> Medicine Group. 2021 Mar 18;2(3):185-192. doi: 10.37871/jbres1208.
Potential Use of Quercetin as Protective Agent against Hydroxychloroquine Induced Cardiotoxicity
Mona G Amer1* and Nader M. Mohamed2
2Department of Pediatrics and Neonatology. Taif University School of Medicine, Taif, Saudi Arabia & General organization of teaching Hospitals and institutions, Egypt
- Hydroxychloroquine
- Curvilinear bodies
- Cardioprotection
- Quercetin
Abstract
The aim of this study is to investigate the protective effects of Quercetin (QCT) on Hydroxychloquine (HCQ)-induced myocardial affection in rats. HCQ has been found to produce toxic effects including cardiac manifestation. Adding QCT to HCQ ameliorates its effects and prevents cardiac manifestations. For this purpose, eighty adult male rats were divided into four groups (n = 20). Group 1 (control) and group 2 (QCT-treated). Group 3 (HCQ treated) received 20 mg/kg of HCQ and group 4 (QCT + HCQ treated) received quercetin (50 mg/kg; orally) combined with HCQ for 4 weeks. Cardiac troponin-I and oxidative markers (Malondialdehyde (MDA), and total serum antioxidant) were estimated in serum. In addition, histopathological and morphometric changes of the rat heart were assessed. The HCQ treated group showed increased serum levels of cardiac troponin-I, MDA and decreased serum levels of total antioxidant. Pathological picture of myocardial hypertrophy and degeneration together with depleted cardiac tissue expression of troponin T were also observed. The characteristic features were presence of whorled myelin bodies and curvilinear bodies by EM examination. These parameters improved better in the group receiving combination of QCT together with HCQ. So, Adding QCT to HCQ could be prophylactic measure against its cardiotoxic effect compared with HCQ treatment alone.
Introduction
Chloroquine (CQ) and Hydroxychloroquine (HCQ) are well known as antimalarial agents. They have similar chemical structure and mechanisms of action with less toxic effect of HCQ. They have been widely used for treatment of rheumatological disease and other connective tissue disorders due to its anti-inflammatory properties [1].
HCQ has anti-viral effects detected in animal models and cell cultures and has been proposed for the treatment of different viral infections. Currently, the scientific community is actively exploring treatments that would potentially be effective in combating COVID-19 which caused a pandemic Severe Acute Respiratory Syndrome Caused by Coronavirus 2 (Sars-Cov-2) infection that is spreading all over the world and raised great public health concerns [2,3]. Hydroxychloroquine as an anti-inflammatory drug has been determined to limit the in vitro replication of SARS-CoV-2 virus. The results enforce the possibility of the efficient of these drugs in COVID-19 treatment. CQ and HCQ are now being tested in clinical trials to assess their effectiveness to combat this global health crisis. Chloroquine and hydroxychloroquine have antiviral characteristics in vitro [4].
The recognized side effects of CQ/HCQ include retinal, neuromyopathic, and cardiac toxicity that are increased with cumulative dose. Specifically, both CQ and HCQ can cause direct myocardial toxicity and exacerbate underlying cardiac dysfunction [5].
Quercetin (Quer; 3',3',4',5,7-pentahydroxyflavone) (QCT) is a naturally essential flavonoids of human diet [6]. It is distributed widely in edible parts of all the plant products such as- roots, bulbs, tubers, leafy vegetables, fruits, tea and cocoa [7]. This flavonoid was found to be a potent antioxidant, anti-inflammatory, anti-blood coagulating, anti-tumoral, anti-apoptotic and an anti-aging biomolecule by Boots, et al. [8] and Kumar Mishra, et al. [9]. They have also claimed the protective effect of QCT on hepatotoxicity induced by CQ therapy. Moreover, QCT and its metabolites exert strong cardioprotective effects in a wide range of experimental models of cardiac injury, likely via their antioxidant, anti-inflammatory and molecular pathways-modulating properties [10].
Although cases with cardiac problems were recorded with HCQ therapy, little was known on the actual cause of associated cardiotoxicity and if the effect is related to HCQ or the underlying disease. So, routine screening of cardiotoxicity is less accepted by cardiology or rheumatology society guidelines as it is not recognized with HCQ therapy. So, the aim of this work is to investigate the toxic effect of HCQ on cardiac structure and function of male albino rats and if QCT has a protective role on HCQ induced toxicity.
Materials and Methods
This study was conducted according to the Institute Review Board Instruction of Care and Use of Laboratory Animals [11]. All experimental stratrgies were approved and implemented in agreement with the guidelines adopted by Faculty of Medicine, Zagazig University, Egypt.
The preliminary studies, animal acclimatization, drug procurement, actual animal experiment and evaluation of results, lasted for a period of two months (March and April, 2020).
Animals
Male albino rats (adult Wistar; weighing about 250 gm) were obtained from animal house of Faculty of Medicine, Zagazig University, Egypt. They were housed under standard laboratory conditions (Tm; 24 ± 2°C and a 12 h day/night cycles). The animals were allowed to access freely to a standard chow diet and water ad libitum. Acclimatization of all animals for 2 weeks was done before starting the experiment for observation of their general health and suitableness for inclusion in the experiment. They were given free. They were randomly assigned into four groups (20 rats each), the negative control group, QCT received group, HCQ treated group and combined HCQ + QCT treated group.
Drug administration
The hydroxyl chloroquine sulphate tablets 200 mg used for this experiment were manufactured by Sanofi Aventis U.S. LLC. Quercetin (3',3'5',7-pentoxyflavone) was purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Rats in the HCQ treated group and combined group received 20 mg/kg body weight of hydroxyl chloroquine sulphate dissolved in distilled water weekly for two months [12]. Distilled water was given to rats of the control group in equal volume using orogastric tube. Rats of QCT and combined HCQ + QCT groups received orally 50 mg/kg dissolved in 2-ml normal saline orally by an orogastric tube once daily [13] in combination with HCQ. The amount of QCT calculated was selected on the basis of reported recommended daily allowances for human beings which are 2000 mg to 5000 mg per day did not show any evidence of toxicity on human health [14]. However, 20-600 mg/kg BW of Quercetin was reported by Willhite [15] to be safe dose and free of any toxicity.
Systolic Blood Pressure (SBP) and Heart Rate (HR) measurements
Systolic Blood Pressure (SBP) was measured weekly in conscious and pre-warmed rats using tail-cuff plethysmography [16]. In every session, seven determinants were made and the mean of the lowest recorded three values within 5 mmHg was considered as SBP. Calculation of Heart Rate (HR) was done from the obtained physiological tracings during measurements of BP [17].
Collection of blood samples
Before sacrificing, Body Weight (BW) of rats were recorded and venous blood samples were taken from the retro-orbital plexus of rats using heparinized capillary tubes and immediately centrifuged then the sera stored at –80C until analyzed for measurement of Cardiac Troponin I (cTnI) using an enzyme-linked immunosorbent assay kit that was obtained from BioMerieux (MO 63042, St. Louis, USA), for quantitative measurement of cTn-I in serum.
Estimation of serum MDA was done through the spectrophotometric measurement of the color generated because of the reaction of thiobarbituric acid with MDA in acidic medium. Quantification of the total antioxidant capacity in serum was done by the reaction of antioxidants with recognized amount of exogenous hydrogen peroxide provided in the sample. Kits for detection of both total serum antioxidants and MDA were received from Biodiagnostic (Cairo, Egypt).
Then rats were anesthetized by via administration of 80 mg/kg body weight ketamine IP. Subsequently, perfusion fixation of the rats was done with a mix of both aldehydes (2.5% glutaraldehyde, 10% neutral buffered formalin saline and, 0.1 M sodium cacodylate, pH 7.2) at 80 mmHg perfusion pressure increased slowly to 130 mmHg to preserve good perfusion [18,19]. After the end of perfusion, the hearts were took out and excess adipose tissue were eliminated, then heart weight was recorded, and the specimens were processed for histological and immunohistochemical study.
Histological analysis
The paraffin sections of all specimens were prepared and stained with Hematoxylin and Eosin (H & E) study of the general morphology and with Van Gieson's (VG) stain to study collagen [20].
Immunohistochemical reaction was done for detection of troponin T. Avidin–biotin-complex immunoperoxidase system was carried out according to Kiernan [21]. The primary antibody used was Mouse Anti-Human/Mouse/Rat Troponin T (Cardiac) Monoclonal Antibody (Catalog # MAB18742). After that, the slides were hatched with the Anti-Mouse IgG VisUCyte™ HRP Polymer Antibody (Catalog # VC001). Staining was completed by incubation with substrate Chromogen Called Diamiobenzidine (DAB) and Mayer's hematoxylin was used as a counter stain. The primary antibody was replaced by phosphate buffer solution for negative control. The specific brown staining appeared in cardiomyocytes cytoplasm.
Morphometric analysis
In H & E stained section, Myocyte Surface Area (MSA) was calculated and appropriate cross sections were outlined as having almost circular nuclei and capillary profiles [22]. “Leica Quin” image analyser computer system (Leica Imaging System Ltd., Cambridge, England) was used to scan each field. The measuring frame of 7286.78 µm² was used as a standard area. Also, Van Geison stained sections were used for measurement of area % of red areas of collagen fibers in a measuring frame area of 118476. 6 in 10 fields for each specimen. For measurement of MSA and area % of collagen fibers, average of 20 regions was used in each rat of all groups.
Statistical analysis
Mean ± Standard Deviation (SD) was used for presentation of all statistical data. One-way Analysis of Variance (ANOVA) was used to assess differences between groups followed by Post Hoc multiple comparison tests (Least Significant Different Test; LSD) to compare changes among individual groups. The significance level was established at p < 0.05.
Results
Biochemical results
Non-significant difference of biochemical parameters was detected between negative group (1) and QCT received group (2). Serum levels of cTn-I (pg/ml) in HCQ treated group significantly increased compared with control group (16.6 ± 11.1 vs. 3.9 ± 1.0, p < 0.01). Serum levels of cTn-I in the QCT + HCQ combined group significantly decreased compared with the HCQ treated group (5.6 ± 2.3 vs. 16.6 ± 11.1, p < 0.01) (Table 1).
Regarding oxidative stress markers, serum levels of MDA (nmol/ml) in the HCQ treated group significantly increased compared with the control group (8.13 ± 0.14 vs . 1.13 ± 0.04, p < 0.01). Serum levels of MDA in the QCT + HCQ combined group significantly decreased compared with the HCQ treated group (1.54 ± 0.02 vs. 8.13 ± 0.14, p < 0.01) (Table 1).
However, serum levels of total antioxidants (mmol/l) in the HCQ treated group significantly decreased compared with the control group (0.81 ± 0.06 vs. 2.68 ± 0.13, p < 0.01). Serum levels of total antioxidants in the QCT + HCQ combined group significantly increased compared with the HCQ treated group (2.16 ± 0.64 vs. 0.81 ± 0.06, p < 0.01) (Table 1).
| Table 1: Showing serum levels of cardiac Troponin I (cTnI), MDA and total antioxidants of studied groups. Data are represented as mean ± SD. | |||||
| Group (1) Negative control |
Group (2) QCT treated group | Group (3) HCQ treated group |
Group (4) Combined HCQ + QCT | p value (ANOVA) |
|
| Serum troponin cTnI (pg/mL) | 3.9 ± 1.0 | 3.1 ± 1.7a | 16.6 ± 11.1b | 5.6 ± 2.3c | <0.001 |
| Serum MDA (nmol/ml) | 1.13 ± 0.04 | 0.9 ± 2.5a | 8.13 ± 0.14b | 1.54 ± 0.02c | <0.001 |
| Total serum antioxidants (nmol/ml) | 2.68 ± 0.13 | 2.76 ± 0.14a | 0.81 ± 0.06b | 2.16 ± 0.64c | <0.001 |
| a) Non-significant relative to group (1) b) Significant relative to group (1) c) Significant from group (3) |
|||||
Measurement of SBP, HR and morphometric results of all studied groups
Calculation of HW/BW ratio and MSA by morphometric study revealed myocardial hypertrophy. Significant increase in in HW/BW ratio and in MSA was detected in HCQ treated group when compared with control group (p < 0.01) table 2. Moreover, significant decrease in both parameters was detected in HCQ + QCT combined group when compared with HCQ treated group (p < 0.01) table 2. Also, HR was significantly decreased and SBP was significantly increased in HCQ treated group when compared with control and with HCQ + QCT combined groups (p < 0.01) (Table 2).
| Table 2: Showing heart weight to body Weight ratio (HW/BW), SBP, heart rate and histomorphometric results of studied groups at time of sacrifice. Data are represented as mean ± SD. | |||||
| Group (1) Negative control |
Group (2) QCT treated group | Group (3) HCQ treated group | Group (4) Combined HCQ+ QCT | p value | |
| HW/BW (x10-3) | 1.54 ± 0.12 | 1.5 ± 0.06a | 2.9 ± 0.8b | 1.8 ± 0.6c | <0.001 |
| SBP (mmHg) | 123 ± 3.9 | 121 ± 5.9a | 164 ± 5.2b | 135 ± 3.8c | <0.001 |
| HR (beat/minute) | 188 ± 3 | 186 ± 6a | 156 ± 9b | 177 ± 6c | <0.001 |
| MSA (µm2) | 198.9 ± 14.8 | 201.1 ± 12.5a | 329 ± 24.2b | 221.6 ± 5.8c | <0.001 |
| Area% of collagen | 1.96 ± 1.02 | 1.81 ± 1.17a | 8.01 ± 0.05b | 2.8 ± 0.6c | <0.001 |
| Area % of troponin T immune expression | 7.9 ± 0.99 | 8.1 ± 0.48a | 3.81 ± 0.18b | 6.8 ± .78c | <0.001 |
| a) non-significant relative to group (1) b) significant relative to group (1) c) significant from group (3) |
|||||
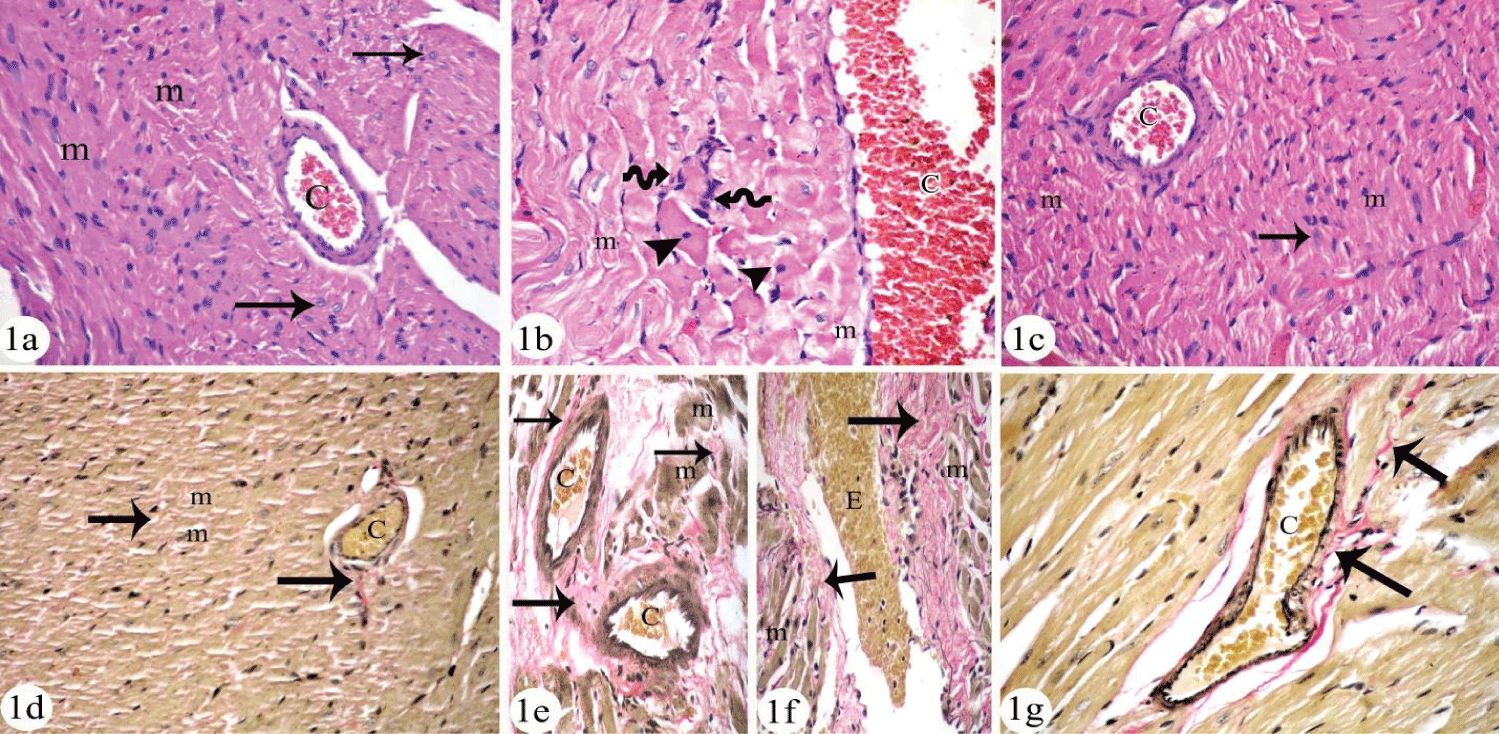
Histological results
Cardiomyocyte of negative control group and QCT treated group appeared with no variations when examined by both light and electron microscope examination. So, figures for negative control group were presented as control. Cardiomyoctes of control group appeared by light microscope examination as polyhedral cells with acidophilic cytoplasm and central nuclei (Figure 1a). On the other hand, cardiomyocyte of HCQ treated group appeared swollen, disorganized and some of them had apoptotic nuclei. Blood capillaries appeared congested and interstitial connective tissue cells were prominent (Figure 2b). The interstitial spaces appeared to have extravasated RBCs and increased connective tissue cells by VG staining. The interstitial spaces were wide and had perivascular fibrosis (Figure 1e). Meanwhile, there was an increase in the fibrous tissue in-between the cardiomyocytes by VG stain (Figure 1b) that was approved quantitatively in table 2 where significant increase in area% of collagen fibers in HCQ treated group when compared to control group was observed (p < 0.01).
Adding QCT to HCQ in combined group (4) showed preservation of cardiac structure in H & E stained section (Figure 1c) and decreased collagen fibers by VG stained section (Figure 1f). Morphometric analysis confirmed the results and significant decrease in area % of collagen fibers was recorded when compared with HCQ treated group (Table 2).
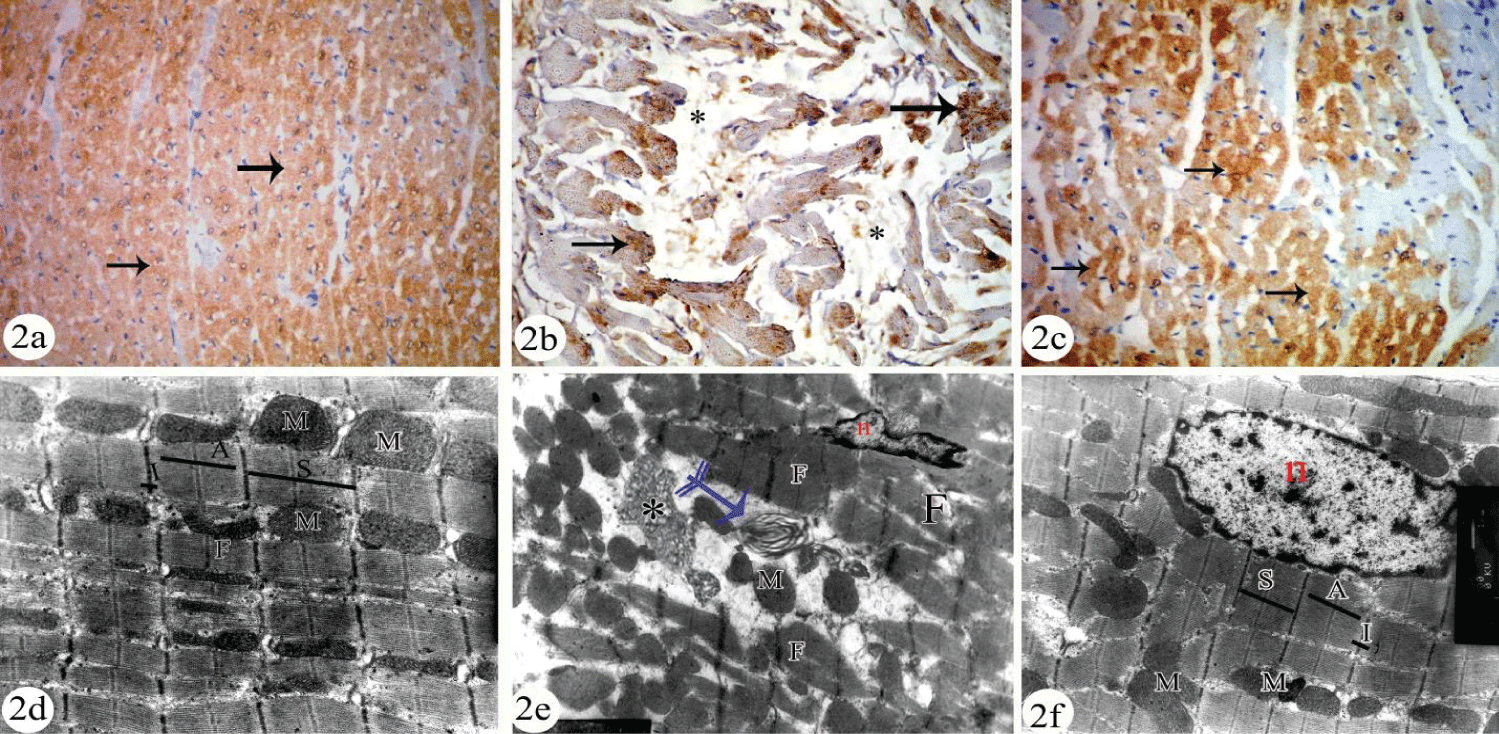
Immunohistochemical analysis of T- troponin revealed apparently decreased cytoplasmic reaction in cardiomycytes of HCQ treated group (Figure 2b) when compared with control (Figure 2a) and combined HCQ + QCT group (Figure 2c). The results were approved quantitatively by measurement of area% of T- troponin immune expression in table 2.
By electron microscope examination, cardiomyocytes of the control group appeared to have sarcoplasm that contains longitudinally arranged myofibrils with mitochondria arranged in rows in between them. Each myofibril consists of successive dark (A) and light (I) bands. Each I band is bisected by dark Z-line and sarcomere is located between two successive Z lines (Figure 2d).
Cardiomyocytes of HCQ treated group were in the form of signs of cellular degeneration. These were disorganized myofibrils and presence of concentric lamellar or myelin bodies and curvilinear bodies. The intercellular spaces appeared to have numerous extravasated RBCs while mitochondria were aggregated in-groups in-between the myofibrils (Figure 2e). Adding QCT to HCQ in the combined group induced prevention of cardiac degeneration. Ultrastructure features of cardiomyocyte of QCT protected group were apparently normal (Figure 2f).
Discussion
Cases of cardiotoxicity have been reported in the literature with HCQ therapy, although less than half of these have been proven on endomyocardial biopsy [23]. Despite increasing recognition, CQ/HCQ cardiotoxicity remains less recognized, and its routine screening is currently not recommended by rheumatology or cardiology society guidelines [24,25]. So, the aim of this study is to investigate the possible toxic effect of HCQ on cardiac structure and function and the possible protective role of QCT when combined with HCQ.
Different structural and functional changes were detected in HCQ treated group of the current study. Signs of myocardial hypertrophy and fibrosis were found in histological and morphometric analysis of HCQ treated rats. Hypertension and heart rate affection were significantly recorded after HCQ treatment. These rats had serum levels of cTnI were significantly higher than the control group. Immunohistochemical expression of troponin T within cardiac muscle revealed significant decrease with HCQ treatment when compared with control.
Similar histological findings were reported in endomyocrdial biopsy of patients treated with HCQ by Joyce, et al. [26]. They found that HCQ induced enlargement and vacuolation in cardiomyocytes. In the current study, presences of myeloid and Curvilinear Bodies (CBs) were obvious within cardiomycytes on transmission electron microscopy of HCQ treated group which was described as abnormal lysosomes by Joyce, et al. [26]. These secondary lysosomes contained a dense material with a lamellar structure, myelin figures, and curvilinear bodies in the cytoplasm of the cardiomyocytes, with disorganization of the myofibrils [27]. Presence of CBs was detected on ultra-structural examination of muscle in patients with HCQ therapy by Khoo, et al. [28] and links their presence to muscle weakness.
Veinot, et al. [29] and Verny, et al. [30] confirmed that the dosage and administration period have not been related to the side effects caused by CQ and HCQ. Moreover, genetic alterations predisposing to cardiac toxicity have not been reported either. Chronic or acute administration of Low or intermediate doses is enough to cause myocardial dysfunction [31].
The available reports on antimalarial drugs explained its side effects by inhibiting hemozoin biocrystallization, which gives rise to toxic free heme accumulation that is responsible for the death of the parasites [32]. Heme is the functional group of different proteins as cytochromes and nitric oxide synthase that induce tissue damage [33]. Heme catalyzed ROS formation, end in oxidative stress and, resulted in cell injury. Furthermore, heme can impair intracellular lipid bilayer, DNA, intermediary metabolic enzymes, and the cytoskeleton lead to tissues' toxic effects [34,35].
The pathophysiology of cardiomyopathy induced by CQ/HCQ- is unwell understood. It could result from suppression of lysosomal enzymes and dysfunction of lysosomes which end in accumulation of different metabolic products within the tissue of the heart results in cardiac pathological manifestations as cardiomyopathy associated with conduction disturbance, concentric hypertrophy resulted in heart failure [5,36].
HCQ inhibit lysosomal phospholipase resulting in formation of indigestible complexes and induce phospholipid accumulation in the cytoplasm. Accumulation of HCQ in lysosomes resulted in inhibition of its enzyme activity and increase of internal PH of lysosomes and inactivation of protein. Depending on their morphology, these are denoted myeloid bodies (forming concentric layers) or curvilinear bodes (comma shape). These depositions lead to vacuolization of the cytoplasm, disorganization of the myofibrils, cell hypertrophy, and, finally, fibrosis [37].
Roos, et al. [38] considered curvilinear bodies as the most specific histological indicator of antimalarial-related cardiotoxicity and these structures are not seen in differential diagnoses of some of the other pathological findings, such as other toxic cardiomyopathies. Antimalarial drugs induce dysfunction of lysosomes resulting in aggregation of Pathological inclusion bodies than can be seen in histological section as cytoplasmic pathognomonic inclusion bodies [39]. Histopathological findings may persist for years after drug discontinuation [40] or could be recovered after drug withdrawal [41].
It was reported that the serum cardiac troponin concentration was an earlier marker of myocardial injury than was the histologic findings. The increase of serum troponin and reduction or loss of troponin expression in tissue is roughly related to disease severity, probably reflecting the extent of inflammatory and degenerative changes of the myocardium. Cardiac troponins I (cTnI) and T (cTnT) have been shown to be highly sensitive and specific markers of myocardial cell injury [42].
Oral supplementation of QCT in this study didn't show any signs of toxicity in group (2) that received QCT alone. Recently Tripathi, et al. [43] found that the safest route for administration of Quercetin is the oral supplementation.
Further, the current work showed that QCT, a plant-based flavonoid, has the potential to prevent the HCQ-induced toxicity and oxidative stress probably through scavenging the free radical generation. In the QCT + HCQ combined group, adding quercetin showed a marked protection against HCQ induced myocardial damage, in which QCT significantly decreased serum levels of MDA and significantly increased total serum antioxidants. These results are in agreement with Panda, et al. [44] who reported that quercetin produced a significant decrease in cardiac MDA and increased GSH content. These beneficial effects of quercetin could be attributed to its antioxidant properties, which are mainly owing to its ability to increase GSH, antioxidant enzyme levels, and scavenge lipid peroxides [45]. In addition, quercetin significantly decreased serum levels of cTn-I and increased cardiac troponin expression by histological analysis in the current work. These findings are in accordance with Zaafan, et al. [13] and with Liu, et al. [45].
In the present work, blood pressure and heart rate of the rats' received QCT in combination with HCQ were significantly less affected than those received HCQ alone. Also, cardiomyocytes were significantly less affected indicating less cardiac damage that was also approved by light and electron microscope examination of cardiac muscle of group 4( combined HCQ + QCT). Similar results were recorded by Kumar Mishra, et al. [35]. They found that QCT revert the toxic effect of CQ. QCT has been reported to be a potent anti-inflammatory and anti-oxidant biomolecule with bioprotective and phytonutrient properties [46,47].
Moreover, the potential use of QCT is clearly evidenced in clinical application by different studies on lung tissue [43] and cardiovascular system [48-51]. They confirmed that QCT and certain QCT-containing food have been shown to exert strong anti-hypertensive effects in both experimental animals and humans through numerous mechanisms such as attenuation of oxidative stress, remodeling of the blood vessels extracellular matrix and affecting cascades of intracellular protein kinase. Moreover, QCT exert strong cardiac protective effect in different heart diseases such as cardiotoxicity induced by doxorubicin, ischemia-reperfusion injury, cardiomyopathy induced by diabetes and others [52-54]. The protective effects of QCT on the heart are connected with affection of different proteins and signaling pathways, including reducing oxidative stress, suppression of apoptosis , as well as having effect on the heart inflammatory proteins [52-54,56].
Conclusion
The present investigation has shown that though HCQ may be a widely used drug, its administration may result in cardiac damage. It is therefore suggested that the drug be prescribed with caution in patients with cardiac abnormality, and QCT prophylaxis was found to be effective in minimizing the cardiotoxic effect of HCQ. Further studies aimed at corroborating this finding should be carried out.
References
- Teixeira RA, Martinelli Filho M, Benvenuti LA, Costa R, Pedrosa AA, Nishióka SA. Cardiac damage from chronic use of chloroquine: A case report and review of the literature. Arq Bras Cardiol. 2002 Jul;79(1):85-88. doi: 10.1590/s0066-782x2002001000009. PMID: 12163948.s
- Quiros Roldan E, Biasiotto G, Magro P, Zanella I. The possible mechanisms of action of 4-aminoquinolines (chloroquine/hydroxychloroquine) against Sars-Cov-2 infection (COVID-19): A role for iron homeostasis? Pharmacol Res. 2020 Aug;158:104904. doi: 10.1016/j.phrs.2020.104904. Epub 2020 May 13. PMID: 32430286; PMCID: PMC7217799.s
- Chen J, Liu D, Liu L, Liu P, Xu Q, Xia L, Ling Y, Huang D, Song S, Zhang D, Qian Z, Li T, Shen Y, Lu H. [A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19]. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020 May 25;49(2):215-219. Chinese. doi: 10.3785/j.issn.1008-9292.2020.03.03. PMID: 32391667s.
- Meo SA, Klonoff DC, Akram J. Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19. Eur Rev Med Pharmacol Sci. 2020 Apr;24(8):4539-4547. doi: 10.26355/eurrev_202004_21038. PMID: 32373993s.
- Yogasundaram H, Hung W, Paterson ID, Sergi C, Oudit GY. Chloroquine-induced cardiomyopathy: a reversible cause of heart failure. ESC Heart Fail. 2018 Jun;5(3):372-375. doi: 10.1002/ehf2.12276. Epub 2018 Feb 20. PMID: 29460476; PMCID: PMC5933951s.
- Sak K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn Rev. 2014 Jul;8(16):122-46. doi: 10.4103/0973-7847.134247. PMID: 25125885; PMCID: PMC4127821.
- Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007 Nov;45(11):2179-205. doi: 10.1016/j.fct.2007.05.015. Epub 2007 Jun 7. PMID: 17698276s.
- Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008 May 13;585(2-3):325-37. doi: 10.1016/j.ejphar.2008.03.008. Epub 2008 Mar 18. PMID: 18417116s.
- Kumar Mishra S, Singh P, Rath SK. Protective effect of quercetin on chloroquine-induced oxidative stress and hepatotoxicity in mice. Malar Res Treat. 2013;2013:141734. doi: 10.1155/2013/141734. Epub 2013 Mar 27. PMID: 23607047; PMCID: PMC3625570s.
- Ferenczyova K, Kalocayova B, Bartekova M. Potential Implications of Quercetin and its Derivatives in Cardioprotection. Int J Mol Sci. 2020 Feb 26;21(5):1585. doi: 10.3390/ijms21051585. PMID: 32111033; PMCID: PMC7084176s.
- Guide for the Care and Use of Laboratory Animals Institute of Laboratory Animal Resources Commission on Life Sciences. Washington DC. National Research Council National Academy Press. 1996.
- Izunya AM, Nwaopara AO, Anyanwu LC, Odike MAC, Oaikhena GA, Bankole JK, Okhiai O. Effect of chronic oral administration of chloroquine on the histology of the heart in Wistar rats. Biology and Medicine. 2011 July 01;3 (4):p.1-6.
- Zaafan MA, Zaki HF, El-Brairy AI, Kenawy SA. Protective effects of atorvastatin and quercetin on isoprenaline-induced myocardial infarction in rats. Bull Fac Pharm. 2013 June; 51(1):35–41. doi:10.1016/j.bfopcu.2013.03.001.
- Morand C, Manach C, Crespy V, Remesy C. Quercetin 3-O-beta-glucoside is better absorbed than other quercetin forms and is not present in rat plasma. Free Radic Res. 2000 Nov;33(5):667-76. doi: 10.1080/10715760000301181. PMID: 11200097.
- Willhite CC. Teratogenic potential of quercetin in the rat. Food Chem Toxicol. 1982 Feb;20(1):75-9. doi: 10.1016/s0278-6915(82)80012-4. PMID: 7200058.
- Kubota Y, Umegaki K, Kagota S, Tanaka N, Nakamura K, Kunitomo M, Shinozuka K. Evaluation of blood pressure measured by tail-cuff methods (without heating) in spontaneously hypertensive rats. Biol Pharm Bull. 2006 Aug;29(8):1756-8. doi: 10.1248/bpb.29.1756. PMID: 16880638.
- Pauline M, Sandhya TA and Maruthy KN. Non Invasive Measurement of Systolic Blood Pressure in Rats: A Simple Technique. Al Ameen J Med Sci. 2011;4(4):365-369.
- Thapar MK, Johnson WW, Sanyal SK. Perfusion-fixation of the heart and its conduction system for ultrastructural studies. Eur J Cardiol. 1980 Feb;11(2):91-104. PMID: 7363928.
- Nasser Hajibagheri MA. Fixatives and fixation. In: Electron microscopy methods and protocols. London, UK: Humana Press Inc;1999. 1st ed. pp 3-7.
- Bancroft JD, Gamble M. Theory and practice of histological techniques. Churchill Livingstone, USA: 2002. 5th ed.
- Kiernan JA. Histological and histochemical methods : theory and practice. Oxford, UK: Butterworth-Heinemann; 1999. 3rd ed.
- Harada K, Komuro I, Shiojima I, Hayashi D, Kudoh S, Mizuno T, Kijima K, Matsubara H, Sugaya T, Murakami K, Yazaki Y. Pressure overload induces cardiac hypertrophy in angiotensin II type 1A receptor knockout mice. Circulation. 1998 May 19;97(19):1952-9. doi: 10.1161/01.cir.97.19.1952. PMID: 9609089.
- Joyce E, Fabre A, Mahon N. Hydroxychloroquine cardiotoxicity presenting as a rapidly evolving biventricular cardiomyopathy: key diagnostic features and literature review. Eur Heart J Acute Cardiovasc Care. 2013 Mar;2(1):77-83. doi: 10.1177/2048872612471215. PMID: 24062937; PMCID: PMC3760572.
- Chang A, Stolin G, Fan J, Larreta BR, Fishbein GA, Wallace WD, Baas AS, Cruz D, Wang J. Hypertrophic cardiomyopathy in a lupus patient: a case of hydroxychloroquine cardiotoxicity. ESC Heart Fail. 2019 Dec;6(6):1326-1330. doi: 10.1002/ehf2.12508. Epub 2019 Sep 7. PMID: 31493341; PMCID: PMC6989295.
- Yogasundaram H, Putko BN, Tien J, Paterson DI, Cujec B, Ringrose J, Oudit GY. Hydroxychloroquine-induced cardiomyopathy: case report, pathophysiology, diagnosis, and treatment. Can J Cardiol. 2014 Dec;30(12):1706-15. doi: 10.1016/j.cjca.2014.08.016. Epub 2014 Aug 23. PMID: 25475472.
- Joyce E, Fabre A, Mahon N. Hydroxychloroquine cardiotoxicity presenting as a rapidly evolving biventricular cardiomyopathy: key diagnostic features and literature review. Eur Heart J Acute Cardiovasc Care. 2013 Mar;2(1):77-83. doi: 10.1177/2048872612471215. PMID: 24062937; PMCID: PMC3760572.
- Baguet JP, Tremel F, Fabre M. Chloroquine cardiomyopathy with conduction disorders. Heart. 1999 Feb;81(2):221-3. doi: 10.1136/hrt.81.2.221. PMID: 9922366; PMCID: PMC1728937.
- Khoo T, Otto S, Koszyka B, Smith C, Blumbergs P, Lester S, Limaye V. The Clinical Significance of Curvilinear Bodies on Ultrastructural Examination of Muscle [abstract]. Arthritis Rheumatol. 2016 Sep 28;68 (suppl 10).
- Veinot JP, Mai KT, Zarychanski R. Chloroquine related cardiac toxicity. J Rheumatol. 1998 Jun;25(6):1221-5. PMID: 9632091.
- Verny C, de Gennes C, Sébastien P, Lê Thi HD, Chapelon C, Piette JC, Chomette G, Godeau P. Troubles de la conduction cardiaque au cours d'un traitement prolongé par chloroquine. Deux nouvelles observations [Heart conduction disorders in long-term treatment with chloroquine. Two new cases]. Presse Med. 1992 May 2-9;21(17):800-4. French. PMID: 1535141.
- Blignaut M, Espach Y, van Vuuren M, Dhanabalan K, Huisamen B. Revisiting the Cardiotoxic Effect of Chloroquine. Cardiovasc Drugs Ther. 2019 Feb;33(1):1-11. doi: 10.1007/s10557-018-06847-9. PMID: 30635818.
- Barennes H, Balima-Koussoubé T, Nagot N, Charpentier JC, Pussard E. Safety and efficacy of rectal compared with intramuscular quinine for the early treatment of moderately severe malaria in children: randomised clinical trial. BMJ. 2006 May 6;332(7549):1055-9. doi: 10.1136/bmj.332.7549.1055. PMID: 16675812; PMCID: PMC1458599.
- Beri R, Chandra R. Chemistry and biology of heme. Effect of metal salts, organometals, and metalloporphyrins on heme synthesis and catabolism, with special reference to clinical implications and interactions with cytochrome P-450. Drug Metab Rev. 1993;25(1-2):49-152. doi: 10.3109/03602539308993973. PMID: 8449148.
- Wagener FA, Volk HD, Willis D, Abraham NG, Soares MP, Adema GJ, Figdor CG. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol Rev. 2003 Sep;55(3):551-71. doi: 10.1124/pr.55.3.5. Epub 2003 Jul 17. PMID: 12869663.
- Kumar S, Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol Lett. 2005 Jul 4;157(3):175-88. doi: 10.1016/j.toxlet.2005.03.004. Epub 2005 Apr 7. PMID: 15917143.
- Page RL, O'Bryant CL, Cheng D, Dow TJ, Ky B, Stein CM, Spencer AP, Trupp RJ, Lindenfeld J. Drugs that may cause or exacerbate heart failure. Circulation. 2016 Jul 11;134:e32-e69. doi:10.1161/CIR.0000000000000426.
- Di Girolamo F, Claver E, Olivé M, Salazar-Mendiguchía J, Manito N, Cequier Á. Dilated Cardiomyopathy and Hydroxychloroquine-induced Phospholipidosis: From Curvilinear Bodies to Clinical Suspicion. Rev Esp Cardiol (Engl Ed). 2018 Jun;71(6):491-493. English, Spanish. doi: 10.1016/j.rec.2017.04.017. Epub 2017 May 18. PMID: 28528883.
- Roos JM, Aubry MC, Edwards WD. Chloroquine cardiotoxicity: clinicopathologic features in three patients and comparison with three patients with Fabry disease. Cardiovasc Pathol. 2002 Sep-Oct;11(5):277-83. doi: 10.1016/s1054-8807(02)00118-7. PMID: 12361838.
- Tönnesmann E, Kandolf R, Lewalter T. Chloroquine cardiomyopathy - a review of the literature. Immunopharmacol Immunotoxicol. 2013 Jun;35(3):434-42. doi: 10.3109/08923973.2013.780078. Epub 2013 May 1. PMID: 23635029.
- August C, Holzhausen HJ, Schmoldt A, Pompecki R, Schröder S. Histological and ultrastructural findings in chloroquine-induced cardiomyopathy. J Mol Med (Berl). 1995 Feb;73(2):73-7. doi: 10.1007/BF00270580. PMID: 7627632.
- Frustaci A, Morgante E, Antuzzi D, Russo MA, Chimenti C. Inhibition of cardiomyocyte lysosomal activity in hydroxychloroquine cardiomyopathy. Int J Cardiol. 2012 May 17;157(1):117-9. doi: 10.1016/j.ijcard.2012.03.112. Epub 2012 Apr 4. PMID: 22481048.
- Tunca R, Sozmen M, Erdogan H, Citil M, Uzlu E, Ozen H, Gokçe E. Determination of cardiac troponin I in the blood and heart of calves with foot-and-mouth disease. J Vet Diagn Invest. 2008 Sep;20(5):598-605. doi: 10.1177/104063870802000510. PMID: 18776092.
- Tripathi A, Kumar B, Sagi SSK. Prophylactic efficacy of Quercetin in ameliorating the hypoxia induced vascular leakage in lungs of rats. PLoS One. 2019 Jun 28;14(6):e0219075. doi: 10.1371/journal.pone.0219075. PMID: 31251771; PMCID: PMC6599121.
- Panda S, Kar A, Banerjee T, Sharma N. Combined effects of quercetin and atenolol in reducing isoproterenol-induced cardiotoxicity in rats: possible mediation through scavenging free radicals. Cardiovasc Toxicol. 2012 Sep;12(3):235-42. doi: 10.1007/s12012-012-9161-3. PMID: 22391854.
- Liu H, Zhang L, Lu S. Evaluation of antioxidant and immunity activities of quercetin in isoproterenol-treated rats. Molecules. 2012 Apr 10;17(4):4281-91. doi: 10.3390/molecules17044281. PMID: 22491677; PMCID: PMC6268199s.
- Dias AS, Porawski M, Alonso M, Marroni N, Collado PS, González-Gallego J. Quercetin decreases oxidative stress, NF-kappaB activation, and iNOS overexpression in liver of streptozotocin-induced diabetic rats. J Nutr. 2005 Oct;135(10):2299-304. doi: 10.1093/jn/135.10.2299. PMID: 16177186.
- González-Gallego J, Sánchez-Campos S, Tuñón MJ. Anti-inflammatory properties of dietary flavonoids. Nutr Hosp. 2007 May-Jun;22(3):287-93. PMID: 17612370.
- Brüll V, Burak C, Stoffel-Wagner B, Wolffram S, Nickenig G, Müller C, Langguth P, Alteheld B, Fimmers R, Naaf S, Zimmermann BF, Stehle P, Egert S. Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-)hypertension: a randomised double-blinded placebo-controlled cross-over trial. Br J Nutr. 2015 Oct 28;114(8):1263-77. doi: 10.1017/S0007114515002950. Epub 2015 Sep 2. PMID: 26328470; PMCID: PMC4594049.
- Calabró V, Litterio MC, Fraga CG, Galleano M, Piotrkowski B. Effects of quercetin on heart nitric oxide metabolism in l-NAME treated rats. Arch Biochem Biophys. 2018 Jun 1;647:47-53. doi: 10.1016/j.abb.2018.03.041. Epub 2018 Apr 3. PMID: 29621523.
- Kim SG, Kim JR, Choi HC. Quercetin-Induced AMP-Activated Protein Kinase Activation Attenuates Vasoconstriction Through LKB1-AMPK Signaling Pathway. J Med Food. 2018 Feb;21(2):146-153. doi: 10.1089/jmf.2017.4052. Epub 2017 Oct 16. PMID: 29035613.
- Pereira SC, Parente JM, Belo VA, Mendes AS, Gonzaga NA, do Vale GT, Ceron CS, Tanus-Santos JE, Tirapelli CR, Castro MM. Quercetin decreases the activity of matrix metalloproteinase-2 and ameliorates vascular remodeling in renovascular hypertension. Atherosclerosis. 2018 Mar;270:146-153. doi: 10.1016/j.atherosclerosis.2018.01.031. Epub 2018 Jan 31. PMID: 29425960.
- Barteková M, Šimončíková P, Fogarassyová M, Ivanová M, Okruhlicová Ľ, Tribulová N, Dovinová I, Barančík M. Quercetin improves postischemic recovery of heart function in doxorubicin-treated rats and prevents doxorubicin-induced matrix metalloproteinase-2 activation and apoptosis induction. Int J Mol Sci. 2015 Apr 13;16(4):8168-85. doi: 10.3390/ijms16048168. PMID: 25872140; PMCID: PMC4425074.
- Wang Y, Zhang ZZ, Wu Y, Ke JJ, He XH, Wang YL. Quercetin postconditioning attenuates myocardial ischemia/reperfusion injury in rats through the PI3K/Akt pathway. Braz J Med Biol Res. 2013 Oct;46(10):861-7. doi: 10.1590/1414-431X20133036. Epub 2013 Sep 24. PMID: 24068165; PMCID: PMC3854307.
- Liu H, Guo X, Chu Y, Lu S. Heart protective effects and mechanism of quercetin preconditioning on anti-myocardial ischemia reperfusion (IR) injuries in rats. Gene. 2014 Jul 15;545(1):149-55. doi: 10.1016/j.gene.2014.04.043. Epub 2014 Apr 24. PMID: 24769323.
- Castillo RL, Herrera EA, Gonzalez-Candia A, Reyes-Farias M, de la Jara N, Peña JP, Carrasco-Pozo C. Quercetin Prevents Diastolic Dysfunction Induced by a High-Cholesterol Diet: Role of Oxidative Stress and Bioenergetics in Hyperglycemic Rats. Oxid Med Cell Longev. 2018 Jan 11;2018:7239123. doi: 10.1155/2018/7239123. PMID: 29576853; PMCID: PMC5821945.
- Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, Liu H, Yin Y. Quercetin, Inflammation and Immunity. Nutrients. 2016 Mar 15;8(3):167. doi: 10.3390/nu8030167. PMID: 26999194; PMCID: PMC4808895.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.