minutes. The maximum equilibrium adsorption capacity of ciprofloxacin is 56.1 mg/g at 308 K according to the Langmuir model. The repeated adsorption experiments show that the gel could still adsorb 80% of the first adsorbed ciprofloxacin after 5 times of elution. These results indicate that the gel can be used as a practical adsorbent for ciprofloxacin in aqueous solution.
Introductıon
With the development of industry and society, environmental pollution has become increasingly serious. Ciprofloxacin is one of fluoroquinolone antibacterial agent and many human lives have been saved since its introduction in the 1980s [1]. However, ciprofloxacin was detected in the aquatic environment with the level of several hundred ng/L [2-6]. The treatment methods of ciprofloxacin pollution include adsorption [7], electrocoagulation [8], photo-Fenton oxidation process [9], ozonation [10], nanofiltration [11]. Due to simplicity, easy operation, regeneration and economic feasibility, adsorption has become a widely studied and applied method in waste water treatment [12,13]. The adsorption property of absorbent is dominated by the physical and chemical properties, which include pore size distribution, specific surface area and the species and density of their functional groups [14].
Polyethylene Glycol Diglycidyl Ether (PEGDE) is well known of their reactivity to amino groups. It is widely used in the synthesis and preparation of polymer materials. Hiroyuki Kono and coworkers prepared a series of water-insoluble cyclodextrin polymers by crosslinking β-cyclodextrin with PEGDE, and the polymers displayed high encapsulation abilities toward bisphenol A in aqueous media [15]. Ali H. Jawad and coworkers prepared a cross-linked chitosan-ethylene glycol diglycidyl ether biofilm, whose maximum adsorption capacities of Reactive Red 120 and Methyl Orange were 165.3 mg/g and 131.2 mg/g, respectively [16]. Therefore, PEGDE can be considered as a kind of material for preparing porous adsorbent with good adsorption capacity. He and co-workers prepared a highly swellable hydrogel using aminoglucan crosslinked with ethylene glycol diglycidyl ether by an epoxy-ring opening reaction under alkaline conditions. The obtained EGDE-crosslinked aminoglucan hydrogels were highly swellable in water owing to a strong water-holding ability and no water was released on compression and breaking of the gels [17]. Using PEG Diglycidyl Ether (PEGDE) cross-linked with a low-molecular-weight diamine and a polyamine-based ionic liquid, Dai et al developed a new family of poly(ethylene glycol) (PEG)-based membranes for CO2 separation in a solvent-free process. The gas permeation results showed that the free PEG dimethyl ether additive acts as plasticizer in the polymeric matrix, resulting in excellent CO2/N2 and CO2/CH4 separation properties [18].
Herein, a porous adsorbent PEGDE-Hypoxanthine Gel (HEG) was designed and prepared by using the PEGDE as the framework, and hypoxanthine-a kind of nitrogen heterocyclic alkaloids as a functional monomer while triethylenetetramine as a crosslinker. The FT-IR, SEM, XRD and BET were performed to study its structure and micromorphology. Besides, the adsorption properties of the HEG toward ciprofloxacin in aqueous solution were investigated and analyzed, including effect of pH (2 to 12), adsorption isotherm, adsorption kinetics and the reusability of HEG towards ciprofloxacin. Moreover, the mechanism was also discussed.
Materials and methods
Materials
Hypoxanthine, polyethylene glycol diglycidyl ether with average molecular weight of 500 and ciprofloxacin were purchased from Aladdin Biochemical Technology Co., Ltd (Shanghai, China). Triethylenetetramine, Hydrochloric Acid (HCl), Sodium Hydroxide (NaOH) and Dimethyl Sulfoxide (DMSO) were purchased from Macklin Biochemical Technology Co., Ltd (Shanghai, China). All of the materials were of analytical grade and used as received. Deionized water made in laboratory was used to prepare all solutions.
Fabrication of the HEG
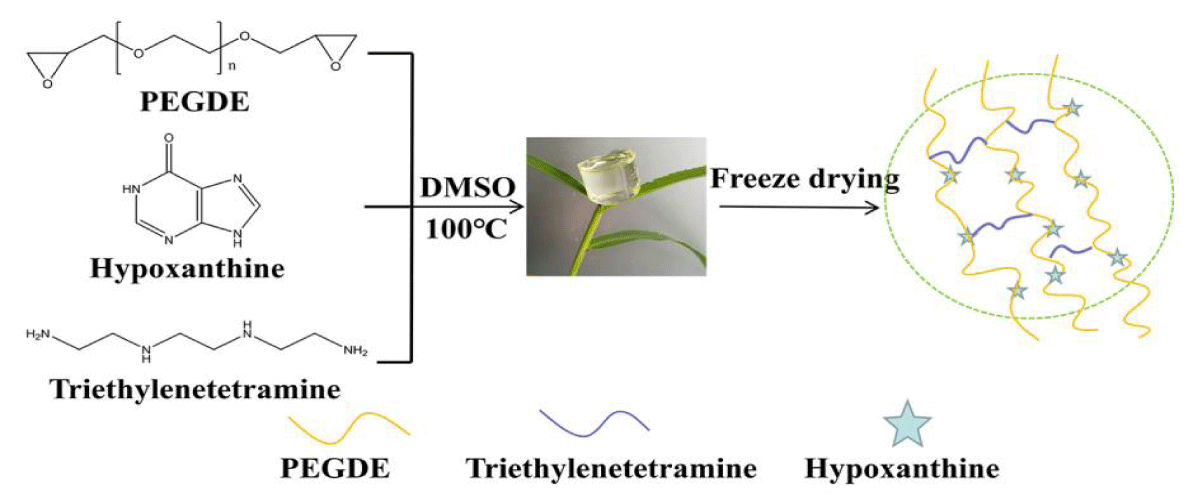
The HEG was prepared by dissolving 0.3267 g hypoxanthine in 5.17 g DMSO, then 3 g of PEGDE and 0.117 g of triethylenetetramine were added. The uniform transparent liquid was obtained by stirring, then cured at 100°C and aged at 140°C for 6 hours to form a gel. The gel was soaked in deionized water for 7 days at atmospheric temperature, and the deionized water was replaced in time to remove the dissolved and unreacted components. Finally, the HEG was obtained by freeze-drying method (Figure 1).
Characterization
The ZEISS sigma 500 Scanning Electron Microscope (SEM) was used to observe the morphology and microstructure of HEG. KBr particles was used to test the chemical structure of the gel on Nicolet IS5 Fourier Transform Infrared Spectroscopy (FT-IR) and X-ray Diffraction (XRD) spectrometer (Panaco X’ Pert PRO). The pore structure and specific surface area of the HEG were analysed on the Micromeritics ASAP2020 BET specific surface area and pore size analysis. Quantitative analysis of ciprofloxacin in solution was conducted on a Shimadzu UV-Vis spectrophotometer.
Adsorption experiment
A batch of adsorption experiments were carried out systematically to evaluate the adsorption capacity of the gel towards ciprofloxacin, including different initial pH, temperature, adsorption time, initial concentration and reusability of HEG. All the adsorption tests were performed on a digital water bath oscillator and conducted three times in parallel with 10 mL ciprofloxacin solution and 10 mg HEG. After reaching the adsorption saturation time, the concentration of ciprofloxacin ions was determined after filtration with 0.22 micron nylon membrane.
In the initial pH study, 0.1 M HCl and 0.1 M NaOH solution were used to adjust the pH of the ciprofloxacin solution to the required range of 2 to 12, and then add 10 mg of gel into 10 mL 100 mg/L ciprofloxacin solution sample bottle and then fully mix and react at 30°C for 24 h. The calculation formula of adsorption capacity (qe, mg/g) is as follows:
(1)
where C0 and Ce are the initial and final concentration of ciprofloxacin (mg/L), respectively. V is the volume (L) of the ciprofloxacin solution, and m is the weight (g) of the absorbent. Linear regression analysis was used to verify the adsorption results.
The adsorption kinetics were carried out with the initial concentration of 100 mg/L at 30°C. After adding 10 mg of HEG, samples were taken at intervals and tested for a certain period of time until the reaction reaches 420 minutes. The Pseudo-first-order, Pseudo-second-order and Intra-particle diffusion adsorption kinetics models were used to describe the ciprofloxacin adsorption kinetic using the following Eqs. (2), Eqs. (3) and Eqs. (4), respectively:
(2)
(3)
(4)
Among them, k1, k2 and ki are the Pseudo-first-order, Pseudo-second-order and internal diffusion rate constant respectively, while qt and qe (mg/g) are the adsorption amount of the HEG at time t and equilibrium time, respectively.
The adsorption isotherms for ciprofloxacin solution were established by initial concentrations ranging from 25 mg/L to 200 mg/L at 25°C, 30°C and 35°C, respectively. Then mixture was blent well and react at 30°C for 24 h. Among the adsorption isotherm models, the Langmuir and Freundlich models are the most widely used. The Freundlich model is suitable for the adsorption of uneven surfaces, while the Langmuir model is suitable for the adsorption of uniform surfaces. Herein, Langmuir isotherm and Freundlich isotherm model were used to investigate the adsorption isotherms, in order to analyze the adsorption form energy of HEG and explore its adsorption mechanism. They are expressed as the following Eqs. (5) and Eqs. (6):
(5)
(6)
Among them, qe and qmax (mg/g) are the equilibrium and maximum adsorption capacity, respectively, Ce (mg/L) is the equilibrium concentration of ciprofloxacin, and b (L/mg) is the Langmuir adsorption constant related to the free energy of adsorption. KF (mg/g) is related to the affinity of the adsorbent to the adsorbate and n is a constant.
With the concentration of the ciprofloxacin solutions were 25 mg/L to 200 mg/L, temperature from 298 to 308 K, reacted for 24 h, the adsorption thermodynamics calculation of ciprofloxacin adsorption experiment was investigated and the the three thermodynamic parameters gibbs free energy (ΔG), enthalpy change (ΔH) and entropy change (ΔS) were determined as following Eqs.(7) and Eqs. (8):
(7)
(8)
where is the thermodynamic equilibrium constant, T (K) is the adsorption temperature, and R (8.314 J/mol K) is the gas constant. For the desorption-resorption test, the ciprofloxacin loaded HEG was soaked in 0.2 M NaOH solution to desorb ciprofloxacin and then washed several times with deionized water, after that, the HEG was dried to constant weight in 60°C oven under normal pressure.
Results and Discussion
Characterization of HEG
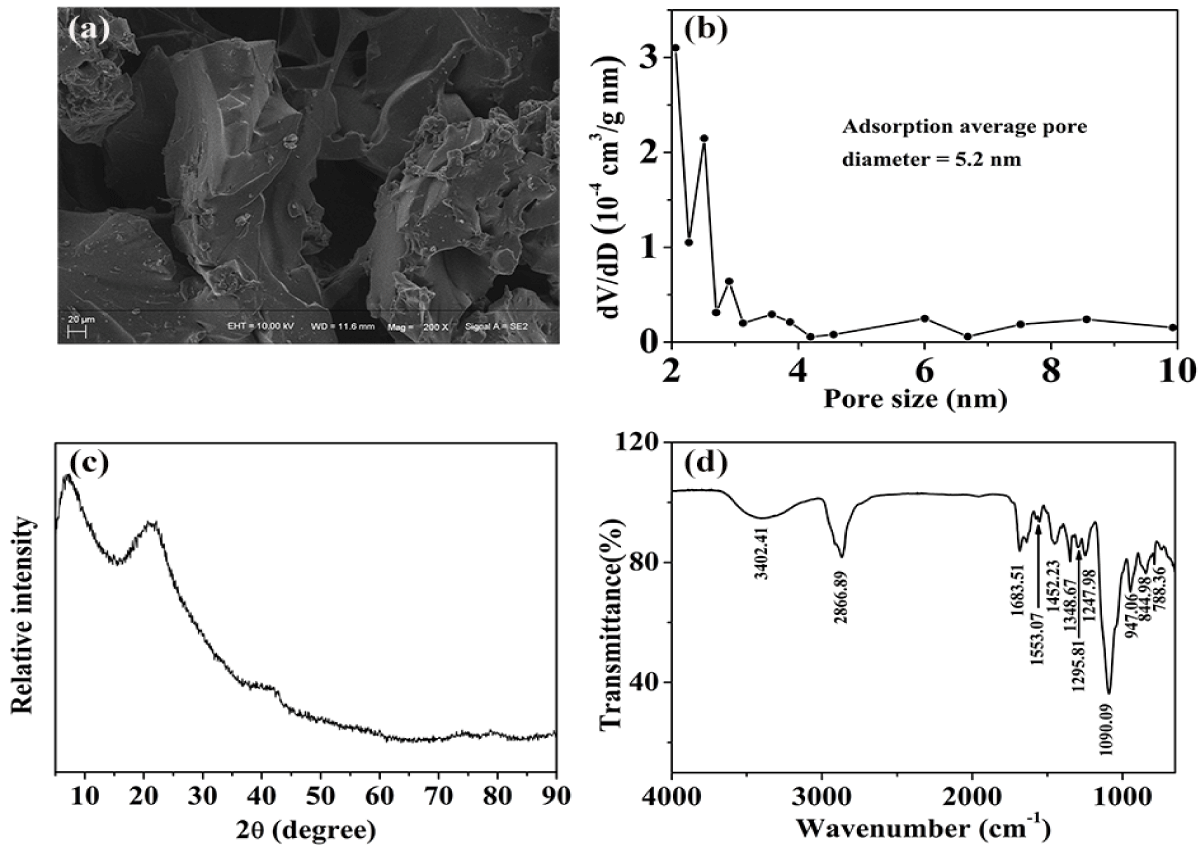
The prepared HEG was characterized by FT-IR, SEM, XRD and BET, and the obtained results are shown below. As shown in figure 2a, HEG has obvious plicated structure and multilevel pore structure, which is beneficial to increase the adsorption. According to the results of the BET test (Figure 2b), the HEG has langmuir surface area of 1.34 m²/g and adsorption average pore diameter of 5.2 nm. The crystallinity can affect its chemical activity and absorption properties. The XRD spectrum was shown in figure 2c, HEG shows only two broad diffraction signals, clearly suggesting the amorphous properties which was beneficial to increase the contact areas with ciprofloxacin. In the FT-IR spectrum (Figure 2d), the HEG shows a broad peak near 3402.41 cm-1, which is a stretching vibration of -OH. Shown at 2866.89 cm-1 is the stretching vibration of -CH2 and -CH-. The peak at 1683.51 cm-1 is assigned to the skeleton vibrations of the aromatic ring, which proves that the existence of hypoxanthine. The adsorption peak at 1452.23 cm-1 is ascribed to the bend vibration of -CH2- and -CH- while the peaks at 1348.67-1247.98 cm-1 are belong to the stretching vibration of C-O and C-N. The characteristic absorption peak of ether is displayed at 1090.09 cm-1.
Adsorption experiment
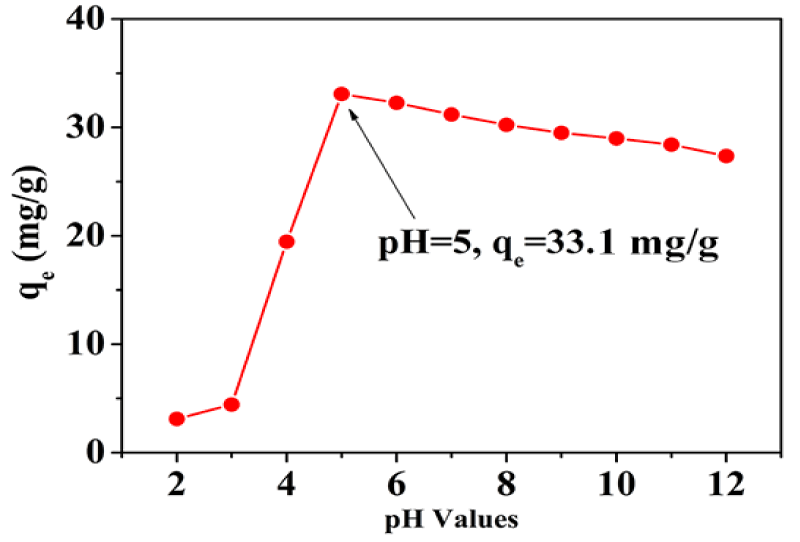
The effect of pH: The initial pH of ciprofloxacin aqueous solution is one of the most important factors that would significantly influence the adsorption of HEG. The adsorption of the ciprofloxacin was investigated at pH 2 to 12 with initial concentration of 100 mg/L at 30°C, as shown in figure 3. It is obvious that the adsorption capacity of HEG toward ciprofloxacin increased and then decreased with the pH value of the solutions changing from 2 to 12. When pH less than or equal to 3, HEG has a poor removal effect on ciprofloxacin, which is due to the protonation of N and O, the hydrogen bond acceptor, under highly acidic conditions. With the increasing pH value, the adsorption of HEG gradually increased as the enhanced interaction between ciprofloxacin and HEG. The maximum adsorption amount of HEG toward ciprofloxacin has reached 33.1 mg/g at pH = 5. However, when the pH value of the solution exceeds this value, the adsorption immediately reduced with the increasing degree of deprotonation which is not conducive to hydrogen bond. The experimental results show that the hydrogen bond between HEG and ciprofloxacin plays a decisive role in the adsorption performance. Base on the above analyses, the pH value of 5 corresponding to the maximum adsorption capacity was selected for subsequent experiments.
The adsorption kinetics: The adsorption kinetics was investigated and the the main influencing factors of the adsorption rate and the speed-limiting steps were also discussed. As we all known, the adsorption process can be divided into three steps: External diffusion, internal diffusion and surface adsorption. Adsorption kinetics mainly discusses the diffusion performance of the adsorbate on the inner and outer surfaces of the adsorbent, the main influencing factors of the adsorption rate and the speed-limiting steps. The rate of the slowest stage determines the total rate of adsorption. Because the adsorption process on the inner surface of the micropores is faster than the liquid membrane diffusion process and the diffusion process in the adsorbent micropores, the adsorption rate mainly depends on the diffusion rate of the adsorbate inside and outside the adsorbent particles. The relevant parameters of adsorption kinetics are important indicators of the adsorption process and cannot be ignored in the design of the adsorption and separation process. Currently, Pseudo-First-Order kinetic model (PFO), Pseudo-Second-Order kinetic model (PSO) and Intra-Particle Diffusion model (IPD) are most commonly used in the adsorption of ciprofloxacin. By comparing the fitting results of different equations, an appropriate model was chosen, as preliminary judgment of the adsorption mechanism.
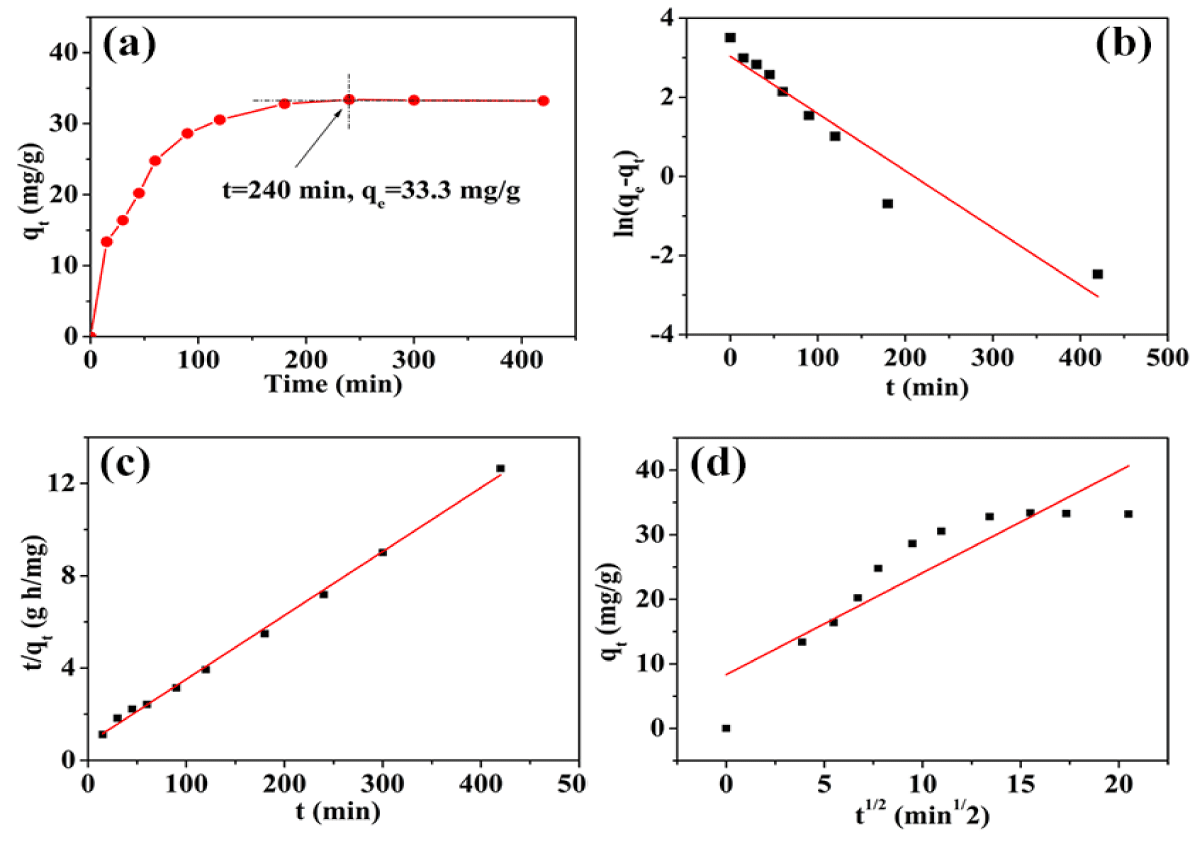
The parameters and plots of the adsorption kinetics results were shown in table 1 and figure 4, respectively. In the first 90 min, the adsorption rate of the gel increased significantly, and the adsorption capacity reached 33.3 mg/g after 240 min. The R2 value of PSO is 0.9970, which is higher than PFO and IPD, with the values are 0.9232 and 0.7939, respectively. The saturation adsorption capacity calculated by PSO simulation is in good agreement with the experimental data, which are 36.1 mg/g and 33.3 mg/g, respectively. Therefore, the PSO model was considered to be the best fitting model for the experimental kinetic data, which indicates that both physical and chemical adsorptions existed in the adsorption process, and the chemisorption was the limiting step in the adsorption of ciprofloxacin by HEG.
| Table 1: Relevant parameters of the kinetic model of ciprofloxacin adsorption on HEG. | |||||||||
| qe (mg/g) | PFO | PSO | IPD | ||||||
| k1 | qe | R2 | k2 | qe | R2 | ki | C | R2 | |
| 33.3 | 0.0145 | 20.8 | 0.9232 | 0.0010 | 36.1 | 0.9970 | 1.5775 | 8.3292 | 0.7939 |
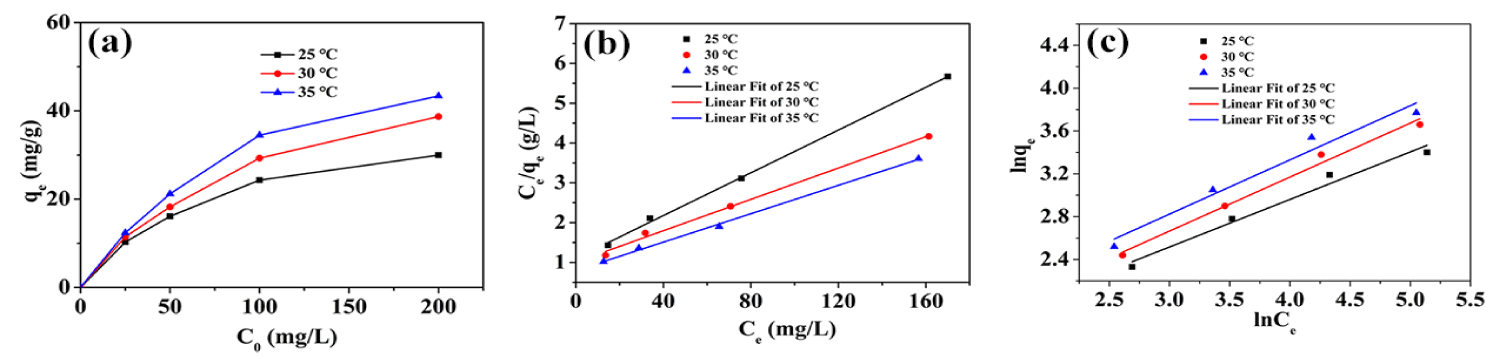
The adsorption isotherms: The adsorption isotherms experiments were also investigated, which included the effect of different initial concentrations of ciprofloxacin on the adsorption at different temperatures. The results are shown in the figure 5. In the process of increasing the temperature from 25°C to 35°C, the maximum adsorption capacity of gels with different initial concentrations increased as the temperature increased. This may be because the HEG adsorption of ciprofloxacin is mainly a chemical adsorption process. Increasing the temperature will improve the activity of the adsorption sites on the gel surface to promote the adsorption reactions.
| Table 2: Langmuir and Freundlich model related parameters for the adsorption of ciprofloxacin on HEG. | ||||||
| T(K) | Langmuir constants | Freundlich constants | ||||
| b | qmax | R2 | 1/n | KF | R2 | |
| 298 | 0.0244 | 37.2 | 0.9979 | 0.4439 | 3.27 | 0.9670 |
| 303 | 0.0195 | 50.8 | 0.9939 | 0.5044 | 3.16 | 0.9826 |
| 308 | 0.0224 | 56.1 | 0.9973 | 0.5064 | 3.69 | 0.9521 |
From the correlation coefficient (R2), compared with the Freundlich model, the Langmuir model is more suitable for description of adsorption. In addition, the maximum adsorption capacity (qmax) increases with the increase of temperature, indicating that higher temperature was beneficial to the adsorption of ciprofloxacin on HEG, and the adsorption process is endothermic. The maximum equilibrium adsorption capacity of ciprofloxacin is 56.1 mg/g at 308 K according to the Langmuir model.
Adsorption thermodynamics: The adsorption thermodynamics experiments were carried out to investigate the the extent and driving force of the adsorption process and try to explain the reasons for the influence of various factors on adsorption. In solid-liquid adsorption, the heat of adsorption is the enthalpy change of the reaction, which is the comprehensive result of the energy change during the adsorption process, and can distinguish between chemical adsorption and physical adsorption.
Plot the logarithm of against the reciprocal of temperature, a straight line can be obtained, and the adsorption thermodynamics can be obtained according to the slope and intercept of the linear equation.
The negative value of ΔG decreases with increasing temperature, indicating that adsorption was a spontaneous process, and higher temperature was more conducive to adsorption. The enthalpy value (ΔH) was calculated to be 25.96 KJ/mol, which was positive, indicating that the adsorption was an endothermic process. The exothermic adsorption process is usually physical adsorption or chemical adsorption, while the endothermic process is chemical adsorption. Therefore, the positive value of ΔH further confirms that the adsorption of ciprofloxacin is chemical adsorption, rather than physical adsorption. The value of ΔS is calculated to be 0.083 KJ/mol, which was a positive value indicating that the prepared gel had a good affinity for ciprofloxacin.
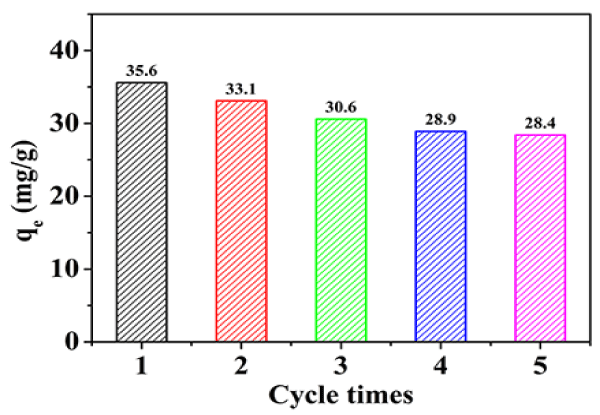
Cyclic adsorption: Reusability is important to reduce the whole cost of HEG in the practical application. The reusability experiments were performed with 200 mg/L ciprofloxacin solution at 35°C. The adsorbed HEG was eluted by dilute hydrochloric acid solution and then repeatedly adsorbed and the results is shown in Figure 6. Although the adsorption capacity of HEG for ciprofloxacin decreased with the increase of repeated experiments, it still maintained 80% of the adsorption capacity. The reusability and simple preparation method of HEG make it a practical adsorbent in ciprofloxacin waste water treatment.
| Table 3: ΔG of ciprofloxacin adsorption on HEG. | |||
| T(K) | 298 | 303 | 308 |
| ∆G (KJ/mol) | -25.06 | -25.50 | -25.92 |
Conclusion
In summary, a hypoxanthine modified polyethylene glycol Diglycidyl Ether Gel (HEG) was prepared by the ring opening polymerization of PEGDE with hypoxanthine in a simple sol-gel method. The structure and composition of the HEG were characterized by SEM, FTIR, BET and XRD. The adsorption experiments of ciprofloxacin by HEG at different pH, temperature, contact time and initial concentration were studied. The results showed that the HEG was mesoporous with the average pore size of 5.2 nm, the optimum pH was 5 and the saturated adsorption time was 240 minutes. The adsorption kinetics experiment is best fit with the quasi second order kinetic model well, while adsorption isotherms prefer to Langmuir model than Freundlich model and the adsorption thermodynamics indicate that the adsorption process is endothermic and spontaneous. The maximum equilibrium adsorption capacity of ciprofloxacin is 56.1 mg/g at 308 K according to the Langmuir model. The results of repeated adsorption experiments showed that HEG could still adsorb 80% of the first adsorbed ciprofloxacin after 5 times cycle. These results indicate that the HEG can be used as a practical adsorbent for ciprofloxacin in aqueous solution.
Acknowledgements
This work was supported by the Laser Fusion Research Center Funds for Young Talents (no. RCFCZ1-2017-16), and Applied Basic Research Program of Sichuan Province (no. 2019YJ003).
References
- Picó Y, Andreu V. Fluoroquinolones in soil--risks and challenges. Anal Bioanal Chem. 2007 Feb;387(4):1287-99. doi: 10.1007/s00216-006-0843-1. Epub 2006 Nov 3. PMID: 17082879.
- Golet EM, Alder AC, Giger W. Environmental exposure and risk assessment of fluoroquinolone antibacterial agents in wastewater and river water of the Glatt Valley Watershed, Switzerland. Environ Sci Technol. 2002 Sep 1;36(17):3645-51. doi: 10.1021/es0256212. PMID: 12322733.
- Karthikeyan KG, Meyer MT. Occurrence of antibiotics in wastewater treatment facilities in Wisconsin, USA. Sci Total Environ. 2006 May 15;361(1-3):196-207. doi: 10.1016/j.scitotenv.2005.06.030. Epub 2005 Aug 8. PMID: 16091289.
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999-2000: a national reconnaissance. Environ Sci Technol. 2002 Mar 15;36(6):1202-11. doi: 10.1021/es011055j. PMID: 11944670.
- Miao XS, Bishay F, Chen M, Metcalfe CD. Occurrence of antimicrobials in the final effluents of wastewater treatment plants in Canada. Environ Sci Technol. 2004 Jul 1;38(13):3533-41. doi: 10.1021/es030653q. PMID: 15296302.
- Renew JE, Huang CH. Simultaneous determination of fluoroquinolone, sulfonamide, and trimethoprim antibiotics in wastewater using tandem solid phase extraction and liquid chromatography-electrospray mass spectrometry. J Chromatogr A. 2004 Jul 9;1042(1-2):113-21. doi: 10.1016/j.chroma.2004.05.056. PMID: 15296395.
- Genc N, Dogan E C. Adsorption kinetics of the antibiotic ciprofloxacin on bentonite, activated carbon, zeolite, and pumice. Desalin Water Treat. 2015;53(3):785-793. https://bit.ly/2QztHff
- Ahmadzadeh S, Asadipour A, Pournamdari M, Behnam B, Rahimi H R, Dolatabadi M. Removal of ciprofloxacin from hospital wastewater using electrocoagulation technique by aluminum electrode: optimization and modelling through response surface methodology. Process Saf Environ Prot. 2017;109:538-547. https://bit.ly/3lX7ovF
- Sun SP, Guo HQ, Ke Q, Sun JH, Shi SH, Zhang ML, et al. Degradation of antibiotic ciprofloxacin hydrochloride by photo-Fenton oxidation process. Environ Eng Sci. 2009;26(4):753-759. https://bit.ly/3lRKg1r
- De Witte B, Van Langenhove H, Demeestere K, Saerens K, De Wispelaere P, Dewulf J. Ciprofloxacin ozonation in hospital wastewater treatment plant effluent: effect of pH and H2O2. Chemosphere. 2010 Feb;78(9):1142-7. doi: 10.1016/j.chemosphere.2009.12.026. Epub 2010 Jan 13. PMID: 20074775.
- Sun SP, Hatton TA, Chung TS. Hyperbranched polyethyleneimine induced cross-linking of polyamide-imide nanofiltration hollow fiber membranes for effective removal of ciprofloxacin. Environ Sci Technol. 2011 May 1;45(9):4003-9. doi: 10.1021/es200345q. Epub 2011 Apr 1. PMID: 21456576.
- Babel S, Kurniawan TA. Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater. 2003 Feb 28;97(1-3):219-43. doi: 10.1016/s0304-3894(02)00263-7. PMID: 12573840.
- Ademoriyo CO, Enyoh CE. Batch Adsorption Studies of Sunset Yellow and Tartrazine Using Coconut and Groundnut Shells. J Biomed Res Environ Sci. 2020;1(5):163-172. https://bit.ly/3lRoZoP
- Gupta VK, Suhas. Application of low-cost adsorbents for dye removal--a review. J Environ Manage. 2009 Jun;90(8):2313-42. doi: 10.1016/j.jenvman.2008.11.017. Epub 2009 Mar 4. PMID: 19264388.
- Kono H, Nakamura T, Hashimoto H, Shimizu Y. Characterization, molecular dynamics, and encapsulation ability of β-cyclodextrin polymers crosslinked by polyethylene glycol. Carbohydr Polym. 2015 Sep 5;128:11-23. doi: 10.1016/j.carbpol.2015.04.009. Epub 2015 Apr 18. PMID: 26005135.
- Jawada AH, Mamata NFH, Hameedb BH, Ismail K. Biofilm of cross-linked Chitosan-Ethylene Glycol Diglycidyl Ether for removal of Reactive Red 120 and Methyl Orange: Adsorption and mechanism studies. J Environ Chem Eng. 2019;7:102965. https://bit.ly/2P5peAG
- He Q, Kusumi R, Kimura S, Kim UJ, Deguchi K, Ohki S, Goto A, Shimizu T, Wada M. Highly swellable hydrogel of regioselectively aminated (1®3)-α-d-glucan crosslinked with ethylene glycol diglycidyl ether. Carbohydr Polym. 2020 Jun 1;237:116189. doi: 10.1016/j.carbpol.2020.116189. Epub 2020 Mar 16. PMID: 32241412.
- Dai Z, Ansaloni L, Gin DL, Noble RD, Deng L. Facile fabrication of CO2 separation membranes by cross-linking of poly(ethylene glycol) diglycidyl ether with a diamine and a polyamine-based ionic liquid. J Membrane Sci. 2017;523:551-560. https://bit.ly/3fi6wQX
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.