> Medicine Group. 2020 November 04;1(7):292-296. doi: 10.37871/jbres1156.
-
Subject area(s):
- Neonatal Care
- Pediatrics
Diagnosing Fetal Skeletal Dysplasia Using Three-Dimensional Computed Tomography: A Study Protocol for an Interventional Study
Miyoko Waratani*, Fumitake Ito, Yukiko Tanaka, Mabuchi Aki, Taisuke Mori and Jo Kitawaki
- Fetal skeletal dysplasia
- Prenatal diagnosis
- Three-dimensional computed tomography
- Shortening of long bones
- Gene expression
Methods: In total, 50 pregnant women who undergo three-dimensional computed tomography for the diagnosis of fetal skeletal dysplasias will be included. The primary outcome is prenatal diagnostic accuracy for fetuses with skeletal dysplasias. The secondary outcome is the safety from radiation exposure.
Results and conclusion: Three-dimensional computed tomography should be considered for the prenatal diagnosis of fetal skeletal dysplasias, as it is important to judge whether the prognosis is favorable or lethal. When considering the risk of radiation exposure, high quality images that are adequate for a diagnosis have been obtained using low-dose three-dimensional computed tomography scans. This approach reduces the level of radiation to which the pregnant woman and fetus are exposed.
Trial registration: University hospital Medical Information Network (UMIN) Center: Trial registration number is UMIN000034744. Data of registration is October 01, 2018. (URL: https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000039610).
FSD: Fetal Skeletal Dysplasia; MIP: Maximum Intensity Projection; 3D-CT: Three-Dimensional Computed Tomography; QUADAS: Quality Assessment Tool for Diagnostic Accuracy Studies; VR: Volume Rendering
Skeletal dysplasias are a group of systemic bone and cartilage disorders. Most skeletal dysplasias are single gene disorders, and a definitive diagnosis can be made by identifying the genetic mutations associated with the diseases. Fetal Skeletal Dysplasias (FSDs) are a group of skeletal dysplasias that occur during the fetal stage, the frequency of which is approximately one in every 5,000 births [1]. The diversity of the FSD phenotypes contributes to the difficulty in diagnosing these disorders. FSDs comprise 436 diseases that are classified into 42 groups, according to the Nosology & Classification of Genetic Skeletal Disorders: 2015 Revision [2]. However, many FSD phenotypes have indistinct definitions, making definitive prenatal diagnosis difficult.
A major role of fetal imaging is to reduce the number of possible diseases in order to allow genetic screening for a definitive diagnosis. Fetal imaging methods that are the basis of diagnosing FSD include ultrasonography and Three-Dimensional Computed Tomography (3D-CT) [3-5]. As fetal ultrasonography has become widely used, the rate of prenatal diagnosis of skeletal dysplasia has increased. However, because ultrasonography utilizes the reflection of sound waves from the bone cortex, it is unsuitable for accurate visualization of the skeleton, and careful attention is required when diagnosing skeletal dysplasias. Due to differences between operators, the low prevalence of FSD, and a variety of disease types, accurate fetal diagnosis is difficult.
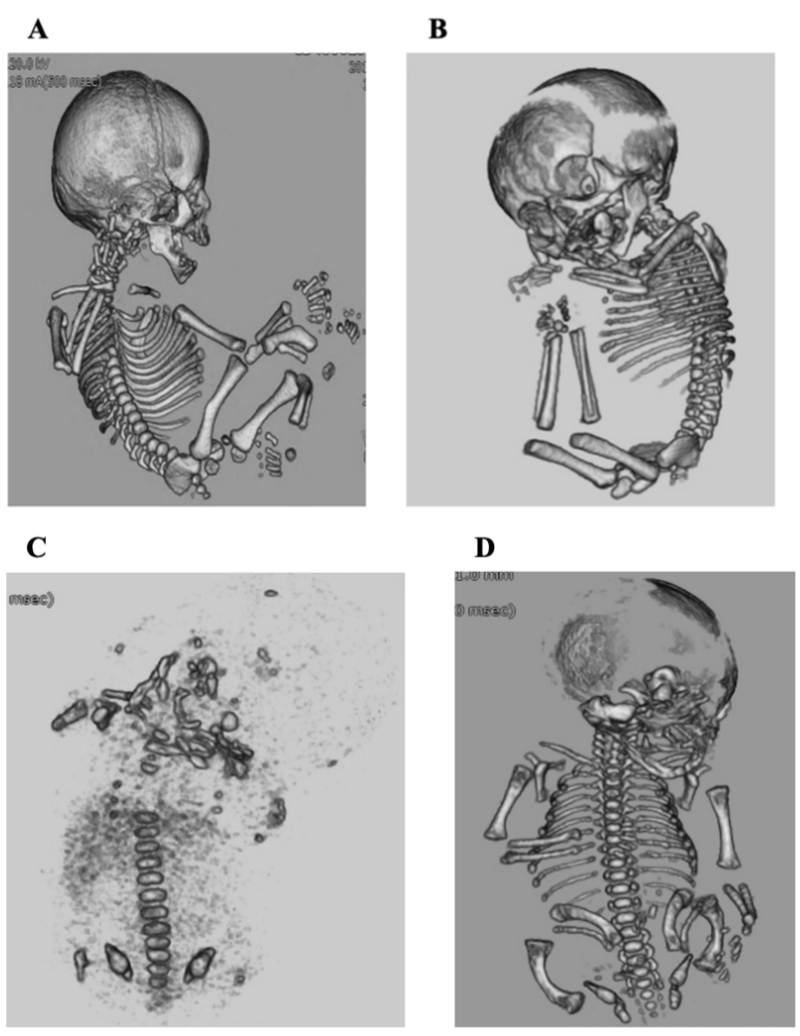
Shoda, et al. [6] first reported a case of fetal 3D-CT in 1977. Imaging by CT is based on X-ray absorption, similar to that of plain radiography (i.e., high absorption in calcified and soft tissues, low absorption in fat and air) (Figure 1A-D). In 3D-CT images, which are obtained by creating 3D images with Volume Rendering (VR) and maximum intensity projection (MIP) techniques, the desired organs and areas can only be visualized from an easily observable direction. In 2004, Ruano, et al. [3] reported the high capability of 3D-CT for diagnosis (accuracy: 94.3%).
There are two concerns regarding radiation exposure during the fetal stage: fetal teratogenicity and the risk of radiation-induced cancer. The CT scans are obtained to diagnose abnormalities of the fetal skeleton at approximately 30 weeks of gestation, at which point there should be no teratogenic effects [7,8]. The rate of childhood cancer at the individual level when exposed in utero is extremely low [7]. Studies have been conducted to further reduce the amount of fetal radiation exposure to a safe level that does not impact diagnostic accuracy. However, no protocol has been established, and results currently depend on the CT imaging techniques available at each facility. In addition, 3D-CT requires specific imaging techniques and cannot easily be performed at all facilities. Therefore, facilities capable of performing 3D-CT examinations should attend to such cases and perform the data collection.
Prenatal diagnosis of FSDs is an important factor for determining whether the prognosis will be favorable or lethal. Based on the diagnosis, a delivery method is selected that allows for the planning of perinatal care. Therefore, the diagnosis may determine the prognosis during the perinatal period. In the present study, we conducted a survey for the preparation of a protocol that has a low risk and a high diagnostic accuracy.
Aims and objectives
The primary aim of the study is to establish the protocol of the diagnosis method using 3D-CT for prenatal skeletal dysplasias. The secondary aim is to reduce the risk of radiation exposure by 3D-CT to both the pregnant woman and fetus. In this study, we aim to establish an accurate prenatal diagnosis method with minimal radioactive exposure.
Design and population
This study is a single arm clinical trial with allocation of intervention. This study began recruitment in October 2018, with a planned final follow-up in December 2024. The number of enrolled subjects is expected to be 50. The number of required patients will be deduced from past medical data, and a practical number will be set.
The protocol was approved by the institutional review board. Written informed consent was obtained from all patients before registration.
The dataset is available from the University Hospital Medical Information Network. This study is funded through a protocol registry: trial registration number UMIN 000034744.
Inclusion criteria
Pregnant women whose pregnancies/childbirths are managed at our hospital and who meet the following selection criteria will be included as participants:
- Pregnancy at age 20 years or older at the time of consent.
- Gestational age > 26 weeks of pregnancy but has not yet delivered.
- Ultrasonography must show fetal short femur (≤ -2.5 standard deviation of the reference of fetal measurements) or an image of a fracture, curvature, or a deformity, in a long bone, skull, rib, or spine.
- Written consent to undergo 3D-CT from the pregnant woman herself.
Exclusion criteria
The following patients were excluded from this study.
- Pregnant women whose childbirth has not been managed at our hospital.
- Pregnant women judged as unsuitable for study participation by an attending physician.
Recruitment of patients
All pregnant women whose fetuses were determined to have abnormal fetal bones using fetal sonography must undergo examination by ultrasonography and 3D-CT. We show the schedule of enrolment, interventions, and assessments in (Table 1).
| Table 1: Schedule of observation and examination. | ||||||||
| STUDY PERIOD | ||||||||
| Enrolment | Allocation | Post-allocation | Close-out | |||||
| TIMEPOINT | -1 week | 0 | 1 week |
2 week |
Every 2 weeks |
~ | Delivery | Infant |
| ENROLMENT: | ||||||||
| Eligibility screen | X | |||||||
| Informed consent | X | |||||||
| Allocation | X | |||||||
| INTERVENTIONS: | ||||||||
| Fetal sonography | X | X | ||||||
| 3D-CT | X | |||||||
| ASSESSMENTS: | ||||||||
| Prenatal diagnosis | X | X | X | X | ||||
| Decision of delivery method |
X | X | ||||||
| Postnatal diagnosis | X | X | ||||||
Outcome measurements
The primary outcome is prenatal diagnostic accuracy of FSD in fetuses with skeletal dysplasia, based on imaging with 3D-CT. The secondary outcome is the safety from radiation exposure in fetuses.
Dose of radiation
Imaging with 3D-CT (Aquilion RXL; TOSHIBA, Japan) will only be performed after thoroughly explaining the effects of radiation exposure to the pregnant woman and obtaining informed consent from the pregnant woman. The 3D-CT images will be obtained by creating 3D images with VR and MIP techniques. In order to minimize exposure of radiation to the fetus without compromising image quality, the following predetermined settings will be used for volume acquisition:
- 1.00-mm thick scans with 1.0-mm intervals for 3.1 s
- 120-mA intensity
- 120-kV voltage
- 0.5-s rotation time
- 1.438 pitch
Evaluating and reporting health damage
Adverse events, regardless of whether there is a causal relationship with the study, include all lethal or unintentional injuries or symptoms that occur to the study's subjects. For this study, radiation-induced cancer is considered a serious adverse event. When an adverse event occurs, the investigator will report it to the principal investigator, who will investigate whether it is related to the examinations performed for this study. If a link between the examinations and childhood cancer is strongly suspected, the ethics committee of Kyoto Prefectural University of Medicine will consider the matter and whether to continue the study or not.
Statistical methods
The methods of statistical analysis are 2×2 χ-squared test, Wilcoxon t-test (Microsoft Excel).
Methodological quality assessment
This study will assess the study quality using the Quality Assessment Tool for Diagnostic Accuracy Studies (QUADAS) by two independent authors. These authors will be categorizing the images into four grades of quality: high, moderate, low, and very low.
Patient and public involvement
Patients or the public were not involved in the design or conduct of the study, and no attempt was made to assess the burden of the intervention by the patients themselves.
Anonymization
Data will be anonymized. Names and other records that could be used to identify a specific individual will be deleted or anonymized by assigning a new code or number. A correspondence table linking the study subjects to these codes (numbers) will be created at the university and safely stored by the personal information manager to prevent external leak.
Security management measures for personal and other information
When handling samples and information related to the study, the principal investigator (Miyoko Waratani, lecturer) will assign numbers that are unrelated to the subjects' personal information, giving due consideration to protecting confidentiality.
If correspondence tables and information are managed on a personal computer, the computer will not be connected to a network. Further, no information that could be used to identify a subject will be included when the findings of the study will be published. Samples and information obtained from the subjects for this study will not be used for any other purpose.
Ethics and dissemination
Institutional review boards of Kyoto Prefectural University of Medicine (ERB-C-991) and all the participating hospitals approved the protocol. Written informed consent was obtained from all patients before registration, in accordance with the Declaration of Helsinki. Results of the study will be disseminated via publication in peer-reviewed journals.
Informed consent status
The study will be conducted after obtaining informed consent from the subjects. Subjects will receive written explanations, and consent will be obtained in writing.
Detailed examination for the screening of FSD and fetal diagnosis is primarily performed using ultrasound. For accurate fetal diagnosis, 3D-CT is considered to be an ancillary examination. When considering the risk of radiation exposure, high quality images that are adequate for a diagnosis have been made by low-dose 3D-CT in recent years. This approach reduces the level of radiation to which the pregnant woman and fetus are exposed.
Prenatal diagnosis of FSDs is important to judge whether the prognosis is favorable or lethal. Based on the diagnosis, a delivery method is selected, which allows for the planning of perinatal care. Therefore, the diagnosis may determine the prognosis of the perinatal period [9].
One limitation of the current study for FSD is the relatively small sample size. It is difficult to collect data in a limited area because it is an infrequent disease. The sample size should be increased in future studies to improve the power of the results.
Thus, 3D-CT can be used as a valuable tool for augmenting sonography in the diagnosis of FSD. Continuation of this study is important to advance the diagnosis of FSD.
Ethics approval and consent to participate. Institutional review boards of Kyoto Prefectural University of Medicine (ERB-C-991) and all participating hospitals approved the protocol. Written informed consent was obtained from all patients before registration, in accordance with the Declaration of Helsinki.
The datasets generated and/or analyzed during the current study are available in the University Hospital Medical Information Network (UMIN). (URL: https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000039610)
The author contributions are as follows: MW conceived and designed the study, wrote the protocol manuscript, and analyzed and interpreted the data. FI, YT, AM, and TM collected the study data. JK provided guidance regarding the study design and ethical approval. All authors read and approved the final manuscript.
We thank the patients, their families, and all investigators involved in this study. We would like to thank Editage (www.editage.com) for English language editing.
- Chen C, Jiang Y, Xu C, Liu X, Hu L, Xiang Y, Chen Q, Chen D, Li H, Xu X, Tang S. Skeleton Genetics: A comprehensive database for genes and mutations related to genetic skeletal disorders. Database (Oxford). 2016 Aug 31;2016:baw127. doi: 10.1093/database/baw127. PMID: 27580923; PMCID: PMC5006089.
- Bonafe L, Cormier-Daire V, Hall C, Lachman R, Mortier G, Mundlos S, Nishimura G, Sangiorgi L, Savarirayan R, Sillence D, Spranger J, Superti-Furga A, Warman M, Unger S. Nosology and classification of genetic skeletal disorders: 2015 revision. Am J Med Genet A. 2015 Dec;167A(12):2869-92. doi: 10.1002/ajmg.a.37365. Epub 2015 Sep 23. PMID: 26394607.
- Ruano R, Molho M, Roume J, Ville Y. Prenatal diagnosis of fetal skeletal dysplasias by combining two-dimensional and three-dimensional ultrasound and intrauterine three-dimensional helical computer tomography. Ultrasound Obstet Gynecol. 2004 Aug;24(2):134-40. doi: 10.1002/uog.1113. PMID: 15287049.
- Krakow D, Lachman RS, Rimoin DL. Guidelines for the prenatal diagnosis of fetal skeletal dysplasias. Genet Med. 2009 Feb;11(2):127-33. doi: 10.1097/GIM.0b013e3181971ccb. PMID: 19265753; PMCID: PMC2832320.
- Macé G, Sonigo P, Cormier-Daire V, Aubry MC, Martinovic J, Elie C, GONZALES M, CARBONNE B, DUMEZ Y, LE MERRER M, BRUNELLE F. and BENACHI A. Three-dimensional helical computed tomography in prenatal diagnosis of fetal dysplasia. Ultrasound Obstet Gyn ecol. 2013 Jul;42: 161-168. doi:10.1002/uog.12298.
- Sohda S, Hamada H, Oki A, Iwasaki M, Kubo T. Diagnosis of fetal anomalies by three-dimensional imaging using helical computed tomography. Prenat Diagn. 1999 Apr;17:670-674. doi:10.1002/(SICI)1097-0223(199707)17:7<670::AID-PD113>3.0.CO;2-8.
- Cassart M. Suspected fetal skeletal malformations or bone diseases: how to explore. Pediatr Radiol. 2010 Jun;40(6):1046-51. doi: 10.1007/s00247-010-1598-6. Epub 2010 Apr 30. PMID: 20432024.
- Guilbaud L, Beghin D, Dhombres F, Blondiaux E, Friszer S, Ducou Le Pointe H, Éléfant E, Jouannic JM. Pregnancy outcome after first trimester exposure to ionizing radiations. Eur J Obstet Gynecol Reprod Biol. 2019 Jan;232:18-21. doi: 10.1016/j.ejogrb.2018.11.001. Epub 2018 Nov 5. PMID: 30453167.
- Bellur S, Jain M, Cuthbertson D, Krakow D, Shapiro JR, Steiner RD, Smith PA, Bober MB, Hart T, Krischer J, Mullins M, Byers PH, Pepin M, Durigova M, Glorieux FH, Rauch F, Sutton VR, Lee B; Members of the BBD Consortium, Nagamani SC. Cesarean delivery is not associated with decreased at-birth fracture rates in osteogenesis imperfecta. Genet Med. 2016 Jun;18(6):570-6. doi: 10.1038/gim.2015.131. Epub 2015 Oct 1. PMID: 26426884; PMCID: PMC4818203.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.