> Environmental Sciences. 2020 September 23;1(5):163-172. doi: 10.37871/jbres1138.
-
Subject area(s):
- Soil Science
- Environmental Impacts
- Environmental Contamination
Batch Adsorption Studies of Sunset Yellow and Tartrazine Using Coconut and Groundnut Shells
Clement Oluwaseun Ademoriyo1 and Christian Ebere Enyoh2*
2Group Research in Analytical Chemistry, Environment and Climate Change (GRACE&CC), Department of Chemistry, Imo State University Owerri, Imo State, Nigeria
- Sunset yellow
- Tartraxine
- Groundnut shell
- Coconut shell
- Adsorption
- Isotherm
This report was based on the comparative study on effectiveness of adsorption of food colors using coconuts and groundnut shell. The activated carbon (coconuts and groundnut shells) were cut into pieces in a furnace at a temperature of 450°C then crushed and sieved using different mesh sizes and activated using hydrochloric acid of different concentration. The food colors (sunset yellow and tartrazine) was prepared with different concentration and calibration curve was drawn, and the required measured concentration was contacted with varied masses of the adsorbent (coconuts and groundnut shell) for an equilibrium adsorption at room temperature on effect of time, pH, shaking speed, and temperature. The results on contact time on the pseudo-first and second using the test mechanism shows pseudo-first order model is more preferable than pseudo second order and the different effect result on the isotherm shows that Freundlich is best fitted for the adsorption process. Overall, groundnut shell showed higher adsorption for both sunset yellow and Tartrazine compared to coconut shell.
Food colouring is used both in commercial food production and in domestic cooking. Due to its safety and general availability, food colouring is also used in a variety of non-food applications, for example in home craft projects and educational settings [1]. People associate certain colours with certain flavours, and the colour of food can influence the perceived flavour in anything from candy to wine. For this reason, food manufacturers add dyes to their products; to give their product better outlook and attraction. Sometimes the aim is to simulate a colour that is perceived by the consumer as natural, such as adding red colouring to glace cherries (which would otherwise be beige), but sometimes it is for effect, like the green ketchup that Heinz launched in 2000 [2]. While most consumers are aware that food with bright or unnatural colours (such as the green ketchup, or children’s cereals such as Fruit Loops) likely contain food colouring, far fewer people know that seemingly “natural” foods such as oranges and salmon are sometimes also dyed to mask natural variations in colour [3]. Colour variation in foods throughout the seasons and the effects of processing and storage often make colour addition commercially advantageous to maintain the colour expected or preferred by the consumer [3].
Food colours can generally be categorized as natural, synthetic and Lakes and dyes. Synthetic food colours also known as artificial food colours are manufactured chemically and are the most commonly used dyes in the food, pharmaceutical and cosmetic industries. Seven dyes were initially approved under the Pure Food and Drug Act of 1906, but several have been delisted and replacements have been found [3,4]. Current seven In the USA, these seven artificial colors are approved for use in food under act of 2007:
Colour additives are available for use in food as either “dyes” or lake pigments (commonly known as “lakes”). Dyes dissolve in water, but are not soluble in oil. Dyes are manufactured as powders, granules, liquids or other special purpose forms [5]. They can be used in beverages, dry mixes, baked goods, confections, dairy products, pet foods, and a variety of other products. Wastewaters from the food colouring, paper, carpet, rubber, plastics, cosmetics and textile industries are polluted by dyes [5,6]. The presence of very low concentrations of dyes in these effluents (less than 1 ppm for some dyes) is highly visible and undesirable [6]. Dyes also have side effects which lakes do not, including the fact that large amounts of dyes ingested can colour stools [7]. The highest rates of toxicity have been found amongst the basic and diazo direct dyes [8].
The removal of dyestuffs from effluents is of great importance in many countries worldwide for both environmental and water reuse concerns [9]. Due to the low biodegradability of dyes, conventional biological treatment processes are not very effective in treating dye wastewaters; therefore, they are usually treated by either physical or chemical processes [9]. Activated carbon is the most widely used physicochemical treatment for the removal of dissolved organics from wastewater, effective even in dilute solution, but commercially available activated carbon is very expensive [10-12]. To decrease the treatment cost of dye removal, attempts have been made to find inexpensive and biodegradable alternative adsorbents [11-13]. In general, a sorbent can be assumed to be inexpensive if it requires little processing, is abundant in nature or is a by-product or waste material from another industry [14,15, 25-28]. It is from this perspective that our research is interested in the removal by adsorption of a class of textile dye that is common and widespread worldwide.
Some previous researches carried on the adsorption of various types of dyes, and some adsorption processes carried out using various adsorbents. Udo and Ogunwale [16] studied the adsorption of methylene blue onto bamboo-based activated carbon. The kinetic equilibrium studies were studied at 30°C. The equilibrium data for methylene blue adsorption well fitted to the Langmuir equation, with maximum monolayer adsorption capacity of 454.2 mg/g. Two simplified kinetic models including pseudo-first-order and pseudo-second-order equation were selected to follow the adsorption processes. The adsorption of methylene blue could be best described by the pseudo-second-order equation. The removal of reactive black and reactive yellow removal from aqueous solution is studied extensively [17], iron adsorption by coconuts and groundnut shell in a batch system: These studies indicate that) using diatomaceous earth the optimum surface area and pH. at zero-point charge are 27.80 m2/g and 4.5 respectively. Also the electrostatic interactions play an important role in the adsorption of dyes onto diatomite. The data obtained from Methylene Blue adsorption onto the tested adsorbents followed the Langmuir and BET equations and the adsorption processes were endothermic and spontaneous in nature. The raw coal sample showed the highest adsorption capacity; generally decreased with increasing pyrolysis temperature and the sample will pyrolysed at 700°Cto exhibit the lowest adsorption capacity. It was concluded that adsorption of methylene blue occurs through physical interactions, and the lignite sample has a meso porous structure. For iron the sorption capacity decreases with an increase in temperature from 20°C to 50°C at the initial Fe(III) sorption. Constants from pseudo-seconds order kinetic model at different temperatures will be evaluated and activation energy will be found to be -13.14 kJ/mol [18]. The kinetics and thermodynamics of the adsorption of dyes from aqueous have been studied extensively the hen egg shell is an excellent adsorbent for removal of the cationic dye (Brilliant green) from aqueous solution. The pH 9 and temperature 303K are fond to be optimum for adsorption Monolayer adsorption capacity is found to be 44.75 mg/g at 303 K. The adsorption from equilibrium reached in 30 min. The adsorption processes follow the pseudo-second rate kinetics. Nasuha, et al. [19] studied the adsorption of Congo red on Aspergillus Niger.
This present work is carried out due to the fact that a lot of wastes generated in the food industries are dyes (food color) which pollute the waste water discharge to the sewers and are not biodegradable. This work aims at using the abundant waste material coconut and groundnut shell to remove the color from the waste and discharge relatively color free solution to the sewers. Categorically industrial this wastes water or the release of harmful substances into the water causes damage to man and its surrounding. The importance of the ecosystem to man is undoubtedly enormous. A research into the elimination of dye concentration in effluents from industrial operations, which inhibits sunlight penetration into the water thus preventing photosynthesis, was the main motivating factor for carrying out this research work.
The coconut palm, Cocos nucifera, is a member of the plantae order- Arecales of the Family Arecaceae (Palm family). It is only accepted species in the genus cocos. The term coconut can refer to the entire coconut palm, the seed, or the fruit which is not a botanical nut but a drupe. Like other fruits it has layers: exo-carp, meso-carp, and endocarp. The exocarp and mesocarp make up the husk of the coconut, and the mesocarp itself is composed of fibers called coir which have many traditional and commercial uses [16]. Coconut coir dust is one of the agricultural waste products often used as adsorbent in waste water treatment. It’s all year round availability and its abundance in the environment makes it a good source of adsorbent for metal ion removal from aqueous solution. It is the light, fluffy material that falls off from the thick mesocarp of coconut fruit when shredded during coir processing. The re-mark able properties of coir dust that enhances its effectiveness as adsorptive/ion exchange capacities include good structural stability, high water absorptivity and highly porous nature. [15].
Groundnut botanically belongs to Araches hypo Gaea Linn of leguminous family. Groundnut is a self-pollinated; annual and herbaceous legume crop. A complete seed of groundnut is called pod and contains one to five kermils which develops underground in a needle like structure called peg which grow into the soil and then converts into a pod. Groundnut has taproot system which has many nodules, present in root and lateral roots. These nodules contain Rhizobium bacterial, which are symbiotic in nature and focus atmospheric nitrogen. Outer layer of groundnut is called groundnut shell: The shell constitute about 25-35% of the pod. The seed accounts for the remaining portion (65-75%) [22]. The shell or pod of the groundnuts contains two, rarely three kernels in individual case like pods .The nuts are egg shaped, and the size depends upon the type of cultivar.
The focus of the present study was to assess the potentiality of coconut and groundnut shell for removal of synthetic dyes (food color) from aqueous solutions with the following objectives;
- To study the adsorption of the food color on coconut and groundnut shell.
- To determine the optimum condition of food unto coconut and groundnut shell using parameters such as temperature, contact time, pH, and shaking speed.
- To determine the adsorption of the food color on the coconut and groundnut shell using adsorption models such as Freundlich and Langmuir isotherms.
Chosen in this study different food color as a model compound because of its strong adsorption on solids and its use in characterizing adsorptive materials. The food colors will be identified in the effluents because it is resistant to fading from exposure to light, water and chemicals due to their complex chemical structure. Therefore, removal of such colored agents from aqueous effluents is a significant environmental importance. The results obtained will be transfer to other food colors with a similar chemical structure. Additionally, a known waste material (coconut and groundnut shell) will be used for the removal of the food color. The coconut and groundnut shell is readily available because the agricultural products are consumed in most homes and in domestic market. Food colors are waste materials especially from industrial effluents, and the harmful effects of this adsorbate (coconut and groundnut shell) on the ecosystem would be limited.
Average people living in this country of today consumed coconut and groundnut shell and the waste generated from it constitutes a kind of pollution to the environment, which is minimized by the use of the shells in this work. Coconut and groundnut shell is relatively the cheapest and most easily accessible material and it is economically wise (waste to wealth).
Collection and pre-treatment of coconut and groundnut shell samples
The waste samples of coconut and groundnut shells were collected at Mile 12 Market, Kosofe Local Government Area of Lagos State, Nigeria. The waste shells were pre-treated by soaking the two solid samples in distilled water for three hours to remove sand and other impurities then put under sun for partial dryness.
Collection and Preparation of adsorbate (food colour)
Sunset yellow and tartrazine are the two food colours used as adsorbate samples, both samples were gotten in powder form of specific mass from LINO Laboratory, Ojo, Lagos State. Solution of both adsorbate were prepared by dissolving 2.0 gram each of sunset yellow and tartrazine in 1 L (1000 mL) of distilled water respectively. The absorbance of both adsorbates was taken at that concentration and others concentrations were also prepared to draw the calibration curve for both adsorbates separately [11].
Preparation of adsorbent
Activation, involving two steps activation scheme was adopted. Firstly, 30 grams of blended raw samples were weighed into five different cleaned and pre-weighed crucibles. They were introduced into a muffle furnace at 450°C for 30 minutes after which they were poured from the crucible into a bath of ice water. The excess water was drained off by filtration then carbonized samples were washed, using 0.1 M HCl to remove surface ash, followed by hot water wash and further washed with distilled water to remove residual acid. The samples were then sun dried for few minutes (30-60 minutes), and further dried in the oven at 100°C for one hour. This process was repeated separately for coconut and groundnut shells until a substantial amount of carbonized sample will be obtained. Thereafter, 25 grams of already carbonized samples were now mixed with 5cm3 of activating agent (1M H2SO4). The samples were allowed to stand for 2 hours, after which it was then introduced into a furnace and heated at 600°C for 5 minutes. The activated samples were allowed to cool with ice-cold water and excess water were drained off by filtration and the samples were dried at room temperature. The above procedure was also done separately for the two wastes samples until substantial amount of activated carbon was obtained. Washing was continued until the pH of samples solution fall within 6-7 using pH metre to avoid further reaction with the food colour [23].
Finally the activated carbon obtained were crushed and sieved into different 5 size particles before further analysis as shown below.
Activated carbon characteristic
The activated carbon was characterized as follow:
w0 = initial weight of sample before activation
wi = final weight of carbon after activation, [8]
w0 = initial weight of sample before activation
wi = final weight of carbon after activation, [8]
Adsorption using batch method
The influence of each parameter such as pH initial dye concentration, adsorbent dose, temperature and constant time were evaluated by varying the parameter under evaluation, while other parameters will be maintained constant.Specific gram of activated carbon of different mesh sizes of each were contacted with required mL of food colour solution of 50 ml/L concentration in a flask separately and these were allowed to stand for 20 minutes. It was then filtered using WHATMAN filter paper (No. 42). The process was repeated at pre-set time (20, 40, 60, 80 and 100 minutes). The concentration and absorbance of in the food colour solution were determined before and after interaction with the activated carbon by a bulk scientific UV-Vis spectrophotometer.
Effect of contact time: For optimization of shaking time, the coconut and groundnut shells of about 2g and sample of 50 ml was taken in five different flasks at different retention time of 10, 15, 20, 25 and 30 minutes. All the samples were subjected to shaking and observed the filtrates absorbance as follows; mass of adsorbent = 2g, volume of adsorbate = 200 ml (0.2 L). Initial concentration of dye solutions was 100 mg/L.
Effect of pH: The influence of pH on the adsorption of food color on coconut and groundnuts powder was determined. The maximum pH for the removal was determined and this was used throughout the experiment. The pH of food color was adjusted to (1, 3, 6, 8 and 10) pH values using 1M Hydrochloric acid. 2g of coconut and groundnut powder of different size particles (300 mic, 600 mic, 1.18mm, 2.36mm and 4.75mm) was mixed with 50ml solution of food color prepared in a separate conical flask with concentration of 100mg/L and stirred at 150rpm at constant temperature and constant time of 35°C and 20 minutes respectively. The dye concentration was determined after the process by testing the absorbance at λmax of 506 nm.
Effect of shaking speed: 2g coconut and groundnuts powder of (300 mic, 600 mic, 1.18 mm, 2.36 mm and 4.75 mm) particle sizes were mixed with 50ml volume of 1000 mg/L food color in 100ml shaking flask at the maximum pH for different time intervals of 5, 10, 15, 30, 60 minutes at 150rpm stirring, and filtrated through a filter paper to separate the remaining solid from liquid phase. The food colour concentration was be determined by measuring absorbance at λmax of 506 nm. The time taken for the maximum adsorption was noted and it was set as the equilibrium time.
Effect of temperature: Also the effect of temperature on the adsorption process was carried out over ranges of 303K, 313K and 323K and initial food color concentrations of 100 mg/L. This was achieved by contacting the food color and the adsorbent inside a water bath at a regulated temperature and at the maximum pH and equilibrium constant time found earlier at 150 rmp stirring.
Determination of the adsorption capacity
The adsorption capacity was calculated using equation
qt = adsorption capacity at time t (mg/g).
ci = concentration of food colour solution before interaction with the activated carbon (mg/L).
ct = concentration of food colour solution after interaction with the activated carbon (mg/L).
V = volume of the food colour solution (L)
W = weight of the activated carbon (g)
Percentage dye removal
where:
co: Initial concentration of adsorbate
co: Concentration of adsorbate at equilibrium
%R: Percentage dye removal
Batch kinetic studies
The kinetic studies were conducted for initial concentration of aqueous solution 100 ml/L at temperature of 25°C. The amount of sorption at time t, qt(mmol/g), was calculated by the following equation:
)
In sorption isotherm studies, solutions with different initial concentrations of sunset yellow and tartrazine (0.05–0.5 mmol/L) were prepared. The equilibrium time was set as 4 hours and mass of all sorbents was 2.0 g. The uptake of the food colour at equilibrium, qe(mg/g), was calculated from equation (2):
The most common model used to fit the kinetic adsorption experiment is Lagergren’s Pseudo first and pseudo second-order model using batch method [9]
Pseudo first-order equation
Pseudo second-order equation
qe = amount of adsorbate adsorbed at equilibrium (mg/g).
qt = amount of adsorbate adsorbed at time t (mg/g).
t = time.
K1 = rate constant for first order reaction (min).
K1 = rate constant for second order reaction (g.mg-1 min-1).
Test of kinetics models: The applicability of pseudo first-order and pseudo second-order kinetic models are verified.
Through the sum of error squares (SSE, %) given by:
SSE = Statistical sum of error
N = number of data point
qe (exp) = adsorption capacity at experimental equilibrium values (mg/g)
qe (cal) = adsorption capacity at equilibrium calculated values (mg/g) [15].
Intra-particle diffusion model: In order to investigate the mechanism of the food colour adsorption onto groundnut Shell and coconut shell, intra-particle diffusion – based mechanism was studied using the equation below
where:
C = Intercept
Kp = intra-particules diffusion rate constant (mg.g-1m-1/2).
Adsorption isotherm
The adsorption test was carried out at the equilibrium conditions of initial pH, contact time and adsorbent dose determined earlier for the dye. The Langmuir and Freundlich isotherms will be used to analyze the data generated from the adsorption studies of the food color on coconuts and groundnut nuts powder using equation (11) and (12) [9].
where Ce (mg/L) is equilibrium concentration of metal ions, qm (mg/g) is maximum adsorption capacity of the adsorbent, K (L/mg) is Langmuir constant, qe (mg/g) is adsorption capacity at equilibrium, Co (mg/L) is initial concentration of metal ions, is relative adsorption capacity of the adsorbent, n is adsorption intensity and KE (L/mg) is Elovich equilibrium constant (Table 1).
Table 1: Artificial colour and colour code of approved Federal Food Drug and cosmetics (FD&C). |
||
| Artificial colourings | Colour code | Colour |
| FD&C Blue No. 1 | Brilliant Blue E133 | Blue shade |
| FD&C Blue No. 2 | Indigotine E132 | Dark blue shade |
| FD&C Green No. 3 | Fast Green E143 | Bluish green shade |
| FD&C Red No. 40 | Allura Red E129 | Red shade |
| FD&C Red No. 3 | Erythrosine E127 | Pink shade |
| FD&C Yellow No 5 | Tartrazine E102 | Yellow shade |
| FD&C Yellow No 5 | Sunset Yellow E110 | Orange shade |
Activated carbon characteristic
Some characteristics of the nut shells are presented in table 2. From table 2, groundnut shell exhibit higher percent burn off than coconut shell due to particles size and surface area particularly the intermolecular surface area of the particles. Also, change in weight for both shell relate to the percent burn off in inversely proportionality. After the activation of both shells with 0.5M of HCL and H2SO4 percent yield ranged from 87.86 % for GS to 93.34 % for CS.
Table 2: Some characteristics of activated nut shells (n=5). |
||
| Parameter | Coconut shell | Groundnut shell |
| % Burn off | 6.64 ± 1.13 | 12.1 ± 1.72 |
| % Yield | 93.34 ± 1.15 | 87.86 ± 1.67 |
Effect of contact time
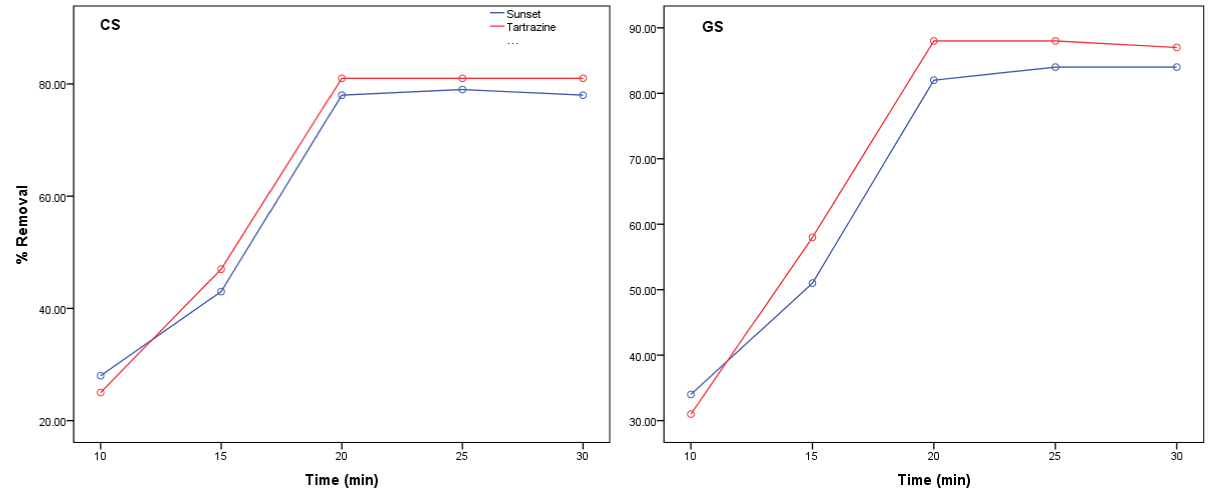
Contact time is inevitably a fundamental parameter in all transfer phenomena such as adsorption. Therefore it is important to study its effect on the capacity of removal of food colour by coconut and groundnut shell adsorbent. The effect of contact time on the removal and maximum adsorption capacity of sunset yellow and tartrazine by coconut (CS) and groundnut (GS) shell at 25°C is shown in figure 1. The adsorption of food colour increase with increase in contact time with rapid removal until 20 mins where maximum adsorption capacity of CS and GS was reached. This observation indicate that at about 20 mins, the available sites on the surface of the absorbents were filled up by the dyes and therefore the amount of dyes being removed by the adsorbent was approximate to the amount of dye desorbing from the adsorbent. However, between the two adsorbent, GS showed relatively higher removal rate than CS. Similarly, in figure 2, the contact time have great influence on absorbates capacity thus influencing the rate of absorbates with time.
Effect of pH on adsorption capacity
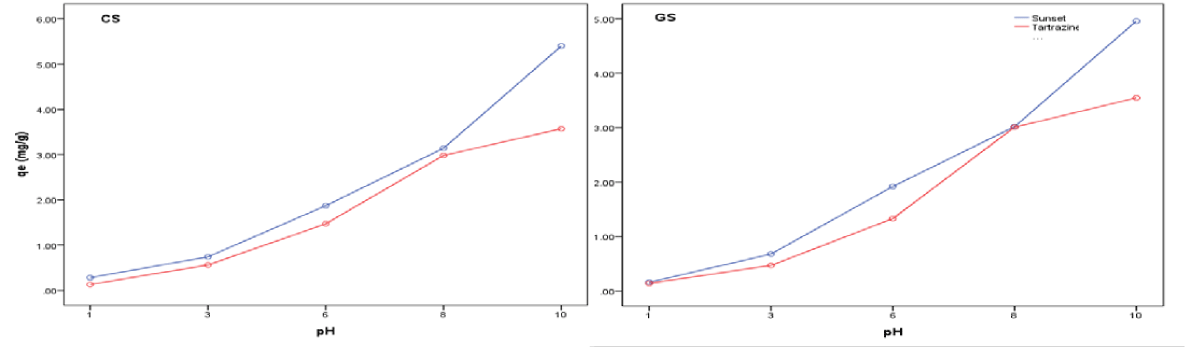
The pH of an aqueous system presents an important variable that may affect the uptake of the adsorbate. The chemical properties of both the adsorbate and the adsorbent vary with pH. The effect of pH on the adsorption of sunset yellow and tartrazine onto CS and GS was studied at pH 1-10. The dependence of the adsorption capacities of the adsorbents on solution pH is shown in figure 2. The adsorption capacity of the adsorbents increased with increasing pH. Rapid increase in the amount of tartrazine at pH 10 was also reported by [23]. Higher adsorption capacity was generally shown for tartrazine than sunset yellow. The optimum pH was 10.
Effect of shaking speed
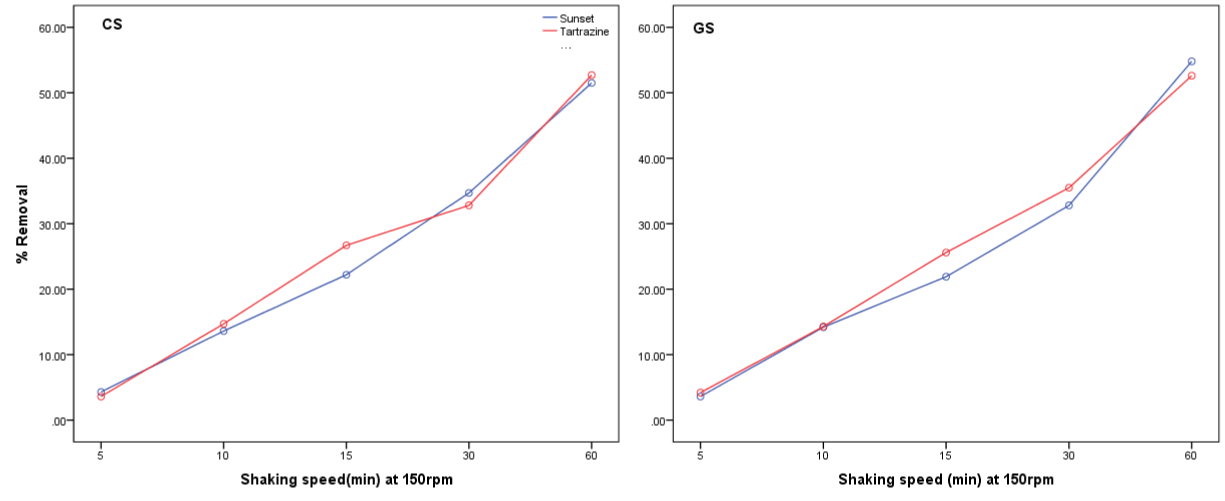
The effect of shaking speed on the removal of the food colors are presented in figure 3. The % removal of sunset yellow and Tartrazine increase as the shaking speed increased. This may be due to an increase in kinetic energy of dye molecule caused by the increased agitation.
Effect of temperature
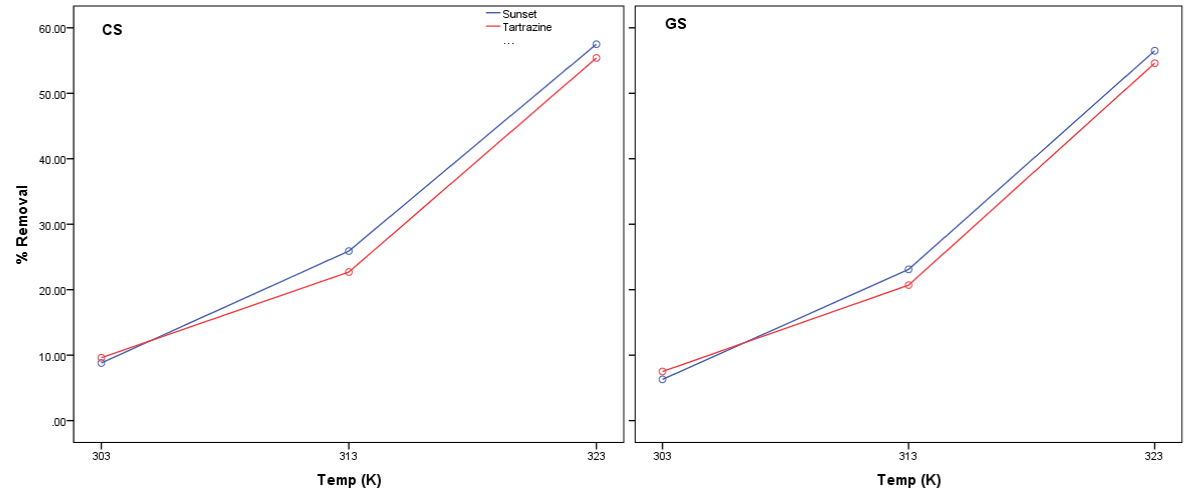
The effect of temperature on the removal of the food colors are presented in figure 4. The % removal of sunset yellow and Tartrazine increase as the temperature was increased from 303 to 323K. An increase in temperature will lead to an increase in kinetic energy of dye molecule and in turn resulting in high interaction with the adsorbents.
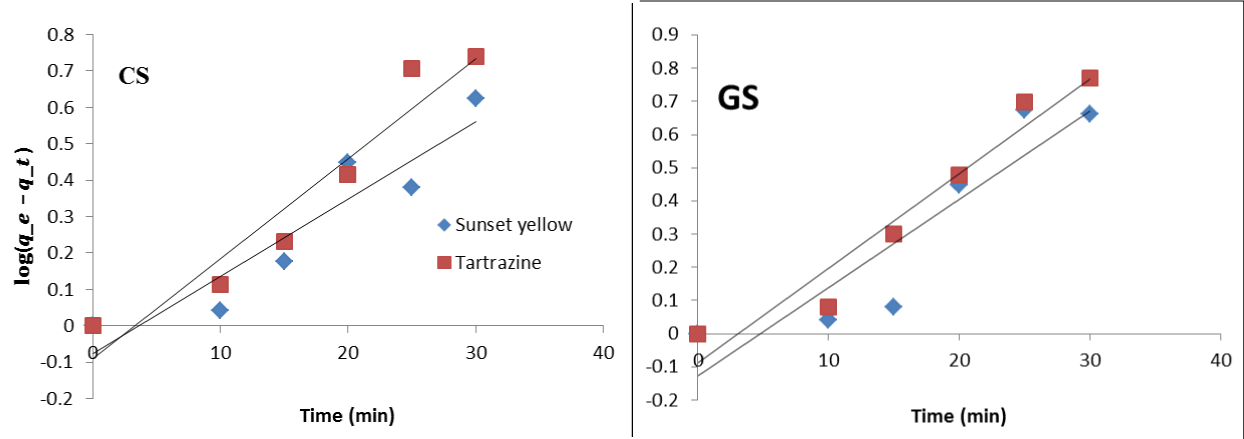
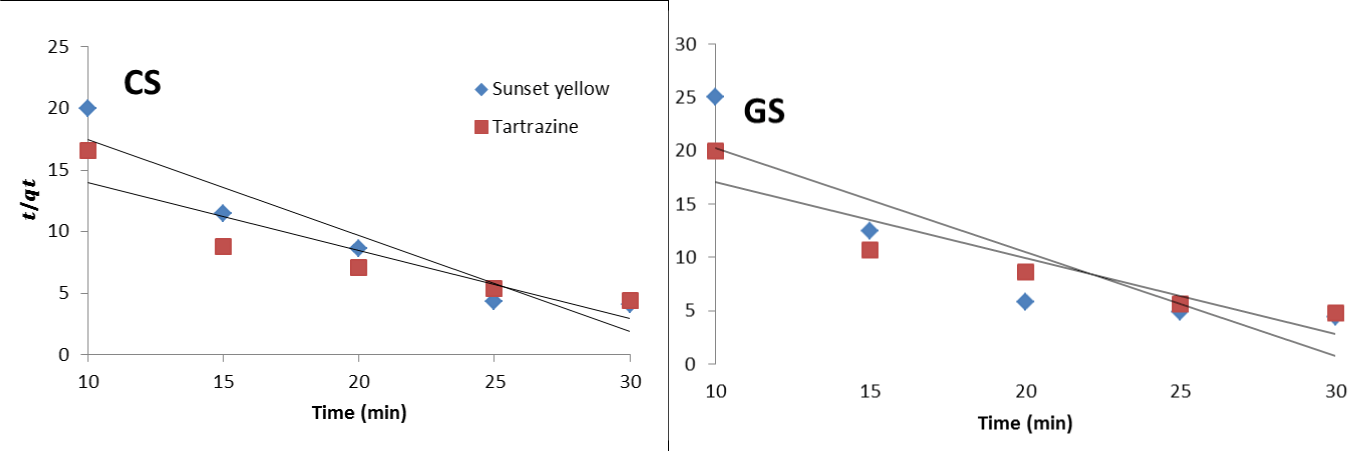
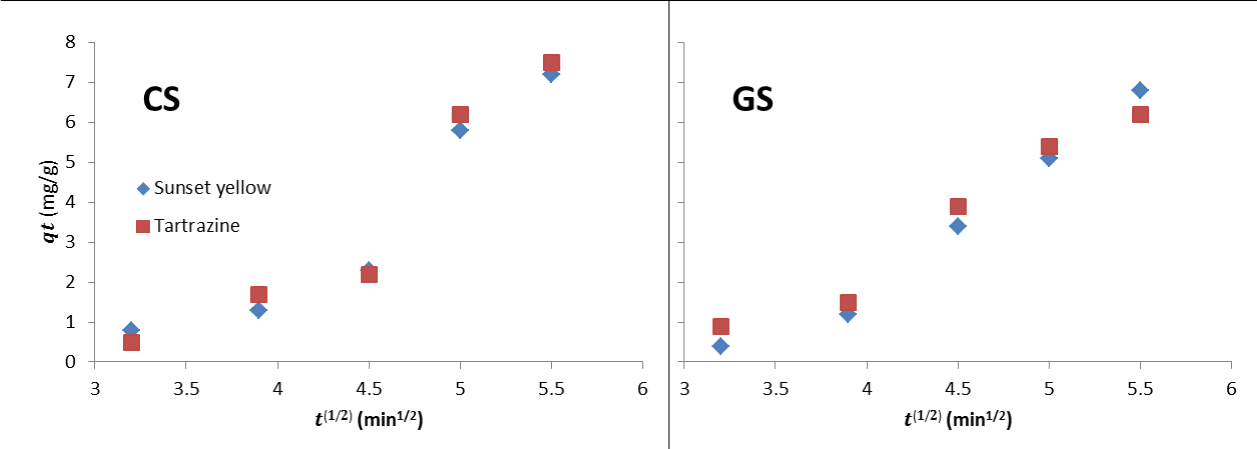
Adsorption kinetic modelling
In order to describe the adsorption kinetics the following kinetic models, pseudo-first figure 5, pseudo second order figure 6 and intraparticle diffusion figure 7 models were considered. The summary kinetic parameters for food colors adsorption by CS and GS at 25 ± 2°C are presented in table 3.
Table 3: Kinetic parameters for sunset yellow and tartrazine adsorption at 25 ± 2°C. |
|||||||||||
| Shells | Food colours | qe (exp) | Pseudo-first order | SSE | Pseudo-second order | Intraparticle diffusion | |||||
| qe (cal) | K1 (1/hr) |
R2 |
|
K2 (g/mg-hr) | R2 | Kip (mg/g.min1/2) | R2 | C | |||
| Coconut | Sunset yellow | 18.12 | 12.42 | 0.0213 | 0.8730 | 5.7 | 0.0241 | 0.8892 | 2.9596 | 0.8795 | -9.6015 |
| Tartrazine | 20.67 | 14.21 | 0.0274 | 0.9247 | 6.46 | 0.0157 | 0.8215 | 3.1756 | 0.8872 | -10.416 | |
| Groundnut | Sunset yellow | 19.02 | 16.45 | 0.0266 | 0.8330 | 2.57 | 0.0319 | 0.7810 | 2.8953 | 0.9685 | - 9.4174 |
| Tartrazine | 21.72 | 18.23 | 0.0286 | 0.9432 | 3.49 | 0.0205 | 0.8468 | 2.5282 | 0.9587 | - 7.5944 | |
Pseudo-first order (PFO): In table 2, for the first order kinetic model, the obtained R2, qe (cal) and removal constant (K1) values as well as the error (sum of square) are presented. The PFO showed significant R2 values were significant (0.8330 to 0.9432 respectively). Therefore, indicating that the model was able to describe the removal process. However, both adsorbents showed stronger correlation for tartrazine than sunset yellow. The rate of pollutants degradation in a treatment system is normally represented by the PFO removal rate constants (k1) [9]. The removal rate constants ranged from 0.0213 to 0.0266 for sunset yellow while for Tartrazine it ranged from 0.0274 to 0.0286. The result indicated higher rate for tartrazine than sunset yellow for both adsorbents. Furthermore, between the two adsorbent higher removal rate constants was obtained by GS. Similar observation was made for the same dyes by using gbafilo (Chrysobalanus icaco) shell [14].
Pseudo-second order (PSO): From table 2, the values of the correlation coefficient for the second-order kinetic model was found to be in the range of 0.7810 to 0.8892, indicating poor applicability of the pseudo-second-order kinetic model to describe the adsorption process when compared to the PFO. The PSO showed stronger correlation and with higher removal rate constants (K2) for sunset yellow than tartrazine.
Intraparticle diffusion model: For intraparticle diffusion model, if the adsorption process follows the intraparticle diffusion model, then qt versus t1/2 will be linear and if the plot passes through the origin, then intraparticle diffusion is the sole rate-limiting step. Otherwise, some other mechanism along with intraparticle diffusion is also involved. In figure 7, the plot for intraparticle diffusion kinetic for the removal of sunset yellow and tartrazine from aqueous solution by CS and GS is presented while the model parameters are summarized in table 2. The regression was not perfectly linear and the lines did not pass through the origin figure 7, suggesting that more than one mode of sorption along with intraparticle diffusion is involved in the dye removal. This could suggest that the adsorption process is complex and may involve more than one mechanism [19]. The Kip values was high for Tartrazine using CS while sunset yellow was high for GS, suggesting greater driving force for the respective adsorbent.
Adsorption isotherm modelling: In order to describe the adsorption isotherm for the effect of pH, shaking speed and temperature, the following isotherm models, Langmuir and Freundlich were considered. These models help to explain how adsorption molecules distribute between the liquid phase and the solid phase in the whole adsorption process, and give a comprehensive understanding of the nature of adsorption. In the use of Langmuir isotherm, it is assumed that the pollutant uptake occurs at specific homogeneous sites within the surface of the adsorbents and once a food color molecule occupies a site, no further adsorption takes place at that site. The Freundlich isotherm is an empirical equation assuming that the adsorption process takes place on a heterogeneous surface through a multilayer adsorption mechanism, stronger binding sites are occupied first and that the binding strength is related to the concentration of dye at equilibrium [22].
The correlation coefficients (R2) for all isotherm models applied on the various factors are summarized in table 4. The highest R2 value was given by the Freundlich isotherm model with temperature have the highest value (0.930 to 0.997). The Freundlich isotherm model therefore is best in describing the removal of sunset yellow and Tartrazine by CS and GS. The slope 1/n and n obtained from the Freundlich model showed values < 1 except for GS for Tartrazine (4.5) highlighting that the binding process followed a chemical [23] except for temperature effect on Tartrazine by GS, which may be physical.
Table 4: Isotherm model parameters for sorption of food colours on nut shells. |
|||||||
| Shell | Food colours | Freundlich | Langmuir | ||||
| N | Kf | R2 | Kmax | qmax | R2 | ||
| pH | |||||||
| Coconut | Sunset yellow | 0.289 | 4.294 | 0.7849 | 0.020 | 0.327 | 0.4833 |
| Tartrazine | 0.150 | 4.983 | 0.8219 | 0.015 | 0.112 | 0.5709 | |
| Groundnut | Sunset yellow | 0.225 | 2.685 | 0.7542 | 0.017 | 0.141 | 0.3619 |
| Tartrazine | 0.216 | 4.335 | 0.8448 | 0.015 | 0.145 | 0.7304 | |
| Shaking speed | |||||||
| Coconut | Sunset yellow | 0.308 | 2.014 | 0.808 | 0.021 | 0.327 | 0.883 |
| Tartrazine | 0.298 | 3.201 | 0.773 | 0.015 | 0.112 | 0.639 | |
| Groundnut | Sunset yellow | 0.376 | 1.752 | 0.8295 | 0.017 | 0.141 | 0.348 |
| Tartrazine | 0.323 | 1.082 | 0.8208 | 0.015 | 0.145 | 0.196 | |
| Temperature | |||||||
| Coconut | Sunset yellow | 0.505 | 1.018 | 0.930 | 0.025 | 0.643 | 0.644 |
| Tartrazine | 0.447 | 2.980 | 0.931 | 0.021 | 0.607 | 0.790 | |
| Groundnut | Sunset yellow | 0.609 | 2.780 | 0.997 | 0.024 | 0.475 | 0.574 |
| Tartrazine | 4.503 | 3.034 | 0.949 | 0.023 | 0.536 | 0.640 | |
From the analysed result, the adsorption capacity indicated that the waste nuts can be used as an alternative sorbents for the removal of color from food color. The adsorption kinetics revealed that the process followed the pseudo-first order kinetic model, whereas, adsorption equilibrium data were found to be well described by the Freundlich isortherm equation. The intra-particle diffusion is not the sole limiting step for the adsorption. The adsorption equilibrium was achieved after 20 minutes. Overall, groundnut shell showed higher adsorption for both sunset yellow and Tartrazine compared to coconut shell.
- Alison D, Collins P. Colouring our foods in the last and next millennium. Int J of Food Sci and Tech. 2001 Dec;35:5-22. doi: 10.1046/j.1365-2621.2000.00373.x
- Meyer Z. Where’s the best place to hit the Heinz? 7 fun facts as ketchup maker turns 150 this year. 2019;USA Today.
- Meggos H.Food colours: An international perspective. The manufacturing nconfectioner. 1995;59-65.
- Barrows JN, Lipman AL, Bailey CJ. Color Additives: FDA’s Regulatory Process and Historical Perspectives. 2009; FDA.
- Apipreeya K, Phussadee P, Prasert P. Removal of metal ion from synthetic wasteWater by activated carbon from Eucalyptus CamaldulensiDehn Bark. The 2nd Joint International Conference on Sustainable Energy and Environment 2006.
- Ozcan AS, Erdem B, Ozcan A. Adsorption of Acid Blue 193 from aqueous solutions onto Na-bentonite and DTMA-bentonite. J Colloid Interface Sci. 2004 Dec 1;280(1):44-54. doi: 10.1016/j.jcis.2004.07.035. PMID: 15476772.
- Isiuku BO, Enyoh CE. Water pollution by heavy metal and organic pollutants: Brief review of sources, effects and progress on remediation with aquatic plants. Analytical Methods in Environmental Chemistry Journal. 2019;2(03):5-38. doi.org/10.24200/amecj.v3.i03.66
- Yulu O, Zabaniotou A. Agricultural precursors for activated carbon production. Renewable and Sustainable Energy Reviews. 2007;11:1966-2005. doi: 10.1016/j.rser.2006.03.013
- Cabe Mc, Smith WL, Harriott P. Unit Operations of Chemical Engineering. 7th Edition. McGraw-Hill. 2006;836-844.
- Omonhenles S, Ofomaja A, Okiemen F. Adsorption of methylene blue by Unmodified and modified citric acid sawdust. Journal of Chemical Soc. of Nigeria. 2006;30:161-164 & Preparation an evaluation of activated carbon from coconut shell and walnut shell. Chemical Journal. 2006;191-196.
- Rahman IA, Saad B, Shaidan S, Sya Rizal ES. Adsorption characteristics of malachite green on activated carbon derived from rice husks produced by chemical-thermal process. Bioresour Technol. 2005 Sep;96(14):1578-83. doi: 10.1016/j.biortech.2004.12.015. Epub 2005 Feb 16. PMID: 15978990.
- Halward T, Stalker T, LaRue E, Kochert G. Use of single-primer DNA amplifications in genetic studies of peanut (Arachis hypogaea L.). Plant Mol Biol. 1992 Jan;18(2):315-25. doi: 10.1007/BF00034958. PMID: 1731991.
- David RD. Walnut production manual. UCANR.ISBN 978-1-879906-27-3. 1997.
- Ekuma FK. Chukwuemeka-Okorie H.O, Okoyeagu A, Chimeziri CC. STUDIES ON THE ADSORPTION OF TARTRAZINE AND SUNSET YELLOW DYES FROM AQUEOUS SOLUTION USING ACTIVATED GBAFILO (Chrysobalanus icaco) SHELL. J Chem Soc.Nigeria. 2019;44:937-947.
- Pearsall Ed. Coconut Concise Oxford Dictionary (10th ed). Oxford: Clarendon Press. 1999.
- Udo EJ, Ogunwale A. Laboratory Manual for the Analysis of Soil, Plant and WaterSamples, second ed. university press limited, Ibadan, Nigeria. AOAC. 1990. Association of Official Analytical Chemist. Official method of Analysis fourteen. Washington DC 1986.
- Diao Y, Walawender WP, Fan LT. Activated carbons prepared from phosphoric acid activation of grain sorghum. Bioresour Technol. 2002 Jan;81(1):45-52. doi: 10.1016/s0960-8524(01)00100-6. PMID: 11708755.
- Fatih D, Sengul K. Removal of Basic Red 46 dye from aqueous solution by pine tree leaves. Chem Eng J. 2011;170: 67-74. doi:10.1016/j.cej.2011.03.029
- Nasuha N, Hameed BH, Din AT. Rejected tea as a potential low-cost adsorbent for the removal of methylene blue. J Hazard Mater. 2010 Mar 15;175(1-3):126-32. doi: 10.1016/j.jhazmat.2009.09.138. Epub 2009 Oct 3. PMID: 19879046.
- Abdel NA, Hendawy EL. Surface and adsorptive properties of carbons prepared from biomass. Appl Surf Sci. 2005 Oct; 252(02): 287-295. doi: 10.1016/j.apsusc.2004.11.092
- Sadaa BH, Amarteyb YD, Bakoc S. An Investigation into The Use Of Groundnut Shell As Fine Aggregate Replacement. Nigerian Journal of Technology. 2013; 32.
- Nadavala SK, Mohammad A, Anesh MP, Madala S, Mansour IAH. Equilibrium and Kinetic Studies of Biosorptive Removal of 2,4,6-Trichlorophenol from Aqueous Solutions Using Untreated Agro-Waste Pine Cone Biomass. Process. 2019; 7(10):1-17. doi: 10.3390/pr7100757
- Asiagwu AK, Emoyan OO, Ojebah CK. Removal of Tartrazine Yellow Dye From Aqueous Solution using Groundnut Shell as Biomass: Kinetic Approach. International Journal of Engineering Research & Technology. 2018 May; 7(05).
- Enyoh CE, Isiuku BO. 2,4,6-Trichlorophenol (TCP) Removal from Aqueous Solution using Canna indica L: Kinetic Isotherm and Thermodynamic Studies. Chemistry and Ecology. 2020 June; doi: 10.1080/02757540.2020.1821673
- Lima DR, Lima EC, Umpierres CS, Thue PS, El-Chaghaby GA, da Silva RS, Pavan FA, Dias SLP, Biron C. Removal of amoxicillin from simulated hospital effluents by adsorption using activated carbons prepared from capsules of cashew of Para. Environ Sci Pollut Res Int. 2019 Jun;26(16):16396-16408. doi: 10.1007/s11356-019-04994-6. Epub 2019 Apr 13. PMID: 30982189.
- Zichao L, Hassan H, Lei Z, Lotfi S, Matias SN, Marcos LO, Moaaz KS, Guilherme LD, Adrian BP, Qun L. Adsorption of congo red and methylene blue dyes on an ashitaba waste and a walnut shell-based activated carbon from aqueous solutions: Experiments, characterization and physical interpretations. Chemical Engineering Journal, 2020 May; 124263. doi: 10.1016/j.cej.2020.124263
- Dai Y, Zhang N, Xing C, Cui Q, Sun Q. The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: A review. Chemosphere. 2019 May;223:12-27. doi: 10.1016/j.chemosphere.2019.01.161. Epub 2019 Feb 5. PMID: 30763912.
- Nobukane H, Yanagihara K, Kunisada Y, Ogasawara Y, Isono K, Nomura K, Tanahashi K, Nomura T, Akiyama T, Tanda S. Co-appearance of superconductivity and ferromagnetism in a Ca2RuO4 nanofilm crystal. Sci Rep. 2020 Feb 26;10(1):3462. doi: 10.1038/s41598-020-60313-x. PMID: 32103095; PMCID: PMC7044234.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.