> Biology. 2020 December 16;1(6):263-270. doi: 10.37871/jbres1172.
Sampling and Characterization of the Environmental Fungi in the Provincial Historic Archive of Pinar Del Río, Cuba
Sofia Borrego1*, Alian Molina1 and Tamara Abrante2
2Conservation Department, Provincial Historical Archive of Pinar del Río. Calle Martí, No. 152 esq. a Ormany Arenado, Pinar del Río city, Cuba
- Airborne fungi
- Archives
- Environmental quality
- Indoor air
- Pathogenic attributes
It has been reported that there is a correlation between indoor airborne fungi and the biodeterioration of valuable documents in archives, libraries and museums, and that these fungi can also cause effects on human health if there are immunological problems or the time of exposure to these environments of low quality is long. The aims of this study were quantifying and characterizing the mycobiota of the indoor air in three repositories of the Provincial Historical Archive of Pinar del Río, Cuba and assessing its impact on the human health. The samplings were made in two different months corresponding to the years 2016 and 2017, one belonging to the rainy season and the other to the season of the little rain using a SAS biocollector and appropriate culture media to isolate fungi. The fungal concentrations and the Indoor/Outdoor (I/O) ratios obtained revealing that the repositories showed good quality environments. In both isolations Cladosporium was the predominant genus followed by Penicillium in the first sampling and Fusarium in the second isolation. The genera Aureobasidium, Sepedonium, Trichaegum and Wallemia were new findings for the Cuban archives. The pathogenic attributes studied showed that 30% of the isolates have spores so small that they can penetrate into the respiratory tract into the alveoli; 10.7% of the taxa obtained in the first isolation and 13.3% of the taxa detected in the second sampling also showed positive results to four virulence tests analyzed "In vitro" (growth at 37°C, hemolytic activity, phospholipase activity and respiratory tract level to which the spores can penetrate). These virulence factors (pathogenic attributes) evidence the risk that environmental fungi represent for the health of personnel in this archive.
The archives, libraries and museums are the institutions in charge of preserving the Memory of a Nation. They contain a large number of documents of heritage value written on various media (papyrus, parchment, paper, etc.), and guard other types of documents such as photographs, maps and plans, engraving as well as digital documents, among others. These supports of an organic, inorganic or synthetic nature are deteriorated over time, but this process is accelerated by the effect of physical (light, temperature and relative humidity), chemical (atmospheric pollution) and biological agents (microorganisms, insects, etc.) [1]. Therefore, the continuous knowledge and control of environmental conditions in these institutions is today one of the most important elements to take into account in the preventive conservation of documentary heritage for a country. The prevalence of inadequate environmental conditions together with the presence of high microbial concentrations in the air of the repositories where this heritage is conserved, has been awakening the attention of researchers and specialists in the area of heritage conservation, due to the risk that this it implies both for the integrity of the heritage preserved and for the health of the personnel that works in these institutions or that receives systematic services in them [2,3]. Specifically, the environmental fungal concentration is one of the main objects of study, since on the one hand, fungal spores and propagules constitute the largest group of all biological material that is transported by air, dust and people towards the indoor environment of the repositories and on the other, it is known that fungi are characterized by a high biodeteriogenic and pathogenic potential [4-6].
Cuba, due to its geographical location, is constantly affected by the dust coming from different parts of the world according to the arrival of winds in different directions, and in particular by the dust of the Sahara desert [7], which together with the high values of Relative Humidity (RH) and Temperature (T) typical of the prevailing climatic conditions at different stages of the year, can lead to high concentrations of viable conidia in the air that, according to the indoor ventilation conditions, are easily deposited on the different substrates, facilitating the development of fungi. These microorganisms have powerful, versatile and adaptable metabolic machinery, which allows them to degrade a great diversity of substrates, both of organic and inorganic origin, leading to the biodeterioration of the different supports preserved in the repositories of heritage institutions [8]. On the other hand, fungi are characterized by having different structures and mechanisms of pathogenicity (virulence factors), which can cause specific suffering to human health [9].
Numerous studies have established a close relationship between environmental conditions, the presence of fungal propagules viable (fungal spores, mycelial cells and their fragments) and their incidence in the eventual triggering of respiratory troubles and on the individuals’ immune system [10,11], managing to associate their presence with the development of symptoms belonging to these types of pathologies and others mycosis [12,13]. Hence, multiple research groups recommend the need to increase the frequency of studies of the environmental conditions in the premises to assess the quality of the environments, in order to guarantee an environmental characterization of them that allow solving early problems associated with the unleashing of pests and/or health conditions of staff. This has been the cause of the multiple studies conducted in the indoor environments of different repositories of the National Archive of the Republic of Cuba [14-16] and other cuban archives [17].
However, for the Cuban archives these studies have not been sufficient. Therefore it was decide since some years, to perform environmental mycological studies in other archives of the country. An example is the case of this study carried out in the Provincial Historical Archive of Pinar del Río (PHA PR) with the aims of quantifying and characterizing the mycobiota of the indoor air in some repositories of this archive as well as assessing the aeromicological dynamics and its impact on the human health.
Characterization of the sampled repositories
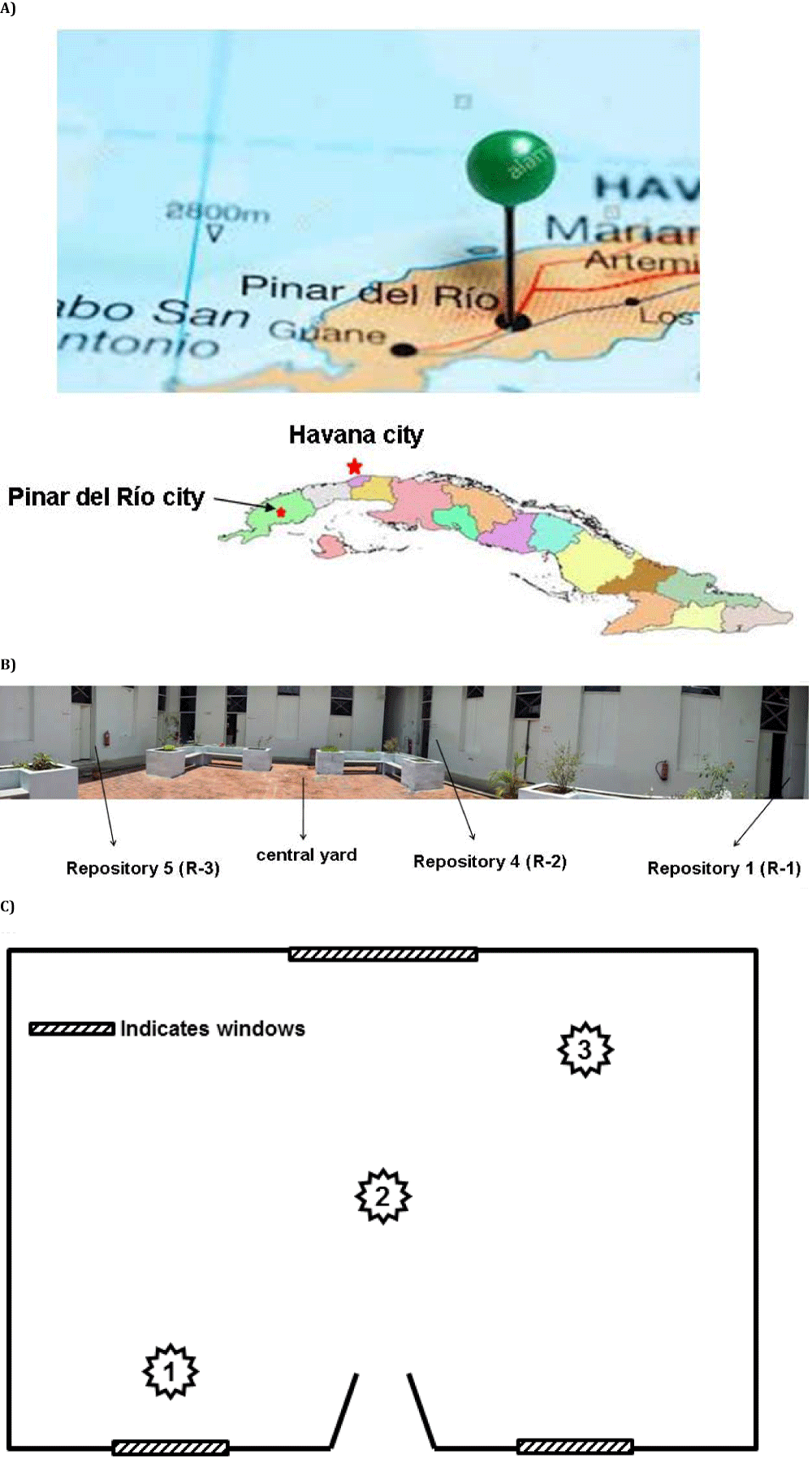
The study was conducted in three repositories of the Provincial Historical Archive of Pinar del Río (PHA PR) located in the westernmost province of Cuba 162 km from Havana, the capital of Cuba (Figure 1A). The archive is located in the center of the Pinar del Río city surrounded by avenues and streets with high vehicular traffic. The building built at the beginning of the 20th century (1910) and is in an eclectic style and consisting of a single floor at the street level with a large central courtyard and around it the repositories are positioned (Figure 1B). The studied repositories measure (length x width x height, in m) the following: Repository 1 (R-1) coincides with repository 1 of the archive, it measures 5.84 x 7.77 x 4.64 m and is located in the south side of the building; Repository 2 (R-2) coincides with repository 4 of the archive, it measures 5.80 x 7.77 x 4.64 m and is located to the east of the building; and Repository 3 (R-3) coincides with repository 5 of the archive, measures 5.64 x 8.65 x 4.64 m and is located on the west side of the building. All have natural cross ventilation.
Sampling of aeromycobiota
The samples were taken in 02/June/2016 (month corresponding to rainy season) and second sampling was done 24/February/2017 (month corresponding at season of the little rainy). The number of points to be sampled was determined according to Sanchis [18], which reports a simple method based on the cube root of the volume of the premises. According to this criterion, a total of 9 points were sampled (3 in each repository) (Figure 1C). Also as a control, a sample of air on the outdoor courtyard was taken. Samplings were done between 10:00 am and 1:30 pm approximately, considering the working hours. Each point of indoor was sampled by triplicate using a biocollector SAS Super 100 (Italy) in vertical position at intervals of one hour between replicates. With this impactor air sampler 100 L of air/min flow rate was collected at a height of 1.5 m approximately. Two variants of Malt Extract Agar (MEA) (BIOCEN, Cuba) were used, one was MEA at pH 5 [19] and the other was MEA supplemented with NaCl (7.5%) [1,16]. This concentration of sodium chloride is used to limit the growth of Mucorales colonies and stimulate the growth of halophilic and xerophilic fungi. Subsequently, the Petri dishes were incubated inverted at 30°C for up to 7 days and the colonies were counted to calculate the fungal concentration per m3 of air expressed in colony forming units (CFU/m3). Then the colonies were isolated and purified.
Together with the microbiological samples, Temperature (T) and Relative Humidity (RH) were measured at the same points analyzed using a digital thermo hygrometer Pen TH 8709 (China).
For the identification, observations were made of the cultural and morphological characteristics of each colony, both front and back, using a stereomicroscope (X14). Conidiophores, conidia and other structures were observed from preparations made with lactophenol or microcultures using a clear field trinocular microscope (Olympus, Japan) at X40 and X100 attached to a digital camera (Samsung, Korea). For the observation of structures hyalines were used lactophenol cotton blue. In the taxonomic identification a group of key mycological was consulted. In the identification up to the genus level was carried out according to the criteria of Barnett and Hunter [20] and Domsch, et al. [21]. For the identification of the species located in the Aspergillus and Penicillium genera, the procedures suggested by Klich and Pitt [22] and Pitt [23] were followed. These methodologies are based primarily on morphological characters and physiological characteristics such as water-temperature relationships, pigmentation and the degree of development of the colonies in certain media. These characteristics were determined 7 days after the strains were inoculated in the Czapek Yeast Extract Agar media, incubated at 5°C, 25°C and 37°C, and in the Malt Extract Agar and Czapek Yeast Extract Agar with 20% Sucrose and incubated at 25°C for Aspergillus and also 25% Glycerol Nitrate Agar at 25ºC for Penicillium. The species located within the genus Cladosporium were identified following the criteria of Ellis [24] and Bensch, et al. [25], those of Fusarium according to Both [26] and that of Nigrospora according to Domsch [21].
Ecological approaches
Relative Density (RD) of taxa isolated from indoor air of each repository was conducted according to Smith [27] where: RD = (number of colonies of one taxon / total number of colonies) x100
Relative Frequency (RF) of the taxa detected on indoor environments was determined according to Esquivel, et al. [28] where: RF = (times a taxa is detected/total number of sampling realized) × 100
The ecological categories are classified as: Abundant (A) with RF = 100-81%, Common (C) with RF = 80-61%, Frequent (F) with RF = 60-41%, Occasional (O) with RF = 40-21%, Rare (R) with RF = 20-0%.
Statistical analysis
The data obtained were processed with the statistical Statgraphics Centurion XV program. Student’s t test was used on comparing the average of the fungal propagules concentrations between the isolations. The ANOVA-1 and Duncan tests were used to compare the obtained RH average during the microbiological sampling of air. A p-value smaller or equal to 0.05 was considered statistically significant.
A multifactorial analysis was performed to obtain possible correlations (Pearson) between the fungal concentrations obtained in both samplings and the thermo-hygrometric parameters recorded during the two years of the study.
Determination of Some Virulence Factor
Spore size of isolated fungal genera: The spore’s dimensions corresponding to several representative strains of the each isolated specie were determined. In all cases at least 20 observations were made distributed in several fields of vision, in preparations of both the young part and the mature area of the colony. These observations were made in trinocular microscope (Olympus, japan) at X100 using a micrometric lens. The sizes conidial of each strain were taken into account for the analysis of the penetration of conidia in the human respiratory tract [14].
Radial growth at 37°C: Strains were grown on Petri dishes containing as culture medium MEA. Strains were incubated for 7 days at 37°C and growth was evaluated [15].
Hemolytic activity: Strains were seeded in Petri dishes with Czapek Agar, which after being sterilized was added 5% defibrinated sheep blood. They were incubated for 7 days at 37°C. In the strains that didn’t grow at 37°C the hemolytic activity was determined at 30°C. The hemolytic activity was evidenced by the appearance of a halo around the colony indicating hemolysis partial if the halo is green (alpha hemolytic), total if it is colorless (beta hemolytic) and absence of hemolysis if there is no halo (gamma hemolytic) [29].
Phospholipase activity: The strains under study were seeded in an agarized culture medium whose composition for 500 ml was 5 g bacteriological peptone, 10 g glucose, 29.3 g sodium chloride and 2.28 g calcium chloride. The pH of the medium was adjusted to 4 and after sterilization, 2 egg yolks were added aseptically. The plates were subsequently incubated at 30°C for seven days. The activity was evidenced by the appearance of a transparent halo around the colony in the light yellow medium, product of the precipitate formed by the salts [29].
In all cases the tests were performed in triplicates.
Behavior of thermo-hygrometric parameters and airborne fungal load in the environment of the repositories
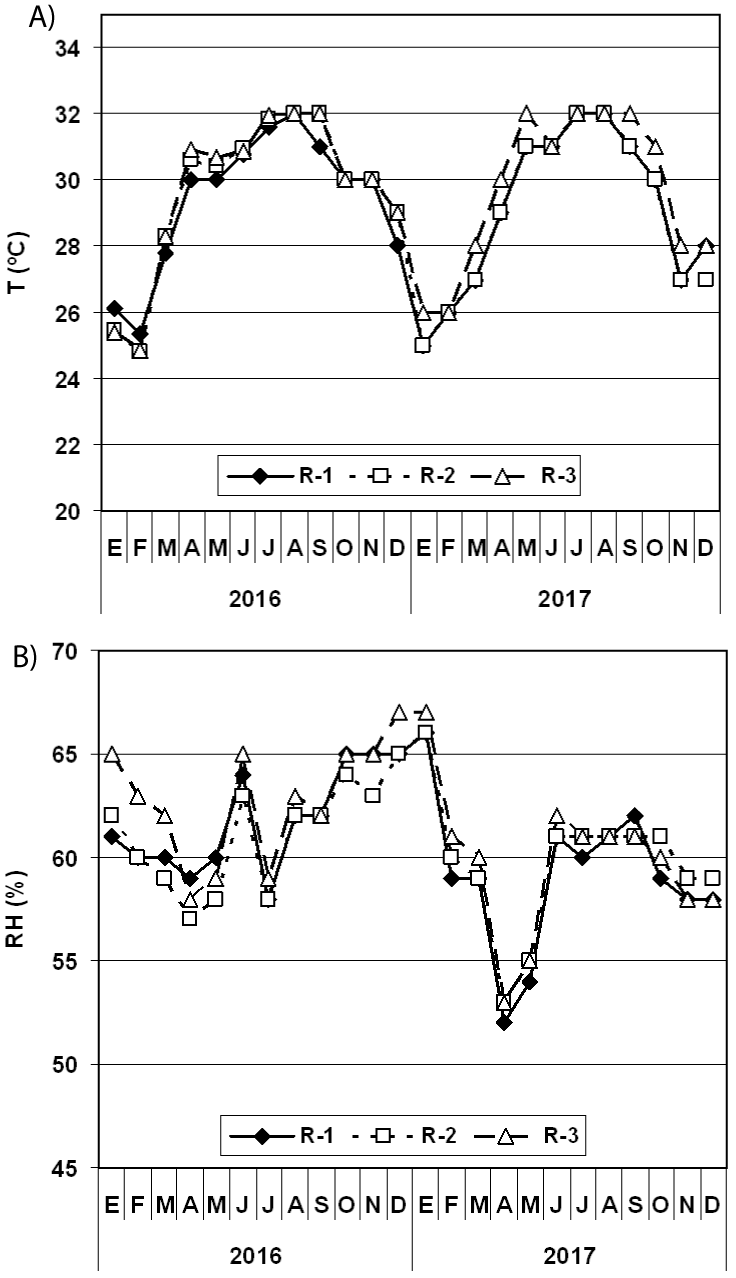
The behavior of the T in the repositories examined shows that the average values obtained are almost superimposed with each other, evidencing the similarity of the values in the three repositories (Figure 2). In 2016, the T average value in the AHP PR was 30°C and in 2017 it was 29°C. However, the T average values in the repositories during the rainy season (from January to May plus December) of 2016 ranged between 27.8°C and 28.2°C whereas in 2017 fluctuated between 27.5°C and 28°C, indicative that there are no differences between the years. In the rainy season (June to November) of 2016 and 2017 the averages were maintained around 31°C (Table 1), and also the values did not show significant differences.
| Table 1: Average values of Temperature (T) and Relative Humidity (RH) in each repository of the PHA PR archive during the little rainy season and rainy season per each studied year. | ||||||||
| 2016 | 2017 | |||||||
| January to May and December (season of little rainy) | June to November (rainy season) |
January to May and December (season of little rainy) | June to November (rainy season) |
|||||
| Repository | T (°C) | RH (%) | T (°C) | RH (%) | T (°C) | RH (%) | T (°C) | RH (%) |
| R-1 | 27.8 | 60.8 | 31.0 | 62.7 | 27.7 | 58.0 | 31.0 | 60.4 |
| R-2 | 28.0 | 60.2 | 31.2 | 62.0 | 27.5 | 58.7 | 30.5 | 60.2 |
| R-3 | 28.2 | 62.3 | 31.2 | 63.2 | 28.0 | 59.0 | 31.0 | 60.5 |
In relation to the RH, the values are also superimposed on one another (Figure 2). In 2016, the RH average value in the PHA PR was 61.9% whilst in 2017 it was 59.5%. On the other hand, the averages values obtained in each repository during the dry season fluctuated between 60.8 and 62.3% in 2016, whilst in 2017 they oscillated between 58 and 59%; although there were no significant differences, during 2017 the values were slightly lower. In relation to the rainy season, in 2016 averages were obtained that fluctuated between 62.0% and 63.2% whilst in 2017 they were between 60.2 and 60.5%; Also, although there were no significant differences between these values, in 2017 the values were slightly lower (Table 1).
In Cuba, the months of June to November are characterized by temperatures above 30°C. This is the season of heavy rains and hurricanes during which the RH is significantly high, sometimes reaching values above 90%, which is why these months are within the rainy season. From December to May are the months that are located in the slightly cold season characterized by little rain, so these months are considered within the season of low rainfall. However, in these years there were no hurricanes in the western region of the country and therefore the thermo-hygrometric values in the archive remained stable.
The first microbiological sampling was conducted on June 2, 2016 (rainy season) and at that time, the T average values in the repositories ranged between 28.5°C and 30.3°C and the RH fluctuated between 61.8% and 67.9% (Table 2). The second analysis was carried out on February 24, 2017 coinciding with the low rainfall season, and the T average ranged between 27.5°C and 29.6°C while the RH average fluctuated between 63% and 70.5%. This day it rained in the morning (from 8:45 to 10:00 am approximately) causing higher values of RH than the monthly average value for each repository (R-1 = 59%, R-2 = 60%, R-3 = 61%). In this case, significant differences (p ≤ 0.05) were obtained when comparing the T and RH averages among the repositories evidenced that R-3 shows the lowest RH values and the highest T values.
| Table 2: Fungal concentrations detected in the indoor air of the studied three repositories of the PHA PR in the two isolations made. | ||||||||||||
| R-1 | R-2 | R-3 | ||||||||||
| Concentrations | Fungi (CFU/m3) |
T (°C) |
HR (%) |
I/O | Fungi (CFU/m3) |
T (°C) |
HR (%) |
I/O | Fungi (CFU/m3) |
T (°C) |
HR (%) |
I/O |
| First isolation was done 02/June/2016 - Rainy season (Fungal concentration outdoor was 66.7 ± 23.1 CFU/m3) | ||||||||||||
| Maximum | 180 | 29.6 | 69.9 | 180 | 29.1 | 69.2 | 140 | 32.3 | 66.3 | |||
| Minimum | 20 | 28.3 | 66.3 | 20 | 27.8 | 62.4 | 30 | 29.3 | 57.1 | |||
| Average | 71.0 ± 53.3 | 28.9 ± 0.4ab | 67.9± 1.2cd | 1.1 | 91.1 ± 73.2 | 28.5± 0.5a | 66.2 ± 2.7bc | 1.4 | 68.6 ± 38.5 | 30.3 ± 1.1b | 61.8± 3.1a | 1.0 |
| Second isolation was done 24/February/2017 - Little rainy season (Fungal concentration outdoor was 133.6 ± 25.2 CFU/m3) | ||||||||||||
| Maximum | 260 | 29.4 | 78.8 | 360 | 31.0 | 76.6 | 220 | 30.4 | 75.4 | |||
| Minimum | 110 | 26.1 | 62.0 | 140 | 26.5 | 56.1 | 90 | 28.0 | 58.1 | |||
| Average | 202.0 ± 53.1* | 27.5 ± 1.3a | 70.5± 2.2d | 1.5 | 226.0 ± 86.5* | 28.4± 1.6a | 66.7 ± 1.6bc | 1.7 | 162.0 ± 66.1* | 29.6 ± 0.9b | 63.0 ± 2.3ab | 1.2 |
| All fungal determination was made in 3 points in each repository by triplicate and the data averaged (n = 9). *: Indicates significant differences according to the Student test (p ≤ 0.05) on comparing the average of the fungal propagules concentrations between the isolations. a,b,c,d,e: Indicate significant differences according to the Duncan test (p ≤ 0.05) on comparing the RH average obtained during the microbiological sampling of air. |
||||||||||||
The environmental fungal concentrations of the first sampling in the repositories indoor air and in outdoors were significantly lower than those obtained in the second sampling (Table 2). In the first isolation the fungal concentrations ranged from 68.6 CFU/m3 obtained in R-3 to 91.1 CFU/m3 detected in R-2 while in R-1 the fungal load was 71 CFU/m3. In the second sampling, the concentrations ranged between 162 CFU/m3 (in R-3) and 226 CFU/m3 (in R-2). Highlighted the fact that regardless of the moment of isolation and that the concentrations obtained in the repositories were similar statistically, in R-3 the lowest concentrations were always obtained, followed by R-1 and finally R-2 (the most contaminated).
Similarly, the indoor/outdoor (I/O) ratios were slightly higher for the second sampling. However, the values in R-1 and R-3 were less than or equal to 1.5 indicative of environments with a very low level of contamination, hence with good quality while in R-2 the I/O index was 1.7, which reveals an environment slightly contaminated. To reduce these indices it is essential to improve natural ventilation and this requires better repositories management.
When correlating T and RH with fungal concentration, a significantly high correlation was obtained between fungal concentration and RH for both isolates (r2 = 0.85 in the 1st sampling and r2 = 0.93 in the 2nd sampling for p < 0.05) while no correlation was found with T (r2 = 0.46 in the 1st sampling and r2 = 0.52 in the 2nd sampling for p < 0.05).
Taxa detected on indoor environment of the repositories and in the outdoor environment
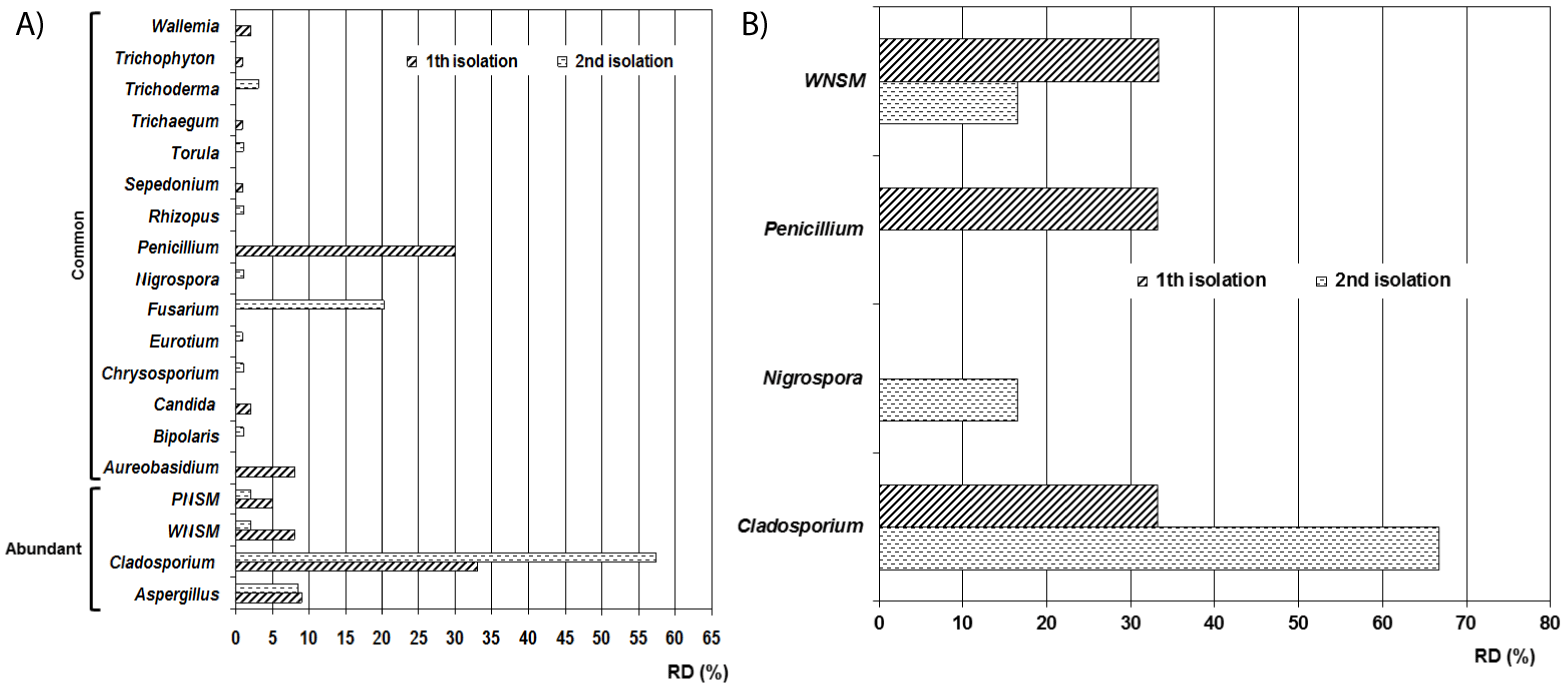
The genera diversity obtained was very close between the two isolates. In the first isolation, nine genera and two non-sporulating mycelia were detected, while in the second, ten genera and the two non-sporulating mycelia mentioned above were detected (Figure 3). In both isolations the preponderant genus was Cladosporium (1st isolation with RD of 33% and 2nd isolation with DR of 57.4%) followed by Penicillium in the first isolation (RD = 30%) and Fusarium in the second sampling (RD = 20.2%). Ecologically the abundant genera were Aspergillus and Cladosporium as well as the White Non-sporulating Septated Mycelium (WNSM) and the Pigmented Non-sporulating Septated Mycelium (PNSM). Aureobasidium, Bipolar, Candida, Chrysosporium, Eurotium, Fusarium, Penicillium, Rhizopus, Sepedonium, Torula, Trichaegum, Trichoderma, Trichophyton and Wallemia were genera classified as common.
In outdoor air the detected taxa in the first isolation were Cladosporium spp. (33.3%), Penicillium spp. (33.3%) and a White Non-Sporulating Septated Mycelium (WNSM) (33.4%) whilst in the second sampling the isolated taxa were Cladosporium spp. (66.8%), Nigrospora spp. (6.6%) and the WNSM (16.6%). The I/O ratios per genus in 1st isolation were Cladosporium spp. = 1, Penicillium spp. = 0.9 and WNSM = 0.2 whilst in the 2nd isolation the ratios were Cladosporium spp. = 0.9, Nigrospora spp. = 0.1 and WNSM = 0.1 revealing values lower than 1.0 indicative that these taxa were not contaminating the repositories environments.
It should be noted that genera Aureobasidium, Sepedonium, Trichaegum and Wallemia were new findings for the Cuban archives.
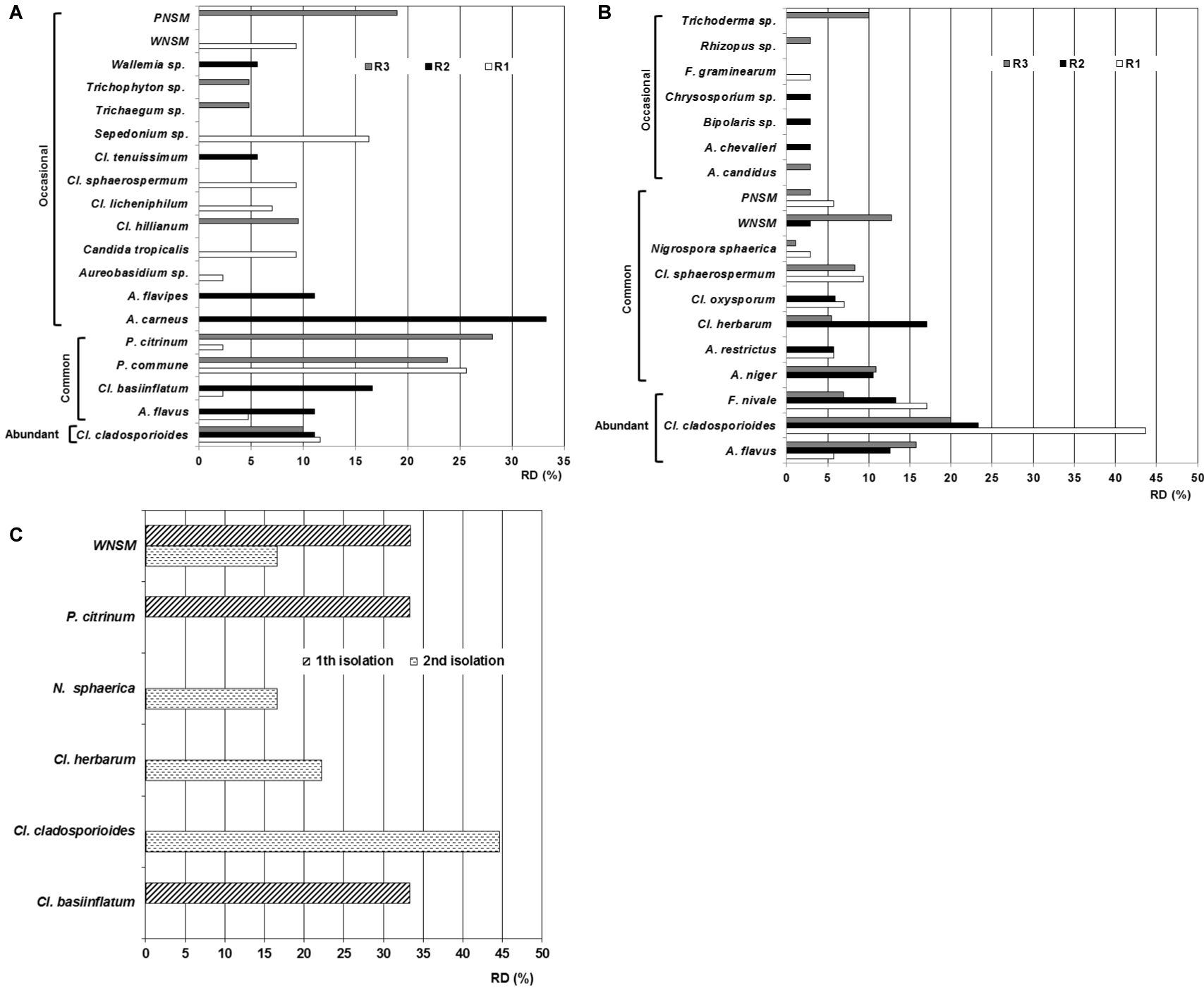
In the first isolation nineteen taxa were obtained. R-1 showed the greatest diversity of species (eleven species and one WNSM) followed by R-2 with seven species and in R-3 six species and a non-sporulating mycelium were isolated (Figure 4). For repositories the predominant specie in R-1 was P. commune followed by Sepedonium sp. and Cl. cladosporioides in second and third place respectively. In R-2 the principal species were Aspergillus carneus and Cl. basiinflatum whilst in R-3 P. citrinum prevailed followed by P. commune and the PNSM. Cladosporium cladosporioides was the most abundant specie since it was found in the three repositories with RD that ranged from 10% to 11.6%. Four species isolated in two of the three repositories were classified as common (Aspergillus flavus, Cladosporium basiinflatum, Penicillium commune and P. citrinum) whilst twelve species and two non-sporulating mycelia were considered as occasional because they were only detected in one of the three repositories; the species were: A. carneus, A. flavipes, Aureobasidium sp., Candida tropicalis, Cladosporium hillianum, Cl. licheniphilum, Cl. sphaerospermum, Cl. tenuissimum, Sepedonium sp., Trichaegum sp., Trichophyton sp. and Wallemia sp.
In the second isolation, eighteen taxa were obtained. Nine species were isolated from R-1 whilst in R-2 and R-3 twelve species were detected in each. This shows that the diversity and quantity of species per repository was slightly greater in this isolation than in the previous one. In R-1 the principal specie was C. cladosporioides followed by Fusarium nivale and Cl. sphaerospermum in second and third place respectively. In R-2 the prevailing species were Cl. cladosporioides, C. herbarum, F. nivale and A. flavus whilst in R-3 the preponderant taxa were Cl. cladosporioides, A. flavus and WNSM.
Three species were classified ecologically as abundant (A. flavus. Cl. cladosporioides and Fusarium nivale), six species and the two non-sporulating mycelia were classified as common (A. niger, A. restrictus, Cl. herbarum, Cl. oxysporum, Cl. sphaerospermum, Nigrospora sphaerica) and eight species were occasional (A. candidus, A. chevalieri, Bipolaris sp., Chrysosporium sp., Fusarium graminearum, Rhizopus sp., Torula sp., Trichoderma sp.). In relation to the prevalence of the taxa per repository it can see that in R-1 the main specie was Cl. cladosporioides followed by F. nivale, in R-2 again Cl. cladosporioides was the predominant specie followed for Cl. herbarum whilst in R-3 equally the preponderant specie was Cl. cladosporioides followed by A. flavus.
From these taxa only five were coincident in the two isolations (A. flavus, Cl. cladosporioides, Cl. sphaerospermum and the two non-sporulating mycelia) revealing a similarity of 26.3% to 27.8% that is below 50% which constitutes a coincidence level very low.
In relation to the obtained taxa in outdoor environment it is necessary to highlight that only three were isolated in the first sampling (Cl. basiinflatum, P. citrinum and WNSM) whilst four were detected in the second isolation (Cl. cladosporioides, Cl. herbarum, Nigrospora sphaerica and WNSM) revealing low taxa diversity in this environment at moment of the samplings. It should be noted that the three taxa isolated in the outdoor environment during the first isolation were detected in the indoor environment of some of the repositories studied, such that Cl. basiinflatum was isolated from the ambient air of R-1 and R-2, P. citrinum was detected in R-1 and R-3 indoor air and WNSM was detected only in R-1. Regarding the second isolation, the same thing happened; the four taxa isolated in the outdoor environment were detected in the indoor environment of some of the analyzed repositories. In this sense, Cl. cladosporioides was also isolated from the environment of the three repositories, Cl. herbarum was detected in the indoor environment of R-2 and R-3, N. sphaerica was isolated from the indoor air of R-1 and R- 3 as well as WNSM was found in the R-2 and R-3 environments.
From all the species found on indoor environments were new records for Cuban archives environments Aureobasidium sp., Cladosporium basiinflatum, Cl. hillianum, Cl. licheniphilum, F. nivale, F. graminearum, Sepedonium sp., Trichaegum sp. and Wallemia sp.
Behavior of the pathogenic attributes (virulence factors) studied in the isolated taxa
Table 3 shows the results obtained from the virulence factors analyzed "in vitro". In the first isolation, four strains (Aureobasidium sp., A. flavus 1, A. flavus 2 and A. flavipes) showed positive results to the growth at 37°C as well as the hemolysins and phospholipases excretion.
| Table 3: Virulence factors (pathogenic attributes) detected in the taxa isolated form repositories indoor air. | ||||||
| Repository | Taxa | Growth at 37°C | Hemolytic activity (hemolysis type) |
Phospholipase activity | Spores size (length x width, μm) |
RT |
| First isolation | ||||||
| R-1 | Aspergillus flavus 1 | + | β | + | 3 x 4.5 | A, B, C |
| Aureobasidium sp. | + | β | + | 7.5 - 15 x 3.5 - 6a 10 - 25 x 5 - 11b |
A, B | |
| Candida tropicalis | + | β | - | 3.8 x 2 - 7 | A, B | |
| Cladosporium basiinflatum 1 | - | - | - | 7 - 10 x 2.5 - 4 | A, B, C | |
| Cladosporium cladosporioides 1 | - | - | - | 3 - 6 x 1.5 - 2.5 | A, B, C | |
| Cladosporium cladosporioides 2 | - | - | - | 3 - 7 x 2 - 3 | A, B, C | |
| Cladosporium licheniphilum | - | - | - | 3 - 5 x 2 - 3 | A, B, C | |
| Cladosporium sphaerospermum 1 | - | β* | - | 4 x 7 | A, B | |
| Penicillium citrinum 1 | + | - | - | 2.2 x 3 | A, B, C | |
| Penicillium citrinum 2 | + | β | - | 2.4 x 3 | A, B, C | |
| Penicillium commune 1 | - | β* | + | 3 x 4 | A, B, C | |
| Penicillium commune 2 | - | β* | - | 3 x 4 | A, B, C | |
| Penicillium commune 3 | - | β* | + | 3 x 4 | A, B, C | |
| Sepedonium sp. | + | - | - | 7 - 17 | A, B | |
| WNSM 1 | - | β* | - | - | - | |
| WNSM 2 | - | - | - | - | - | |
| R-2 | Aspergillus carneus | + | β | - | 2 - 2.5 x 3 - 3.5 | A, B, C |
| Aspergillus flavus 2 | + | β | + | 3 x 5 | A, B, C | |
| Aspergillus flavipes | + | β | + | 2 x 3 | A, B, C | |
| Cladosporium basiinflatum 2 | - | - | + | 6.5 - 10 x 3 - 4 | A, B, C | |
| Cladosporium cladosporioides 3 | - | - | - | 3 - 7 x 1.5 - 2.5 | A, B, C | |
| Cladosporium tenuissimum | - | - | - | 10 - 25 x 3 - 6 | A, B, C | |
| Wallemia sp. | - | - | - | 1.5 - 2.4 | A, B, C | |
| R-3 | Cladosporium hillianum | - | - | - | 6.5 - 11 x 2.5 - 4 | A, B, C |
| Trichaegum sp. | + | - | - | 18 - 20 | A | |
| Trichophyton sp. | + | - | + | 26 - 50 x 5 - 8 | A | |
| PNSM 1 | - | - | + | - | - | |
| PNSM 2 | - | - | - | - | - | |
| Second isolation | ||||||
| R-1 | Aspergillus flavus 3 | + | β | + | 3 x 4.5 | A, B, C |
| Aspergillus restrictus 1 | - | - | - | 4 - 10 x 3 - 5.5 | A, B, C | |
| Cladosporium cladosporioides 4 | - | - | - | 3 - 7 x 1.5 - 2.5 | A, B, C | |
| Cladosporium cladosporioides 5 | - | β* | + | 3 - 6 x 1.5 - 3 | A, B, C | |
| Cladosporium sphaerospermum 2 | - | α* | + | 3 x 4.5 | A, B, C | |
| Fusarium nivale 1 | - | - | - | 15 - 20 x 3 - 5 | A, B | |
| Fusarium nivale 2 | - | - | - | 15 - 30 x 3 - 5 | A, B | |
| Nigrospora sphaerica | - | - | - | 15 x 20 | A | |
| PNSM 3 | - | - | + | - | - | |
| R-2 | Aspergillus niger 1 | + | β | - | 3.5 x 4.5 - 5 | A, B, C |
| Aspergillus restrictus 2 | - | - | + | 5 x 5.5 | A, B | |
| Bipolaris sp. | + | β | + | 14 - 30 x 6 - 11 | A | |
| Cladosporium cladosporioides 6 | - | β* | - | 3 - 7 x 1.5 - 3 | A, B, C | |
| Cladosporium herbarum | - | - | - | 5.5 - 10 x 4 - 5 | A, B | |
| Cladosporium oxysporum | - | β* | + | 3 - 5 x 2 - 3 | A, B, C | |
| Chrysosporium sp. | + | β | + | 3.5 - 7.5 x 3 - 4.5 | A, B | |
| Fusarium nivale 3 | - | - | + | 15 - 30 x 3 - 5 | A, B | |
| Torula sp. | - | - | + | 4 x 6 | A, B | |
| WNSM 3 | + | β | - | - | - | |
| R-3 | Aspergillus candidus | - | - | + | 2.5 x 3.5 - 4 | A, B, C |
| Aspergillus niger 2 | + | β | + | 3.5 x 5 | A, B | |
| Fusarium graminearum 1 | - | - | - | 30 - 40 x 4 - 5 | A | |
| Fusarium graminearum 2 | - | - | - | 30 - 50 x 3.5 - 5 | A | |
| Fusarium nivale 4 | - | - | + | 15 - 20 x 3 - 5 | A | |
| Rhizopus sp. | + | - | + | 6 - 8 x 4.5 - 8 | A, B | |
| Trichoderma sp. | - | - | + | 3.6 - 4.5 x 3.5 - 4.5 | A, B | |
| WNSM 4 | - | α* | - | - | - | |
| WNSM 5 | + | β | - | - | - | |
| PNSM 4 | - | α* | - | - | - | |
| PNSM 5 | + | β | + | - | - | |
| In the growth at 37°C: +: indicates growth, -: indicates no growth. Hemolysis type: α: alpha hemolytic (hemolysis partial), β: beta hemolytic (hemolysis total). *: Indicates that the excretion of hemolysins in this strain was determined at 30°C. RT: Respiratory Tract level to which the spores can penetrate. A: Upper Respiratory Tract. B: Trachea, Bronchi and Bronchioles. C: Alveoli. a: Indicative of hyaline conidia. b: Indicative of dark brown conidia. | ||||||
The P. commune 1strain revealed a different behavior than the P. commune 2 and 3 strains, the same happened between strains Cl. basiinflatum 1 and 2, between WNSM 1 and 2 as well as between PNSM 1 and 2 demonstrating the metabolic diversity that may exist among different strains of the same species or taxon even when they have been isolated from the same ecosystem and emphasizes the need to perform each physiological analysis to different strains representative of the colonies number of the same morphological type or morphotype. In the second isolation, 5 strains showed positive results at these three analyses; they were A. flavus 3, Bipolaris sp., Chrysosporium sp., A. niger 2 and the PNSM 5. Similarly, several strains of the same taxon showed different behaviors, such was the case A. niger 1, A. restrictus 1 and 2, Cl. cladosporioides 4, 5 and 6 as well as between WNSM 3 and 4, and PNSM 3 and 4. It should be noted that the strain of Cl. cladosporioides 5 showed β-hemolysis and excretion of phospholipase, attributes that are of a pathogenic interest. On the other hand, it is important to highlight that WNSM 5 grew at 37°C and showed β-hemolysis, dangerous attributes for human health.
In relation to the spore’s size and their penetration power in the respiratory tract, it can appreciate that in the upper respiratory tract 100% of the detected spores can penetrate; however in the first isolation only four species could enter until the trachea, bronchi and bronchioles (Aureobasidium sp., Candida tropicalis, Cladosporium sphaerospermum, Sepedonium sp.) because their size exceeds 3.5 μm; this is indicative of 14.3% of the total of the evaluated taxa. On the other hands eighteen species possesses spores so small (≤ 3 µm) that they can penetrate until the alveoli, representing a 64.3%; highlighting among them, all the species of Aspergillus and Penicillium as well as the most of the Cladosporium species. In second isolation ten species showed very small dimensions revealing that they can enter until the trachea, bronchi and bronchioles representing a 33.3% of the total of the examined taxa, and nine species (30%) are able to penetrate until the alveoli; in this group were the most of the analyzed species of Aspergillus and Cladosporium.
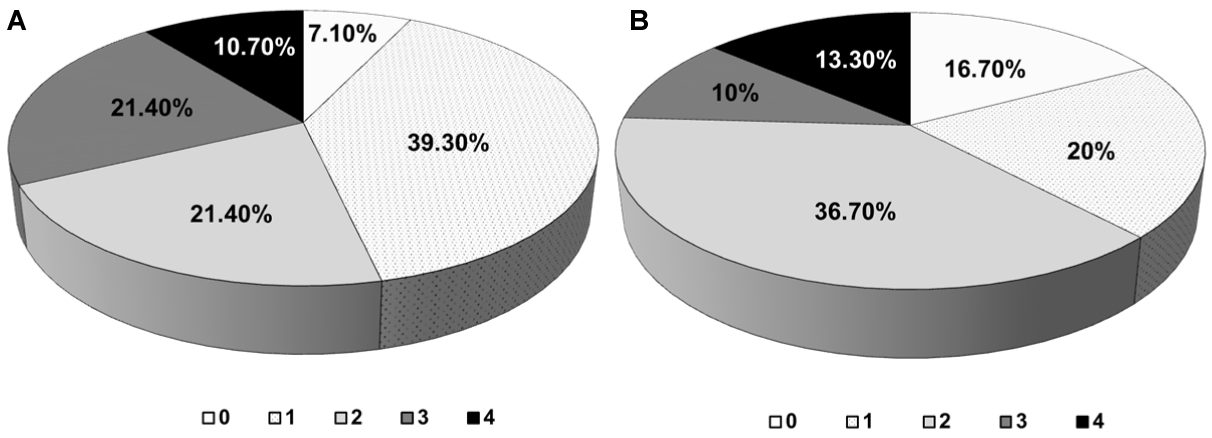
A species that shows positivity to the four tests carried out will always be more risky for health. In that sense, 10.7% of the taxa obtained in the first isolation and 13.3% of those detected in the second, showed positive results to the four virulence test "in vitro" (Figure 5), showing the potential pathogenic risk.
The environmental microbiological quality of a room or premises depends on different factors such as the activity that is carried out, the number of people that access or remain in the room, the location of the room within the building, the building location, the dusting level on the surfaces, the type of ventilation/acclimatization, the geographic and climatic zone, and time of the year, etc [30]. That is why, although work environments with similar characteristics (such as some documentary repositories) may have similarities in the environmental mycobiota, it cannot be assumed that their composition is homogeneous. To obtain accurate results, timely and frequent studies must be carried out, even more so when it comes to detecting microorganisms with implications for human health.
It is known that microclimatic conditions that induce a very high aw can be generated in a room with a low RH, therefore, the measurement of indoor RH alone can be a poor predictor of mold problems [31] and simply decreasing the RH could not be enough. In addition, many fungal spores are resistant to changes in RH and T [32]. In Cuba, the T and RH are high all year, but the natural ventilation and in particular the natural cross ventilation plays an important role in slowing down the microbial growth on the different materials that coexist in the repositories. Therefore, in this study these thermo-hygrometric variables were taken into account in their relation to the concentration and variability of the environmental micobiota of the studied repositories.
Despite this, the monthly averages of T and RH were stable in the different repositories of the PHA PR, mainly due to the characteristics of the building. The relatively small dimensions of the repositories, the distribution of repositories around the central courtyard and the existence of large windows and the entrance/exit door located in opposite positions within the repositories guarantees not only the natural ventilation but also an air flow that moves from the cooler areas to the hot yard, favoring the cooling of the environment in the repositories and promoting the stability of these thermo-hygrometric parameters.
Mycological sampling of both indoor and outdoor air showed that the concentrations were significantly lower in the rainy season. This could be due to the systematic washing of the atmosphere during the rainy months causing a decrease in the external bioaerosols, and since the repositories have natural ventilation, the decrease of the fungal load in the indoor air is favored. These results are contrary to those previously obtained in some repositories ventilated naturally in the National Archive of Cuba [1,15].
Although in the first isolation the average of fungal concentration was significantly lower, it is noteworthy that the fungal concentration showed a significant positive correlation with the RH for both isolation (85% in the first sampling and 93% in the second) while the temperature not affected significantly the fungal concentration. A similar result was previously obtained [33,34]. Although the temperature usually influences the dynamics of the mycobiota in indoor air [35], it seems to have a secondary effect by acting on other factors such as RH and air currents, which explains its behavior.
As in Cuba there is no standard that allows establishing parameters of environmental microbiological quality for cultural heritage institutions, the criteria of some authors regarding the indoor/outdoor microbial concentrations ratios (I/O) were taken into account to define when an environment is contaminated or not [36-38. In general, it is accepted that the microbial concentration on indoor air is similar to that in the open air and that most indoor fungi come from sources external [33], so the total number of fungi in indoors is lower than outdoors [39]. According to Sobral, et al. [40] when the I/O ratio is equal to or less than 1.5 is indicative that the indoor environmental conditions are good, that is, that the indoor environment is not contaminated. This behavior was obtained in this study in which most of repositories showed I/O ratios equal to or less than 1.5 (with the exception of R-2 in the second sampling) evidencing a good environmental quality due to good ventilation of repositories. When comparing the I/O ratios by taxon, it was obtained that for all of them (Cladosporium spp., Nigrospora spp., Penicillium spp. and WNSM) the obtained values were equal to or less than 1.0, revealing good quality environments, an aspect that corroborates what before exposed.
In this study, open windows and doors were one important factor which may explain the variation in prevalence and concentration of different species. It is known that air circulation can promote spore dispersion and their transportation, but at the same time it can desiccate fungal mycelium by preventing the establishment of microclimates and surface-driven humidity gradients [41]. Therefore, this air circulation ensures that the fungal propagules that enter to the environment of the repositories come out and do not deposit on the documents and furniture. The R-2 in the second sampling showed a ratio of 1.7 that reveals a slightly contaminated environment, therefore of regular quality [36,40], possibly because this repository was not efficiently ventilated in some moments and it is known that a suitable ventilation and air circulation is essential to prevent fungal spores from settling on books. This indicates the need to maintain an adequate environmental and conservation management to guarantee the correct natural ventilation and air circulation in all repositories if fungal growth on the documents is to be avoided, even in extreme situations such as heavy rains and hurricanes. It is important to highlight that in this study, fortunately, no "amplification sites" of the fungal load in the repositories environments were detected and this was due to the good circulation of natural air within the repositories. This result is contrary to those obtained in a Cuban museum [42].
In this study the fungal prevalence, in particular of the principal genera (Aspergillus and Cladosporium), is similar to other studies carried out in indoor environments of Cuban libraries, archives and museums [1,16,34,42]. Besides Aspergillus and Penicillium, Cladosporium is considered among the commonest genera found indoors with some species being predominate under these environmental conditions [4,8,32,36,37]. Also in this case the prevalence of two types of non-sporulating mycelia, one white (WNSM) and other pigmented (PNSM) was obtained. These mycelia types were also obtained in other studies in indoor environments of Cuban archives and museums [15-17,34,40] as well as in environments of archives and libraries of other countries [37,43-46].
Ecologically Cl. cladosporioides, Cl. sphaerospermum, Cl. herbarum, Cl. oxysporum and Cl. tenuissimum were abundant, common or occasional species in the samplings; but they were detected previously in other archives, libraries and museum environments [5,8,15]. However, other species of the Cladosporium genus (Cl. basiinflatum, Cl. hillianum, Cl. licheniphilum) were detected for the first time in the environment of this archive and it has not seen any report of them in the literature consulted for the indoor environments of archives hence they are new findings for the archival environments.
Three fungal species (A. flavus, Cl. cladosporioides, Cl. sphaerospermum) and the two mycelia types were common in both environmental sampling, which represents a similarity of 26.3% to 27.8%, which according to Esquivel et al. [28] is low.
Other genera were isolated in smaller quantity; such is the case of Sepedonium, Trichaegum, Trichophyton, Wallemia, Bipolaris, Chrysosporium, Rhizopus and Trichoderma that were classified ecologically as occasional.
Chrysosporium and Bipolaris genera were previously detected in the environment of the National Archive of Cuba [14,15] as well as archives and libraries of other countries [32,44,46] whilst Rhizopus has been also isolated from indoor environments of archives and library [2,6,37]. However, there are reports that some species of these genera are pathogens or opportunistic pathogens in humans hence the importance of their findings [12,13,47,48].
Among the Fusarium species detected are F. nivale and F. graminearum, both isolated for the first time from archive environments in Cuba. Fusarium graminearum (detected only in R-1 environment) was a species isolated from the environment of Havana [19], a city near to the Pinar del Río city, which evidences the possibility that it has penetrated to this repository environment from the outside at some time prior to sampling and has remained in that environment. This specie has also been reported as plant pathogen, particularly of cereals, and may cause adverse health effects in humans and domestic animals [49].
It is highlights that, for the first time in the air of Cuban archives, filamentous fungi belonging to the genera Sepedonium, Trichaegum and Wallemia were isolated. Sepedonium spp. has been isolated from the environment of Poland libraries [32]; although it is a saprophytic fungus that inhabits soil, plant material and mushroom compost [50] some cases of infection in human by this fungus have been reported [51]. On the other hands, Trichaegum spp. was detected in the Albuquerque environment, New Mexico [52] and in plants grown that grow in very humid lands [53]. Ecologically, Wallemia is a ubiquitous genus that is usually isolated from different types of foods, hypersaline water, soil, plants and indoor environments; particularly from house dust [10]. Also, this genus has been isolated from libraries and archives environments [6,43] and it has pathogenic species as it is for example Wallemia sebi [54].
In comparison to filamentous fungi, and in particular considering the airborne species, for yeast genera there is little data on their diversity and abundance. Only in the first sampling also two yeast species were detected one was Candida tropicalis and the other Aureobasidium sp. Instead, in the second isolation only a yeast species was isolated (Torula sp.). This species was detected on indoor air of the National Library of Poland by Zerek [32] and was noticed previously on indoor environment of repositories of the National Archive of Cuba [14].
Candida species have been isolated from archival, library and museum environments [5,55,56]; even some species was isolated from the dust of various libraries in Brazil [46]. These species are considered opportunistic pathogens in immunocompromised people [47,57]. Species of Aureobasidium (one of several genera of "black yeasts") have also been detected in the air of libraries and museums in Cuba [42], in the museums indoor air of another countries [5,56], in libraries indoor air [6,8,37,46] and archives [43,44,58]. Aureobasidium is a widely distributed fungal genus usually found in soil, fresh water, dead plant material, marine estuary sediments and wood [59]. This genus has also been observed to grow on textiles, foodstuffs, fruits and painted surfaces. In the indoor environment, Aureobasidium growth is commonly found in moist places such as bathrooms and kitchens, especially on shower curtains, tile grout and windowsills. The spores are usually disseminated by wind (when dry) and water and some species have been reported as human pathogenic [60].
In the first sampling, from the R-1 and R-2 were isolates A. flavus and Cl. basiinflatum but in the outdoor environment only were isolated Cl. basiinflatum and the white mycelium with a high predominance, and in a smaller proportion the pigmented mycelium. This evidences the entry of these taxa into the repositories air from the outdoor environment. However, Cl. cladosporioides that was abundant in the indoor air of the repositories, or A. flavus that was detected in two repositories (R-1 and R-2) were not species detected in the outdoor, the same happened with the rest of the species identified inside the different repositories; this result is contrary to previous reports that indicate to this Cladosporium species as the most abundant in outside environments of many countries [36] and of Cuba [61]. This may be due to the loss of viability of the spores in the air due to the high radiation and temperature of the tropics, particularly in the months of intense heat such as June, which manifests with greater emphasis at 11:00 am [19], approximate time in which sampling was done.
Cladosporium basiinflatum was a species first detected in the indoor environment of a Cuban archive, and was also recently isolated for first time from the outdoor Havana environment, capital of Cuba [19]. This species is European, particularly from Germany where it has been isolated from plants. Although in the Pinar del Río province there is no station that monitors the fungi in the outdoor environment, this finding made it possible to know that this species was found in the outdoor environment of the province but it was detected in the indoor environments. On the other hand, its finding in the indoor environment of the PHA PR indicates that it has dispersed in other environments outside the Capital.
In the first sampling, Cl. basiinflatum, P. citrinum and WNSM were taxa isolated from outdoor environment and were detected also on indoor environments whilst in the second isolation Cl. cladosporioides, Cl. herbarum, Nigrospora sphaerica and WNSM were the taxa detected in both outdoor and indoor environments revealing a 100% coincidence for both isolations. This result can be indicative that these species came from the outdoor environment.
The survival ability of fungi in the air on indoor environments is influenced by the RH factor. Relative humidity values in the repositories can support fungal growth because there are some of them that can grow at RHs of ≤ 90%, even < 70% [6]. On the other hand, the materials biodeterioration in archive and library is more commonly caused by fungi. Depending on individual characteristics of each species, they may grow in a wide range of moisture conditions, from xerophilic to hydrophilic. Those more hazardous to documentary heritage are xerophilic fungi. According to these approaches some isolated species of the repositories environments in the PHA PR has potentialities to be pioneer, secondary and tertiary colonizer according to informed by Górny [62].
Cabral [38] and Pinzari [41] reported that fungi can serve as bioindicators of air quality because, depending on the presence and concentration of some genera, wet environments and diseased buildings can be discovered (Sick Building Syndrome, SBS); therefore they constitute an advertisement of dangerousness environmental for health. These authors suggest that the predominance of Aspergillus spp. and Penicillium spp. is indicative of an indoor environment with humidity in the building and evidence that the building is sick; on the contrary if Cladosporium is the predominant genus then the building is healthy. On the other hand, Guild and MacDonald [63] and Karbowska-Berent et al. [33] reported that up to 50 CFU/m3 is the concentration accepted for each species that is detected so that it does not represent a risk to health whilst other authors indicate that for individual fungi. According to this criterion, the repositories showed healthy environments particularly in the second sampling, since most of the species were detected in concentrations lower than 50 CFU/m3 with the exception of Cl. cladosporioides which reached concentrations of up to 210 CFU/m3, but this genus is indicative of a healthy environment as mentioned earlier.
Generally, the archive and library workers are at no particular health risk if the collections are stored under appropriate hygienic and climatic conditions and the construction standards for this type of facilities are complied with. If the storage conditions change, e.g. the indoor T or RH increases, the collections may become colonized with microscopic fungi presenting a health hazard to the personnel. In addition, when already contaminated documents are removed for restoration and maintenance, workers are exposed to these microorganisms that exist on the documents, in the dust and on the surface of the wall of the repositories. Under conditions of increased RH, the microorganisms, including fungi, form bioaerosols that are inhaled by the workers. Colonization with microorganisms and formation of bioaerosols can also proceed when the level of indoor humidity is normal but the volume of dust on the documents and inside the archive building is high. The type of work that make archive and library workers particularly exposed to microscopic fungi through skin contact and bioaerosols inhalation include: restoration and maintenance activities, transportation to and from the storage site, destroying damaged documents with a shredder, examination and remediation of contaminated documents, maintenance and repair of ventilation systems. Therefore these microenvironments constitute reservoirs of spores and other fungal propagules that can have a marked allergenic character and become an important risk factor considering the high dose of allergens and the long exposure time of the individuals who access the repository during the working day. For these reasons, personal protective devices, particularly suitable masks, should be used whenever possible as they protect the health of employees efficiently.
The inhalation of the fungal spores, fragments of hyphae and other fungal fragments even in conditions of non-viability can trigger several health affectations of allergen type in pre-sensitized individuals fundamentally. These spores and fungal fragments can come into contact with the nasopharyngeal mucosa and trigger allergies or infections [64,65]. Also, it is known that these spores can penetrate the human respiratory tract, particularly those of smaller size (≤ 3 µm) and can reach the alveoli causing invasive mycosis on immunocompromised people principally [66]. Likewise, fungi may colonize the human body and may damage airways by the production of toxins, catalases, proteases, phospholipases, lipases and other important enzymes as well as volatile organic compounds. Thus, fungi have a greater impact on the people’ immune system than any other allergenic sources [11].
The individual capacity of a microorganism to cause diseases depends on a series of mechanisms known as virulence factors or pathogenic attributes [9,67]. In filamentous fungi these are diverse and vary according to the group, the genus or the species [68].
In this study, the hemolysins and phospholipases production at 37°C were evaluated using common solid test media because these analyses count among the virulence factors of many human pathogenic fungi. It is known that the growth of fungi at 37°C is a virulence factor necessary and essential to consider a potentially pathogenic fungal strain, because this is the body temperature of humans and other warm-blooded animals [10,69]. The development under this condition allows the invasion of epithelial, endothelial and blood vessels surfaces. Furthermore, according to Brunke et al. [67] the expression of some genes involved in invasive mechanisms occur at that temperature. For these reason, this parameter was evaluated in all strains isolated from repositories air.
Hemolysins are toxins produced by certain microorganisms and are secreted into the extracellular medium producing erythrocytes rupture [70]. The imbalance in the osmotic pressure of the cell due to the pores formed by these proteins has been suggested as the mechanism of hemolysis. This phenomenon compromises the transport of oxygen to the tissues, triggers episodes of anemia and facilitates the invasion of blood vessels by the pathogen because iron is a very necessary compound for metabolic processes, as it requires it as an enzymatic cofactor [70].
All cells are surrounded by a membrane, which in turn is composed of lipids, phospholipids, among other biomolecules. Its functions include nutrient transport and power generation, in addition to receiving signals, etc. It is therefore the integrity of the cell membrane is vital to the cell. Phospholipases are a group of enzymes secreted by various pathogenic microorganisms have the ability to damage cell membranes of the host. The ability to produce and secrete enzymes with phospholipase activity of certain fungi has been considered as a potential virulence factor that contributes significantly to their pathogenicity [71], as they are involved in important physiological processes such as fungal dissemination in the host and the defense or evasion to mechanisms of the immune system [72].
From the obtained isolates, some taxa were able to grow at 37°C and the enzymes hemolysins and phospholipases were secreted evidencing the high risk that they represent to human health. Among the most risky species highlighted Aureobasidium sp., Aspergillus flavus 1 and 2, and Aspergillus flavipes obtained in first sampling as well as Aspergillus flavus 3, Bipolaris sp., Chrysosporium sp., Aspergillus niger 2 obtained from the second isolation. It is important to highlight that in this last isolation the taxa Aspergillus niger 1, Rhizopus sp., and PNSM 5 could also be dangerous for the health although they show only three pathogenic attributes (75% of the total). This result is consistent with the reports of de Hoog et al. [47] and Gostinčar et al. [60]. In this study the size of the spores shows that the most species belonging to the genera Aspergillus, Cladosporium and Penicillium can penetrate to the alveoli increasing the risk to health. Similar results were reported in previously studies done in Cuban archives [14,17].
With relation to the non-sporulating mycelia Pounder et al. [73] determined by molecular biology that these mycelia can be non-sporulating forms of pathogenic fungi, so they can be potential emerging pathogens. Besides, other authors have been reported similar results [74]. This may explain the fact that PNSM 5 showed positivity to the most "in vitro" virulence tests performed.
These obtained results with the Aspergillus species are agree with those reported by other authors, who detected hemolysin production in strains of Aspergillus flavipes, A. flavus and A. niger using a methodology similar to what was used in this research [15,17,75]. Moreover, it has been reported the detection of more than one type of hemolysin in strains of Aspergillus flavus and A. niger, producing species par excellence such mycotoxins which can be inhaled and absorbed by skin [76] and clinical importance widely cited [77]. Bomogolova and Kirtsideli [29] studied the phospholipase activity in fungi isolated from outdoor environments of the city of St. Petersburg. They found that a representative of the genus Chrysosporium showed positive enzymatic activity, which is consistent with this study.
It should be noted that some species of Cladosporium also showed a marked hemolytic activity constituting this an interesting result. They were Cl. sphaerospermum 1, Cl. cladosporioides 5, Cl. cladosporioides 6 and Cl. oxysporum. Of these, two species also showed phospholipase activity (Cl. cladosporioides 5, Cl. oxysporum). The phospholipase activity in Cl. cladosporioides was reported previously [29,78].
In filamentous fungi, the virulence factors and the pathogenicity strategies are diverse and vary according to the group, the genus or the species [9]. The union of several virulence factors in the same strain is a necessary condition to classify them as potentially pathogenic [64]. Although other pathogenic attributes that determine the potential and extent of the infection can be shown in the infection process, the pathogenic attributes examined in this study are of vital importance in fungi with multiple host capacity [79].
With the virulence factors analyzed (pathogenic attributes) it was obtained that 100% of fungal spores can be inhaled and are trapped in the upper respiratory tract and that only about 45% have sizes that allow them to reach the lower respiratory tract . It was also obtained that 28% of isolated fungal strains can grow at 37°C and excretes hemolysins but 10.7% of the obtained strains in the first isolation and 13.3% of the obtained strains in second isolation evidenced the four studied pathogenic attributes. Although the immune system of the personnel protects them from possible respiratory diseases, the detected attributes show the potential risk to which the personnel are exposed during the working day, hence the need for the personnel not to trust the environmental fungi just because they remain in the environment air since they represent a potential health risk, therefore, it is important that personnel protect themselves during the working day using suitable protective equipment and in particular correct masks, fundamentally to avoid inhalation of fungal propagules during handling and manipulation of documents.
These results emphasize the need to perform microbiological studies periodically and to know the fungal variability (including their physiological and pathogenic characteristics) that may exist between apparently similar indoor environments.
The authors want to thank the logistical support that the CITMA Delegation of the of Pinar del Río city provided.
Funding Information
This research project is financially supported by the Ministry of Science, Technology and Environment (CITMA) of Cuba (grant number I-2118025001).
- Borrego S, Perdomo I. Aerobiological Investigations Inside Repositories of the National Archive of the Republic of Cuba. Aerobiologia. 2012;28(3):303-316. https://bit.ly/37fYnIn
- Kadaifciler DG. Bioaerosol Assessment in the Library of Istanbul University and Fungal Flora Associated with Paper Deterioration. Aerobiologia. 2017;33(1):151-166. https://bit.ly/3nhn1xW
- Okpalanozie OE, Adebusoye SA, Troiano F, Cattò C, Ilori MO, Cappitelli F. Assessment of Indoor Air Environment of a Nigerian Museum Library and its Biodeteriorated Books Using Culture-Dependent and Independent Techniques. Int Biodeter Biodegr. 2018;132:139-149. https://bit.ly/3mkPCkp
- Lavín P, Gómez de Saravia SG, Guiamet P. An Environmental Assessment of Biodeterioration in Document Repositories. Biofouling. 2014;30(5):561-569. https://bit.ly/3qQBB1w
- Skóra J, Gutarowska B, Pielech-Przybylska K, Stępień Ł, Pietrzak K, Piotrowska M, Pietrowski P. Assessment of Microbiological Contamination in the Work Environments of Museums, Archives and Libraries. Aerobiologia. 2015;31:389-401. https://bit.ly/2K1c59g
- Rahmawati SL, Zakaria L, Rahayu ES. The Diversity of Indoor Airborne Molds Growing in the University Libraries in Indonesia. Biodiversitas. 2018;19(1):194-201. https://bit.ly/2W8S6YK
- Sullivan TS, Ramkissoon S, Garrison VH, Ramsubhag A, Thies JE. Siderophore Production of African Dust Microorganisms over Trinidad and Tobago. Aerobiologia. 2012;28(3):391-401. https://bit.ly/3gM8SGk
- Micheluz A, Manente S, Prigione V, Tigini V, Varese GC, Ravagnan G. The Effects of Book Disinfection to the Airborne Microbiological Community in a Library Environment. Aerobiologia. 2018;34:29-44. https://bit.ly/3oQ1756
- Heitman J. Microbial Pathogens in the Fungal Kingdom. Fungal Biol Rev. 2011 Mar 1;25(1):48-60. doi:10.1016/j.fbr.2011.01.003. PMID: 21528015;PMCID: PMC3081590.
- Yang C, Pakpour S, Klironomos J, Li DW. Microfungi in Indoor Environments: What is Known and What is Not. In:Li DW. (Ed.), Biology of Microfungi. Switzerland: Springer International Publishing; 2016. p. 373-412. https://bit.ly/37dgvT8
- Dey D, Ghosal K, Bhattacharya SG. Aerial Fungal Spectrum of Kolkata, India, Along with their Allergenic Impact on the Public Health: A Quantitative and Qualitative Evaluation. Aerobiologia. 2019;35:15-25. https://bit.ly/2LyeCbs
- Suchonwanit P, Chaiyabutr C, Vachiramon V. Primary Cutaneous Chrysosporium Infection following Ear Piercing: A Case Report. Case Rep Dermatol. 2015 Jul 7;7(2):136-40. doi:10.1159/000436989. PMID: 26269703;PMCID: PMC4519602.
- Andrianaki AM, Kyrmizi I, Thanopoulou K, Baldin C, Drakos E, Soliman SSM, Shetty AC, McCracken C, Akoumianaki T, Stylianou K, Ioannou P, Pontikoglou C, Papadaki HA, Tzardi M, Belle V, Etienne E, Beauvais A, Samonis G, Kontoyiannis DP, Andreakos E, Bruno VM, Ibrahim AS, Chamilos G. Iron restriction inside macrophages regulates pulmonary host defense against Rhizopus species. Nat Commun. 2018 Aug 20;9(1):3333. doi:10.1038/s41467-018-05820-2. Erratum in:Nat Commun. 2018 Nov 22;9(1):5015. PMID: 30127354;PMCID: PMC6102248.
- Borrego S, Molina A. Determination of Viable Allergenic Fungi in the Documents Repository Environment of the National Archive of Cuba. Austin J Public Health Epidemiol. 2018;5(3):1077. https://bit.ly/2IKKUii
- Borrego, S, Molina A, Santana A. Fungi in Archive Repositories Environments and the Deterioration of the Graphics Documents. EC Microbiol. 2017;11(5):205-226. https://bit.ly/349zcFj
- Borrego S, Perdomo I. Airborne microorganisms cultivable on naturally ventilated document repositories of the National Archive of Cuba. Environ Sci Pollut Res Int. 2016 Feb;23(4):3747-57. doi:10.1007/s11356-015-5585-1. Epub 2015 Oct 24. PMID: 26498813.
- Borrego S, Molina A. Behavior of the Cultivable Airborne Mycobiota in Air-Conditioned Environments of Three Havanan Archives, Cuba. Journal of Atmospheric Science Research. 2020;3(1):16-28. https://bit.ly/3a7Njil
- Sánchis J. Los Nueve Parámetros Más Críticos en el Muestreo Microbiológico del Aire. Rev Tecn Lab. 2002;276:858-862. https://bit.ly/2K1XSc7
- Sánchez KC, Almaguer M, Pérez I, Rojas TI, Aira MJ. Diversidad Fúngica en la Atmósfera de la Habana (Cuba) Durante Tres Períodos Poco Lluviosos. Rev Int Contam Ambie. 2019;35(1):137-150. https://bit.ly/3qSrWHR
- Barnett HL, Hunter BB. Illustrated Genera of Imperfect Fungi. 4th ed. Minneapolis: Burgués; 2003. https://bit.ly/3gIggm8
- Domsch KH, Gams W, Anders TH. (Eds.). Compendium of Soil Fungi. Vol. 1. London: Academic Press LTD; 1980. https://bit.ly/38gnRVx
- Klich MA, Pitt JI. A Laboratory Guide to the Common Aspergillus Species and their Teleomorphs. North Ryde, New South Wales, Australia: Commonwealth Scientific and Industrial Research Organization. 1994.
- Pitt JI. A Laboratory Guide to Common Penicillium Species. 3rd ed. North Ryde, New South Wales, Australia: Food Science. 2000.
- Ellis MB. More Dematiaceous Hyphomycetes. Kew, Surrey, England: Commonwealth Mycological Institute. 1976. https://bit.ly/3a8KzkP
- Bensch K, Groenewald JZ, Dijksterhuis J, Starink-Willemse M, Andersen B, Summerell BA, Shin HD, Dugan FM, Schroers HJ, Braun U, Crous PW. Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales). Stud Mycol. 2010;67:1-94. doi:10.3114/sim.2010.67.01. PMID: 20877444;PMCID: PMC2945380.
- Both C. Fusarium. Laboratory Guide to the Identification of the Major Species. Kew, England: Commonwealth Mycological Institute. 1977. https://bit.ly/34b7qZ5
- Smith G. Ecology and Field Biology. 2nd ed. New York: Harper & Row; 1980. https://bit.ly/3oSf6Hw
- Esquivel PP, Mangiaterra M, Giusiano G, Sosa MA. Microhongos Anemófilos en Ambientes Abiertos de Dos Ciudades del Nordeste Argentino. Bol Micol. 2003;18:21-28. https://bit.ly/3qRlKjb
- Bogomolova EV, Kirtsideli I. Airborne Fungi in Four Stations of the St. Petersburg Underground Railway System. Int Biodeter Biodegr. 2009;63(2):156-160. https://bit.ly/3gIbUeS
- Grbić ML, Stupar M, Vukojević J, Maričić I, Bungur N. Molds in Museum Environments: Biodeterioration of Art Photographs and Wooden Sculptures. Arch Biol Sci Belgrade. 2013;65(3):955-962. https://bit.ly/2K20iYf
- Fog Nielsen K. Mycotoxin production by indoor molds. Fungal Genet Biol. 2003 Jul;39(2):103-17. doi:10.1016/s1087-1845(03)00026-4. PMID: 12781669.
- Zerek BF. Shortmo uldre View. In: The Preservation and Protection of Library Collections. A Practical Guide to Microbiological Controls. Chandos Publishing, Elsevier Ltd; 2014. p. 23-96. https://bit.ly/37fmlmW
- Karbowska-Berent J, Gorny RL, Strzelczyk AB, Wlazło A. Airborne and Dust Borne Microorganisms in Selected Polish Libraries and Archives. Build Environ. 2011;46:1872-1879. https://bit.ly/3a4OI9r
- Rodríguez JC, Rodríguez B, Borrego S. Evaluación de la Calidad Micológica Ambiental del Depósito de Fondos Documentales del Museo Nacional de la Música de Cuba en Época de Lluvia. AUMGMDOMUS. 2014;6:123-146. https://bit.ly/3nhvQb2
- Stryjakowska-Sekulska M, Piotraszewska-Pająk A, Szyszka A, Nowicki M, Filipiak M. Microbiological Quality of Indoor Air in University Rooms. Pol J Environ Stud. 2007;16(4):623-632. https://bit.ly/3gLHpEJ
- Salonen H, Duchaine C, Mazaheri M, Clifford S, Morawska L. Airborne Culturable Fungi in Naturally Ventilated Primary School Environments in a Subtropical Climate. Atmos Environ. 2015;106:412-418. https://bit.ly/3nhw6H2
- Pyrri I, Tripyla E, Zalachori A, Chrysopoulou M, Parmakelis A, Kapsanaki-Gotsi E. Fungal Contaminants of Indoor Air in the National Library of Greece. Aerobiologia. 2020; doi: 10.1007/s10453-020-09640-0.
- Cabral JP. Can we use indoor fungi as bioindicators of indoor air quality? Historical perspectives and open questions. Sci Total Environ. 2010 Sep 15;408(20):4285-95. doi:10.1016/j.scitotenv.2010.07.005. Epub 2010 Jul 23. PMID: 20655574.
- Sobral LV, Melo KN, Souza CM, Silva SF, Silva GLR, Silva ALF, Wanderley KAA, Oliveira IS, Cruz R. Antimicrobial and enzymatic activity of anemophilous fungi of a public university in Brazil. An Acad Bras Cienc. 2017;89(3 Suppl):2327-2340. doi:10.1590/0001-3765201720160903. Epub 2017 Oct 26. Erratum in:An Acad Bras Cienc. 2018 Jul-Sep;90(3):3223-3239. PMID: 29091106.
- Borrego S, Molina A. (2019). Fungal Assessment on Storerooms Indoor Environment in the National Museum of Fine Arts, Cuba. Air Qual Atmos Health. 2019;12:1373-1385. https://bit.ly/37YPEJK
- Pinzari F. Microbial Ecology of Indoor Environments. The Ecological and Applied Aspects of Microbial Contamination in Archives, Libraries and Conservation Environments. In: Abdul-Wahab SA. (Ed.). Sick Building Syndrome in Public Buildings and Workplaces. Springer Berlin Heidelberg; 2011. pp. 153-178. https://bit.ly/3mc4Qbr
- Rojas TI, Aira MJ, Batista A, Cruz IL, González S. Fungal Biodeterioration in Historic Buildings of Havana, Cuba. Grana. 2012;51(1):44-51. https://bit.ly/3qNFBjg
- Roussel S, Reboux G, Millon L, Parchas MD, Boudih S, Skana F, Delaforge M, Rakotonirainy MS. Microbiological evaluation of ten French archives and link to occupational symptoms. Indoor Air. 2012 Dec;22(6):514-22. doi:10.1111/j.1600-0668.2012.00781.x. Epub 2012 Apr 18. PMID: 22429323.
- Pinheiro AC, Sequeira SO, Macedo MF. Fungi in archives, libraries, and museums:a review on paper conservation and human health. Crit Rev Microbiol. 2019 Sep-Nov;45(5-6):686-700. doi:10.1080/1040841X.2019.1690420. Epub 2019 Dec 9. PMID: 31815562.
- Micheluz A, Manente S, Tigini V, Prigione V, Pinzari F, Ravagnan G, Varese GC. The Extreme Environment of a Library:Xerophilic Fungi Inhabiting Indoor Niches. Int Biodeter Biodegr. 2015;99:1-7. https://bit.ly/2We9qeY
- Leite-Jr DP, Pereira RS, Almeida WS, Simões SAA, Yamamoto ACA., Souza JVR, et al. Indoor air mycological survey and occupational exposure in libraries in Mato Grosso-Central Region-Brazil. Advances in Microbiology. 2018;8:324-353. https://bit.ly/37g5PmV
- de Hoog GS, Guarro G, Gene J, Figueras MJ. Atlas of Clinical Fungi. 2nd edn. España: Universidad Rovira I Virgili Reus. 2000. https://bit.ly/3mgSDlY
- da Cunha KC, Sutton DA, Fothergill AW, Cano J, Gené J, Madrid H, De Hoog S, Crous PW, Guarro J. Diversity of Bipolaris species in clinical samples in the United States and their antifungal susceptibility profiles. J Clin Microbiol. 2012 Dec;50(12):4061-6. doi:10.1128/JCM.01965-12. Epub 2012 Oct 10. PMID: 23052310;PMCID: PMC3502984.
- Keller MD, Bergstrom GC, Shields EJ. The aerobiology of Fusarium graminearum. Aerobiologia. 2014;30:123-136. https://bit.ly/2WexCh2
- EMLab. Sepedonium sp. In: Fungal Library. EMLab P & K; 2019. https://bit.ly/3gO8HdJ
- Yogo N, Shapiro L, Erlandson KM. Sepedonium intra-abdominal infection:a case report and review of an emerging fungal infection. J Antimicrob Chemother. 2014 Sep;69(9):2583-5. doi:10.1093/jac/dku138. Epub 2014 Apr 30. PMID: 24788660.
- Dupont EM, Field RC, Leathers CR, Northey WT. A survey of the airborne fungi in the Albuquerque, New Mexico, metropolitan area. J Allergy. 1967 Apr;39(4):238-44. doi:10.1016/0021-8707(67)90017-2. PMID: 5228670.
- Mazurkiewicz-Zapałowicz K, Wróbel M, Silicki A, Wolska M. Studies on Phytopathogenic and Saprotrophic Fungi in Rush Associations of Lake Glinno (NW Poland). Acta Mycol. 2006;41(1):125-138. https://bit.ly/3gJSnKW
- Nguyen HD, Jančič S, Meijer M, Tanney JB, Zalar P, Gunde-Cimerman N, Seifert KA. Application of the phylogenetic species concept to Wallemia sebi from house dust and indoor air revealed by multi-locus genealogical concordance. PLoS One. 2015 Mar 23;10(3):e0120894. doi:10.1371/journal.pone.0120894. Erratum in:PLoS One. 2015;10(5):e0129752. PMID: 25799362;PMCID: PMC4370657.
- Harkawy A, Górny RL, Ogierman L, Wlazło A, Ławniczek-Wałczyk A, Niesler A. Bioaerosol assessment in naturally ventilated historical library building with restricted personnel access. Ann Agric Environ Med. 2011;18(2):323-9. PMID: 22216807.
- Gutarowska B, Skora J, Zduniak K, Rembisz D. Analysis of the sensitivity of microorganisms contaminating museums and archives to silver nanoparticles. Int Biodeter Biodegr. 2012;68:7-17. https://bit.ly/3qWJTEW
- Pouranik M, Mishra G. Onychomycosis: Review. EC Microbiol. 2017;5(3):81-85.
- Paiva de Carvalho H, Mesquita N, Trovão J, Fernández S, Pinheiro AC, Gomes V, et al. Fungal Contamination of Paintings and Wooden Sculptures Inside the Storage Room of a Museum: A Recurrent Norms and Reference Values Adequate? J Cult Herit. 2018;34:268-276. https://bit.ly/2Ltrak9
- Yang CS, Li D-W. Ecology of Fungi in the Indoor Environment. In: Yang CS, Heinshon PA. (Eds.). Sampling and Analysis of Indoor Microorganisms. John Wiley & Sons, Inc; 2007. p. 191-214. https://bit.ly/3oTkeev
- Gostinčar C, Ohm RA, Kogej T, Sonjak S, Turk M, Zajc J, Zalar P, Grube M, Sun H, Han J, Sharma A, Chiniquy J, Ngan CY, Lipzen A, Barry K, Grigoriev IV, Gunde-Cimerman N. Genome sequencing of four Aureobasidium pullulans varieties: biotechnological potential, stress tolerance, and description of new species. BMC Genomics. 2014 Jul 1;15:549. doi:10.1186/1471-2164-15-549. PMID: 24984952; PMCID: PMC4227064.
- Almaguer M, Aira MJ, Rodríguez-Rajo FJ, Rojas TI. Temporal Dynamics of Airborne Fungi in Havana (Cuba) During Dry and Rainy Seasons: Influence of Meteorological Parameters. Int J Biometeorol. 2014;58:1459-1470. https://bit.ly/3gIivWR
- Górny RL. Filamentous microorganisms and their fragments in indoor air--a review. Ann Agric Environ Med. 2004;11(2):185-97. PMID: 15627323.
- Guild S, MacDonald M. Mould Prevention and Collection Recovery: Guidelines for Heritage Collections. Technical Bulletin No. 26. Canada: Canadian Conservation Institute (CCI). 2004. https://bit.ly/380qJpl
- Green BJ, Tovey ER, Sercombe JK, Blachere FM, Beezhold DH, Schmechel D. Airborne fungal fragments and allergenicity. Med Mycol. 2006 Sep;44 Suppl 1:S245-55. doi: 10.1080/13693780600776308. PMID: 17050446.
- Lin WR, Chen YH, Lee MF, Hsu LY, Tien CJ, Shih FM, Hsiao SC, Wang PH. Does Spore Count Matter in Fungal Allergy?:The Role of Allergenic Fungal Species. Allergy Asthma Immunol Res. 2016 Sep;8(5):404-11. doi:10.4168/aair.2016.8.5.404. PMID: 27334778; PMCID: PMC4921694.
- Twaroch TE, Curin M, Valenta R, Swoboda I. Mold allergens in respiratory allergy:from structure to therapy. Allergy Asthma Immunol Res. 2015 May;7(3):205-20. doi:10.4168/aair.2015.7.3.205. Epub 2015 Mar 11. PMID: 25840710;PMCID: PMC4397360.
- Brunke S, Mogavero S, Kasper L, Hube B. Virulence factors in fungal pathogens of man. Curr Opin Microbiol. 2016 Aug;32:89-95. doi:10.1016/j.mib.2016.05.010. Epub 2016 May 31. PMID: 27257746.
- Woodcock A. Moulds and asthma:time for indoor climate change? Thorax. 2007 Sep;62(9):745-6. doi:10.1136/thx.2007.079699. PMID: 17726167;PMCID: PMC2117312.
- Köhler JR, Hube B, Puccia R, Casadevall A, Perfect JR. Fungi that Infect Humans. Microbiol Spectr. 2017 Jun;5(3). doi:10.1128/microbiolspec.FUNK-0014-2016. PMID: 28597822.
- Nayak AP, Green BJ, Beezhold DH. Fungal hemolysins. Med Mycol. 2013 Jan;51(1):1-16. doi:10.3109/13693786.2012.698025. Epub 2012 Jul 9. PMID: 22769586;PMCID: PMC4663657.
- Ghannoum MA. Potential role of phospholipases in virulence and fungal pathogenesis. Clin Microbiol Rev. 2000 Jan;13(1):122-43, table of contents. doi:10.1128/cmr.13.1.122-143.2000. PMID: 10627494;PMCID: PMC88936.
- Webb BJ, Blair JE, Kusne S, Scott RL, Steidley DE, Arabia FA, Vikram HR. Concurrent pulmonary Aspergillus fumigatus and mucor infection in a cardiac transplant recipient:a case report. Transplant Proc. 2013 Mar;45(2):792-7. doi:10.1016/j.transproceed.2012.03.056. Epub 2012 Sep 15. PMID: 23267784.
- Pounder JI, Simmon KE, Barton CA, Hohmann SL, Brandt ME, Petti CA. Discovering potential pathogens among fungi identified as nonsporulating molds. J Clin Microbiol. 2007 Feb;45(2):568-71. doi:10.1128/JCM.01684-06. Epub 2006 Nov 29. PMID: 17135442;PMCID: PMC1829023.
- Jeyaprakasam NK, Razak MF, Ahmad NA, Santhanam J. Determining the Pathogenic Potential of Non-sporulating Molds Isolated from Cutaneous Specimens. Mycopathologia. 2016 Jun;181(5-6):397-403. doi:10.1007/s11046-016-9984-8. Epub 2016 Feb 3. PMID: 26847667.
- Theeb BO, Farooq IM, Hashim AJ. Hashim S. Purification of Hemolysin from Aspergillus fumigatus and Study its Cytotoxic Effect on Normal Cell Line (REF) in Vitro. Egypt. Acad. J. Biol. Sci., G Microbiol. 2013;5:35-43. https://bit.ly/2W9fXYi
- Masaphy S, Ezra R. Targeted Inspection of Environmental Mycological Load for Mitigation of Indoor Mold Toward Improved Public Health. J Microb Biochem Technol. 2016;8(5):449-458. https://bit.ly/3qVBLVd
- Oliveira M, Caramalho R. Aspergillus fumigatus:A More Airborne Particle or a Powerful Biohazard?. NACC. 2014;21:20-34. https://bit.ly/345y0D5
- Pérez I, Sánchez KC. Aspectos Fisiológicos del Género Cladosporium Desde la Perspectiva de sus Atributos Patogénicos, Fitopatogénicos y Biodeteriorantes. Rev Cub Cienc Biol. 2019;7(1):1-10. https://bit.ly/2JRRIeL
- Valencia-Guerrero MF, Quevedo-Hidalgo B, Franco-Correa M, Díez-Ortega H, Parra-Giraldo C.M, Rodríguez-Bocanegra MX. Evaluación de Actividades Enzimáticas de Fusarium spp., Aislados de Lesiones en Humanos, Animales y Plantas. Universitas Scientiarum. 2011;16(2):147-159. https://bit.ly/3gHoMC6
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.